Study on the Structure and Characteristics of Recyclable Copper Removal Adsorbent①
XIE Ji-Ling LUO Peng ZHENG Yue-Guo CHEN Fei-Yun YOU Rui-Rong WU Qiu-Ping YU Yan
(School of Material Science and Engineering, Fuzhou University(New Campus in University Town of Fuzhou), Fuzhou 350108, China)
1 INTRODUCTION
Copper ions and their compounds were toxic and universally exist in the contaminated water body,and they entered the organisms through respiratory tract and alimentary canal, causing great harm to human survival and biological diversification[1]. As there are many methods to treat copper-containing wastewater, the applied treatment method will be different for different forms of copper ions. Currently, the main methods to treat wastewater containing heavy metals domestic and abroad are chemical precipitation, iron oxidation, adsorption,ion exchange, electrolysis, photocatalysis, new mesoporous materials, gene technology, and so on.Each method has its own advantages and disadvantages, but after overall comparison, adsorption became the preferred technique in industrial treatment because of its wide applicable range, simple operation, recyclability of adsorbent and recyclability of useful heavy metals[2-6].
Adsorption is a method to purify wastewater by physical or chemical adsorption. The atoms or molecules on the surface of adsorbent will show a certain residual surface activity as an unbalanced force, so when the copper ions in wastewater contact with the surface of absorbent, they will be attracted by the attraction from the surface of adsorbent and then absorbed, so as to reach the purpose of purification of wastewater[7].
Aluminum sludge is obtained after precipitation and filtration of a large amount of colloid aluminum-containing wastewater produced in the process of aluminum surface treatment. Its main component is amorphous γ-AlOOH, which may change into such intermediates as γ-Al2O3, δ-Al2O3, θ-Al2O3, etc and into stable α-Al2O3at different sintering temperature.It has ultrafine particle size and very high activity to be used in many fields as the substitute of industrial aluminum oxide[8-9]. With huge specific surface area and strong adsorption, diatomite is widely applied in such fields as wastewater treatment, various fillers,porous ceramics, and so on[10]. With wide variety of sources and cheap price, the composite diatomite made from aluminum sludge followed by sintering at a certain temperature may be used for the preparation of wastewater adsorbent with ideal strength and sound adsorption. Such shaped adsorbent is easy to separate from water body, so it can be recyclable,and solve the problem that the conventional powder adsorbent is difficult to regenerate and recycle. In this study, we will discuss the effect of such adsorbent in different formulas and conditions on copper removal, so as to provide basic data for the development of low-cost and efficient adsorbent of wastewater purification.
2 EXPERIMENTAL METHODS AND PROCESS
2.1 Formulas of raw materials and preparation of samples
Aluminum sludge was obtained from Fujian Nanping Alumium Co., Ltd., and diatomite was purchased from Zhedong Diatomite Company (Food Grade), with their chemical compositions listed in Table 1.
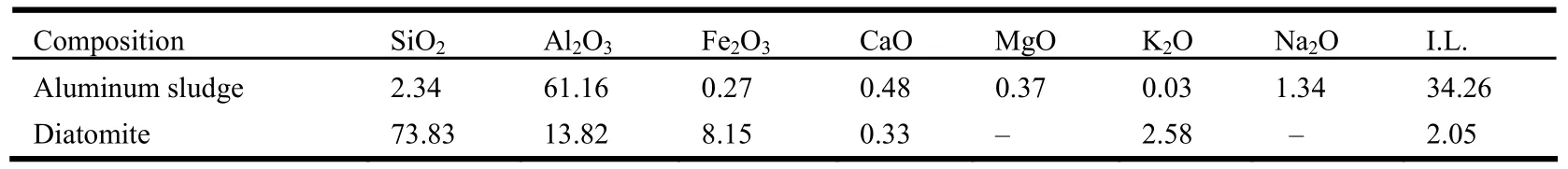
Table 1. Chemical Composition of Aluminum Sludge and Diatomite
The sludge pre-sintered at 900 ℃ and diatomite(100 sieves) are prepared according to mass ratios of 30:70, 40:60, 50:50, 60:40 and 70:30, respectively,and then well mixed with oyster shell powder (100 sieves) of 30% of the total quantity. After being pelletized by adding 5% polyvinylalcoho, it was weighed 1.0 g and shaped to be donut samples with diameter of 20.00 mm (shaping pressure: 2.0 MPa).Then the samples were placed in the muffle furnace and calcined to 1000 ℃.
