晶种导向干凝胶法制备成型ZSM-5分子筛
岳明波 杨 娜 王一萌,*
1 Introduction
Zeolites and related microporous materials have been extensively studied for industrial applications such as adsorbents, catalysts,and separation media.Recently,more work focused on the improvement of the bulky molecule diffusion in zeolites.1,2Hierarchical porous zeolites with mesoporosity and/or macroporosity or nanoscale zeolites with high external surface areas are alternatives to break these diffusion limitations in the intrinsically molecular-sized micropores of zeolites.3-9In addition,zeolites synthesized via conventional techniques are usually obtained as powders.To make them suitable for practical applications,zeolites are usually shaped into large shapes or granules with the help of inorganic binders to avoid excessive pressure drops in fixed bed reactors and/or dusting problems.The addition of binders may result in partial blocking of the zeolite pore system and also leads to dilution of the active species. Therefore,it could be interesting to develop a mechanically stable and self-supporting shaped zeolite with both uniform shape and hierarchical structure.
In general,there were two strategies to fabricate shaped zeolites.One involves nanocrystal zeolites being assembled as shaped zeolites and another involves the transformation of shaped amorphous aluminosilicate into zeolites by in situ crystallization.In the former strategy,extra templates,such as nonionic surfactants,10carbon,11-14and polymers,15-17can be introduced to assemble the nanoscale zeolite into monoliths or to shape the zeolite nanocrystals in confined syntheses,which increases the cost of the preparation process and limits its industrial application.In the latter approach,amorphous aluminosilicate precursors were extruded or molded to certain shape and were then converted to zeolites through an appropriate crystallization process.The precursor shape was retained and the amorphous microstructure was transferred into the crystalline zeolite through this in situ crystallization process.By this strategy, various zeolites with hierarchical pore structures were obtained by adjusting the composition of the precursor.18-21For example, hollow zeolite capsules with three-dimensionally ordered macropores were prepared through transformation of zeolite-seeded silica spheres.22Closely packed nanocrystalline ZSM-5 and beta zeolites were also prepared from amorphous silica grains.23,24In addition,macroporoussilica-alumina compositeswith MFI-type crystals were prepared by seed-induced in situ and layer-by-layer synthetic methods.25However,attempts to control the zeolite crystal size in such shaped zeolites are seldom reported.
Apart from hierarchically porous zeolites,nanocrystalline zeolites with higher surface area were also expected to solve the diffusion limitations involved in catalytic reactions.26However, the difficulties of separation and recovery of nanosized zeolites cause problems in industrial application.Therefore,it is highly desirable to synthesize nanocrystalline zeolite aggregates with a monolithic shape in one step,which would avoid the recovery steps.This binderless nano-crystalline shaped zeolite would exhibit a high catalytic activity in the bulk molecular reaction and curtail the difficulties in the processing to the shaped catalyst.
A hydrothermal synthesis process was usually used to convert the shaped aluminosilicate precursor to crystalline zeolites.However,limited by the diffusion,the growth rate of zeolite on the surface was always higher than that in the inner region of the cylindrical extrudates in the hydrothermal treatment process.23This means that the zeolites always aggregate on the surface of extrudates and the zeolite size distribution is not uniform.In the dry gel conversion(DGC)method,the transformation to the zeolite was accomplished in the presence of water vapor below the saturation condition.Compared with hydrothermal synthesis method,the impact of diffusion on zeolite growth rate was minimized in the DGC method because of higher diffusion rate of gas than liquid.In the DGC process,no solid contacted directly with water.Usually,capillary condensation of water vapor enabled the surface of the amorphous silica gel to be covered with a thin layer of water during the steaming treatment.Hence,the zeolitization of the silicate gel was performed just in the microareas of the precursor gel,with the digestion of the aluminosilicate gel and crystallization of the zeolite being located in this micoarea.A seed surface crystallization approach can be used for rapid synthesis of ZSM-5 zeolites with controllable crystal size and morphology.27The crystallization of ZSM-5 zeolites occurred on the surface of zeolite seeds with rapid crystallization rate.So,in this work,we plan to combine the seed surface crystallization and DGC method for the preparation of zeolites and to fabricate binderless nanocrystalline shaped zeolites.
2 Experimental
2.1 Shaped zeolite preparation
The chemicals used in this work were sourced commercially and used without further purification.Tetrapropylammonium hydroxide(TPAOH,25%solution)was purchased from Shanghai Cainorise Chemicals Co.,Ltd.Silica sol(mass fraction of SiO2:30%),silica gel(mass fraction of SiO2:90%),and boehmite(mass fraction of Al2O3:70%)were purchased from the Qingdao Haiyang Chemicals Co.,Ltd.Tetraethylorthosilicate (TEOS,AR grade)was purchased from the Sinopharm Chemical Reagent Co.,Ltd.The seed gel was prepared by mixing TPAOH,distilled water,and TEOS following the molar composition:0.35TPAOH:1TEOS:20H2O.After additional stirring at room temperature for 10 h,the mixture was hydrothermally treated at 100°C for 24 h to obtain the seed gel.
The fabrication of shaped ZSM-5 zeolite was generally divided into two processes:the preparation of the amorphous aluminosilicate extrudates(the shaped zeolite precursor,denoted as SZP)and the followed crystallization process by the DGC method.The SZP extrudates with the molar composition of 0.025TPAOH:1SiO2:0.011Al2O3were typically prepared as follows:silica gel and boehmite were well mixed,and then silica sol,seed gel and/or TPAOH solution were added.The total molar ratio of TPAOH to SiO2was fixed at 0.025.When the seed gel was added,the mixtures were kneaded carefully to form a gel and extruded into cylindrical shaped extrudates with diameter of 2 mm.After drying at room temperature for 48 h,the extrudates(1.2 g)were put in a polytetrafluoroethylene(PTFE)-lined stainless steel autoclave,containing 10 mL pre-added water at the bottom to produce saturated steam/water(caution:no solid contacted directly with water).After heating at 175°C for different times,the auto clave was cooled to room temperature and the solid was separated,air-dried and finally calcined in air at 550°C for 5 h to ensure the complete removal of the template and other organic material.The obtained shaped zeolite was denoted SZ-x-y,where x represents the percentage of TPAOH added from seed gel to total added TPAOH and y represents the heating time.