2.2 Copper removal experiment
Cu(NO3)2(3.799 ± 0.001 g) was accurately weighed in 1 L volumetric flask and prepared to be copper stock solutions with concentration of 1 g/L after adding distilled water. Then the obtained solution was diluted to be copper-containing solution with different concentration.
The conditions for testing copper adsorptive capacity were as follows: the ambient temperature for experiment was 22 ℃, and the ratio of sample and wastewater usage was 1 g/25 mL. The copper removal effects of the samples were determined at a regular interval to get influence of different pH values (2, 3, 4, 5, 6, 7 and 8), different initial concentration of copper-containing wastewater (3, 5, 10,15 and 20 mg/L) and different adsorption time (20,40, 60, 90 and 180 min) on the adsorptive capacity.The concentration of copper ions was determined by TAS-986 atomic absorption spectro- scopy, and the adsorption capacity was represented as Qe(mg/g).Then the formula was as below:
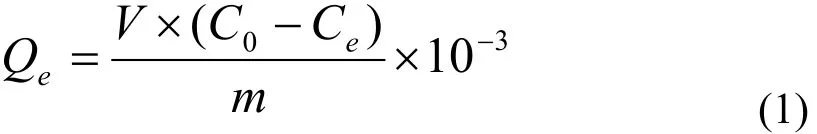
Where: V — the volume of solution (mL);
C0— the concentration of Cu2+(mg/L) in the wastewater before treatment;
Ce— the concentration of Cu2+(mg/L) in the wastewater after treatment;
m — the mass of adsorbent (g).
2.3 Structure and property characterization
The crystal phases in all samples were determined by XD-5A X-ray Powder Diffractometer (made by Shimadzu in Japan) (CuKα target, current: 40 mA,voltage: 40 kV, range of diffraction angle: 10~80°,scanning speed: 4 °/min). The microstructures of samples before and after copper removal were observed by Philips XL30ESEM Environmental Scanning Electron Microscopes. And the strength,water absorption rate and apparent porosity of the samples donut were also determined
3 RESULTS AND DISCUSSION
3.1 Influence of pH values on the adsorptive capacity of Cu2+
The influence of diatomite and aluminum sludge composites on the rate of Cu2+removal with different formulas under different pH conditions are shown in Fig. 1 (adsorption time was controlled to be 90 min). From Fig. 1 we can see that with increasing the pH value of the solutions, the rate of Cu2+removal is also increased. When the pH value is 6, the rates of Cu2+removal in 5 formulas have reached the maximum. Thereafter, the adsorptive rate begins to decrease because when the pH value is relatively low, the concentration of H+ions is relatively high, and H+and Cu2+thus exhibit competitive adsorption, which leads to lower adsorptive rate. And with further pH value increase, such competition weakens, and the adsorptive rate gets lager. After the pH value reaches 6, Cu2+starts to form the precipitation of Cu(OH)2that is always in the form of colloid flocculation and scatters in the water body. Cu2+is wrapped to some extent, which is not good for adsorption. Therefore, when the pH value is higher than 6, the removal rate of Cu2+shows a decreasing trend. Under the same pH values,compared with copper removal capacity of all formulas, the proportion of aluminum sludge:diatomite= 40:60 is relatively higher with the maximum adsorption capacity of about 0.46 mg/g.
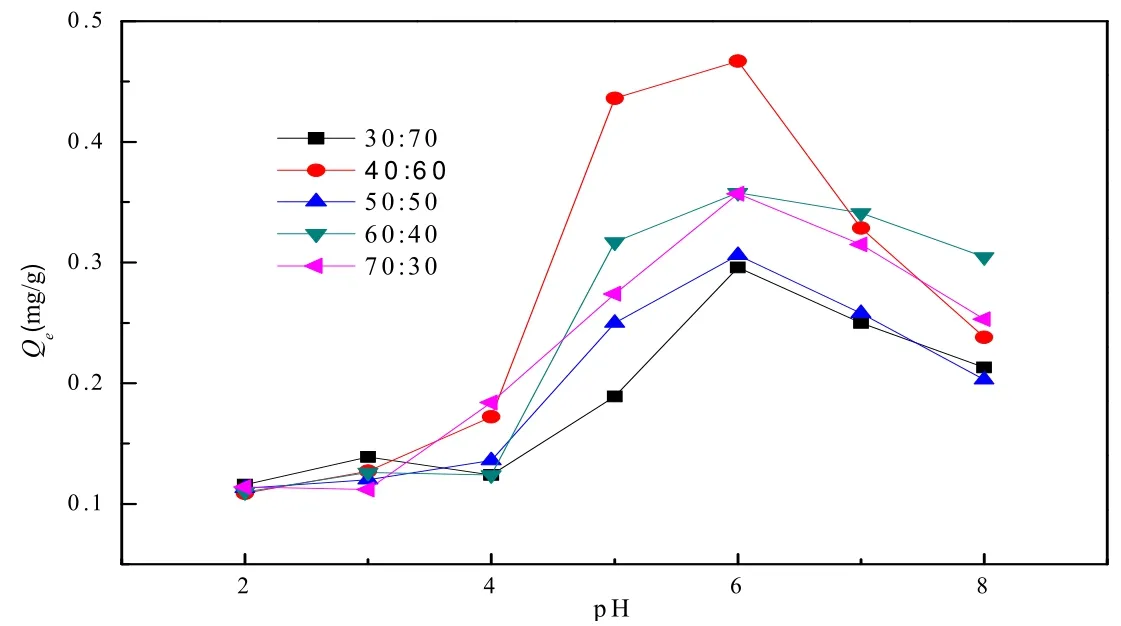
Fig. 1. Influence of pH on the Cu2+ adsorption capacity
3.2 Influence of the initial concentration on the Cu2+ adsorption capacity
When the pH value of copper-containing solution is controlled to be 6, the influence of samples of all formulas on Cu2+adsorption capacity with different initial concentration of copper-containing solutions is shown in Fig. 2, which displays that when the initial concentration of Cu2+is in the range of 3~10 mg/L, the adsorption capacity of all samples increases gradually with the rise of Cu2+concentration,but starts to decrease when the initial concentration of Cu2+reaches 15 mg/L. However, the adsorption capacity of solution with the initial concentration of 20 mg/L is still higher than that with lower initial concentration (< 10 mg/L). For the copper-containing wastewater with the same initial con- centration, the sample with formula of aluminum sludge:diatomite = 40:60 has higher copper removal capacity than other formulas.
3.3 Influence of adsorption time on the Cu2+adsorption capacity
When pH = 6, the Cu2+adsorption capacity of copper-containing wastewater with the initial concentration of 5 mg/L for different adsorption time is shown in Fig. 3. From Fig. 3, we can see that with the prolongation of adsorption time, the Cu2+removal rate of all formulas firstly rises and then maintains at a relatively stable level. When adsorption time reaches 90 min, the adsorption reaches equilibrium. And then, the removal rate fluctuates in a very small range. The copper adsorption capacities of all samples have no big difference. In particular,the adsorption capacity of the sample with aluminum sludge:diatomite = 40:60 is about 0.46 mg/g.
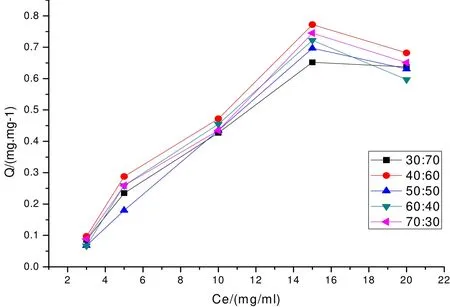
Fig. 2. Influence of Cu2+ initial concentration on its adsorption capacity
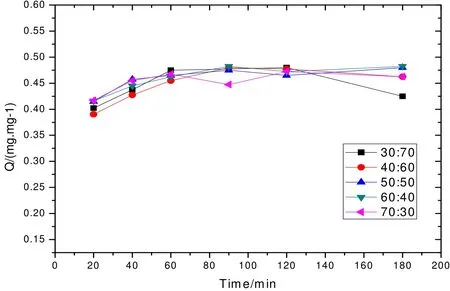
Fig. 3. Influence of adsorption time on the Cu2+ adsorption capacity
3.4 Sample property test results
The strength, porosity, water absorption rate and bulk density of different samples are tested, with results listed in Table 2.
Table 2 shows that with the increase in the content of diatomite in the formulas, the compressive strength and bulk density of samples present the trend of decrease, but the apparent porosity and water absorption rate are in an opposite trend. In particular, the sample with aluminum sludge: diatomite = 40:60 has its compressive strength of 8.20 MPa, apparent porosity of 47.02%, water absorption rate of 0.54% and bulk density of 1.08 g/cm3. And compared with other formulas, its strength may bear such forces as erosion, collision, etc. in the water body treatment process, so as to ensure longer service life. At the same time, its higher porosity and copper adsorption capacity make it become an ideal formula.
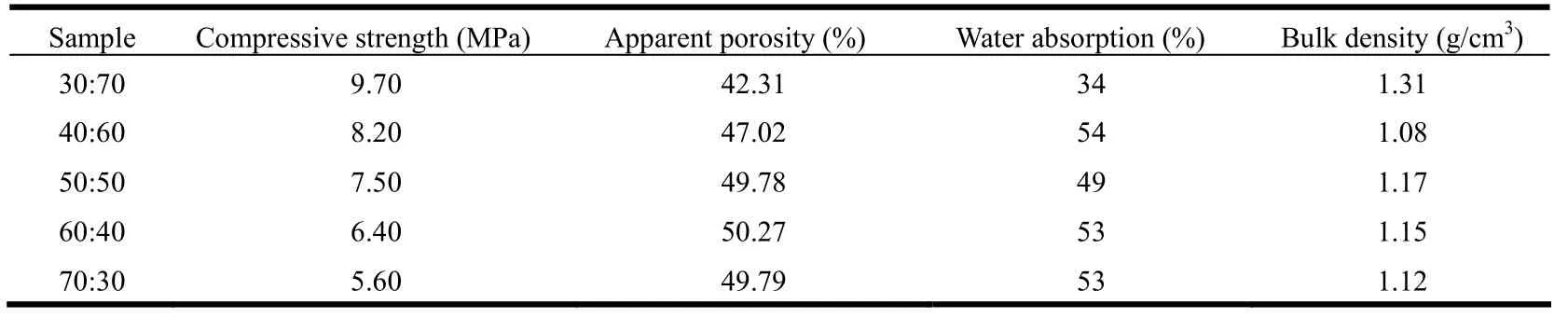
Table 2. Properties of Each Sample
3.5 X-ray analysis
XRD analysis is made to the samples with different formulas before and after adsorption, and the results are shown in Fig. 4 (a) and (b).
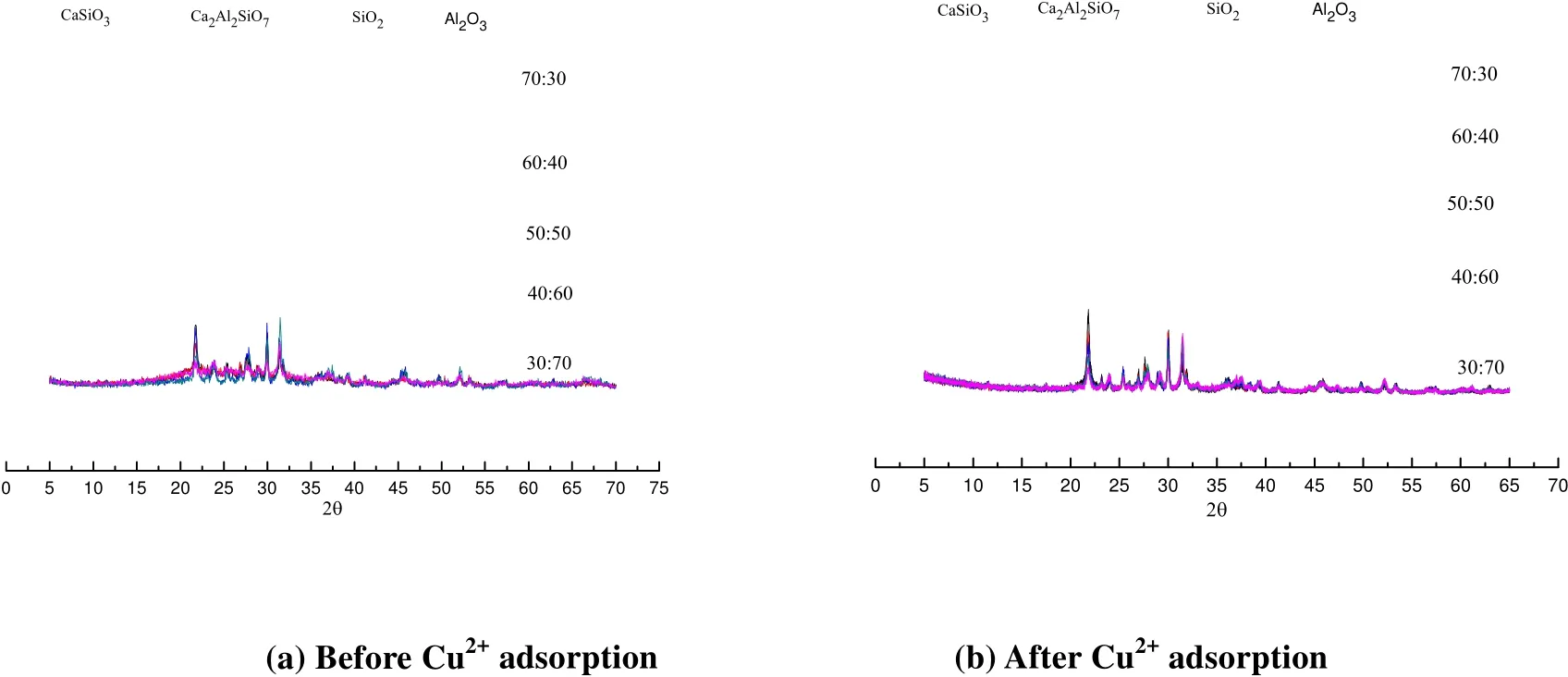
Fig. 4. XRD patterns of each sample before and after Cu2+ adsorption
From Fig. 4, we can see that before and after adsorption, there are four major phases in the absorbent such as CaSiO3, Ca2Al2SiO7, SiO2and Al2O3(CaSiO3(34-0612), Ca2Al2SiO7(34-1236),SiO2(47-1144) and Al2O3(04-0877). In particular,SiO2is the main component of diatomite, Al2O3is transformed from aluminum sludge after calcination at high temperature, and SiO2and Al2O3react with CaO obtained after calcination of oyster shell to produce corresponding calcium-containing phase CaSiO3or composite product of Ca2Al2SiO7. As the composite material has a lot of silicon-oxygen bonds and aluminum-oxygen bonds which may have complex reaction with copper ions, the samples thus present better copper removal property. In particular,calcium aluminosilicate has the structure similar to zeolite, not only keeping the porous structure of silicon oxide, but also having the activity of active aluminum oxide, which can help the adsorption. In formula 5, due to different proportions of raw materials, the contents of all crystalline phases in corresponding XRD pattern peaks are also different.However, before and after adsorption, the crystalline phases of the materials do not have any changes,indicating that the physical adsorption takes the lead in the process of adsorption.
3.6 SEM analysis
The microstructures of the sample with formula of aluminum sludge:diatomite = 40:60 before and after adsorption of copper are shown in Fig. 5(a) and 5(b).
From Fig. 5, we can see that the copper removal material prepared by aluminum sludge and diatomite is of porous structure and large specific surface area to provide rich adsorption points which can help the adsorption. Before and after Cu2+adsorption, the samples still have higher porosity, so they have high Cu2+adsorption capacity to ensure longer service life.
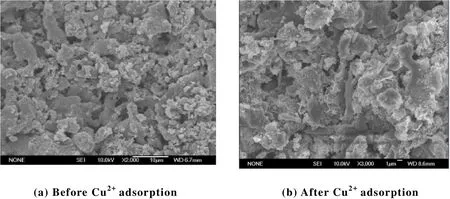
Fig. 5. SEM images of samples before and after Cu2+ adsorption
4 CONCLUSION
(1) Aluminum sludge and diatomite can be used to prepare copper removal material with higher strength, longer service life and high copper removal efficiency. The formula of aluminum sludge:diatomite = 40:60 has the proportion of efficient adsorption, with compressive strength reaching 8.20 MPa,apparent porosity of 47.02%, water absorption rate reaching 0.54%, and bulk density of 1.08 g/cm3.
(2) The optimal external conditions to remove copper are pH = 6, adsorption time of 90 min and initial concentration of Cu2+not higher than 15 mg/L,then the Cu2+adsorption capacity may reach 0.46 mg/g when the initial concentration is 5 mg/L.
(1) Wang, D. H.; Xu, X. H.; Song, S. Manual of Special Contaminate Treatment in Industrial Wastewater.Beijing, Chemical Industry Press 2000, 54–65.
(2) Wen, M. B.; Wang, D. S. Overview of heavy metal wastewater treatment methods. Industrial Water Treatment 2011, 65–68.
(3) Paul, C. J.; Wu, S. N. Simultaneous adsorption of copper ions and humic acid onto an activated carbon.Journal of Colloid and Interface Science 2004, 334–342.
(4) Xu, X. Y.; Shang, A. (Zambia). Study on the treatment of acid copper-containing wastewater from mines. Metal Mines 2006, 76–78.
(5) Guo, R. D.; Wu, H.; Zhang, X. Y. Study on the treatment methods of high-concentrated copper-containing wastewater. Modern Chemical Industry 2004, 33, 280–282.
(6) Song, C. L.; Chen, Z. W.; Fan, H. M. Overview of copper-containing wastewater treatment technology. Chemical Defense on Ships 2008, 22–25.
(7) Ye, Z. H.; Song, Q. Adsorption and Ion Exchange (17thChapter in Chemical Engineering Manual). Chemical Industry Press, Beijing 1985.
(8) Yu, Y.; Ruan, Y. Z.; Huang, Q. M. Study on crystalline structures of aluminum sludge at different calcinating temperatures. Chin. J. Struct. Chem. 2003, 22 , 607–613.
(9) Yu, Y.; Ruan, Y. Z.; Wu, R. P.; Liu, S.; Zeng, H. R. Research of high temperature crystalline structure and property evolution of magnesium aluminate spinel. Chin. J. Struct. Chem. 2008, 26, 1455–1460.
(10) Wu, H. P.; Wu, R. P.; Yu, Y.; Huang, M. L. Preparation of and study on diatomite-based porous ceramic. Bulletin of the Chinese Ceramic Society 2009, 28, 641–645.
- 结构化学的其它文章
- Synthesis of a Weak Basic uPA Inhibitor and Crystal Structure of a Complex with uPA①
- Crystal Structure of PAI-1 in Complex with Gallate①
- Interesting Weak Interactions in Three Silver Coordination Polymers Based on Tetrafluorobenzenedicarboxylate and Benzonitrile Ligands①
- Synthesis and Crystal Structure of (Z)-N-(2-(diethylamino)ethyl)-7-(5-fluoro-2-oxoindolin-3-ylidene)-2-methyl-4,5,6,7-tetrahydro-1H-indole-3-carboxamide Methanol Solvate①
- Synthesis, Crystal Structure, and Biological Activity of 4-Chlorobenzaldehyde(2-trifluoromethylTrifluoromethyl-5,6,7,8-tetrahydrobenzo[4,5]- thieno[2,3-d]pyrimidin-4-yl)hydrazone Monohydrate①
- DFT Study on the Electronic and Structural Properties of MoS6-/0 Clusters①