2.2 Characterization
The X-ray diffraction(XRD)measurements were carried out on a Rigaku-Ultima diffractometer with Cu Kαradiation in the 2θ range from 5°to 35°.The relative crystallinity was estimated by comparing the five peaks areas(22°-28°)of these samples with those obtained for a commercial ZSM-5 sample,purchased from the Catalyst Plant of Nankai University.Infrared tests were performed on a Nicolet Fourier transform infrared (FTIR)spectrometer(NEXUS 670)using the conventional KBr wafer technique.Nitrogen adsorption-desorption isotherms were measured at-196°C on a Quantachrome Autosorb-3B volumetric adsorption analyzer.Before the measurements,the sample was out gassed for 4 h in the degas port of the adsorption apparatus at 300°C.The BET specific surface area was calculated using adsorption data acquired at a relative pressure (p/p0)range of 0.05-0.22.The total pore volume was determined from the amount adsorbed at a relative pressure of about 0.99.The micropore volume and external surface were obtained by t-plot analysis using a relative pressure range of 0.05-0.33.The pore size distribution(PSD)curves were calculated from the analysis of adsorption branch of the isotherm using the Barrett-Joyner-Halenda(BJH)algorithm.Thermogravimetric(TG)and derivative thermogravimetric(DTG)analyses were performed using a Perkin-Elmer TGA analyzer with a heating rate of 10°C·min-1up to 800°C under an air flow to discover the various decomposition steps occurring in the as-dried precursor as a function of temperature.Scanning electron microscope(SEM)was performed by an S-4800 field emission scanning electron microscope(FE-SEM,Hitachi,Japan)with an acceleration voltage of 3 kV.Transmission electron microscopy(TEM)was performed by an S-4800 field emission microscope(FE-SEM,Hitachi,Japan)with an acceleration voltage of 30 kV.The ammonia temperature programmed desorption(NH3-TPD)experiments were carried out in a flow apparatus with helium as carrier gas(30 mL·min-1). These samples were transformed into H-type zeolite before NH3-TPD experiments through NH+4exchange and calcination at 550°C.
3 Results and discussion
Powder XRD was used to monitor the phase structure changes taking place in the DGC process.There are four series of samples with different amounts of seed gel addition:SZ-0, SZ-10,SZ-20,SZ-100,where 0,10,20,100 represent the percentages of TPAOH added from seed gel to total added TPAOH. Fig.1 shows the XRD patterns of these samples with different crystallization times.As seen from Fig.1,the wide dispersive peak of amorphous aluminosilicate at 15°-30°disappeared and the diffraction peaks of ZSM-5 zeolite appeared for these four series samples after 24 h crystallization.However,the relative crystallinity and the crystallization ratio of these four series samples are different.After a heating time of 2 h,the SZ-0-2h sample exhibits the broad peak of amorphous aluminosilicate without diffraction peaks of ZSM-5 zeolite(Fig.1A);SZ-10-2h and SZ-20-2h samples display the diffraction peaks of both amorphous aluminosilicate and ZSM-5 zeolites(Fig.1(B, C))and in the case of the SZ-100-2h sample,the diffraction peaks of amorphous aluminosilicate disappear and only the diffraction peaks of ZSM-5 zeolite are seen.Fig.2 gives the crystallization curves of these four samples loaded with different seeding gels.The crystallization process proceeded more rapidly with higher levels of seed gel addition in the precursor.For example,SZ-100 series sample achieve 100%relative crystallinity within 3 h.With the same amounts of TPAOH in the precursor,SZ-0-3h only displays 50%relative crystallinity after 3 h crystallization.Even after 24 h heating,the relative crystallinity of the obtained SZ-0-24h sample just achieved 80%.The reason can be ascribed to the addition of seed gel which pro-motes the growth of zeolite.The crystallization of ZSM-5 zeolites can occur on the surface of seeds with a rapid crystallization rate.27

Fig.1 XRD patterns of a series of samples heated for different times
TG and DTG analyses of the as-prepared SZ-100 and SZ-0 series samples are shown in Fig.3 and Fig.4,respectively. These TG curves can be divided into three zones based on the DTG curves of these samples.The mass loss in zone I(25-100°C)corresponds to the removal of physisorbed water. Zone II(100-300°C)is associated with the thermal decomposition of the structure-directing agent(TPAOH)in the amorphous aluminosilicate gels.Zone III(300-800°C)involves the decomposition of TPA+ions within the micropore of zeolite formed.As shown in Fig.3 and Fig.4,the mass loss in zone II decreased with a prolonging of the crystallization time.In contrast,the mass loss in zone III increased with increasing crystallization time.After 24 h crystallization,the mass loss of SZ-100-24h sample in zone II almost decreased to zero.Correspondingly,the relative crystallinity of this sample was over 100%.However,after the same 24 h crystallization period,the mass loss of SZ-0-24h sample in zone II was about 23%and the relative crystallinity of this sample was 80%.So,the TPAOH was occluded gradually into the micropores of the formed zeolite in the crystallization process.When the amorphous aluminosilicate gels were completely transformed into zeolite crystals,most TPAOH entered the micropores of the zeolite in this crystallization process.

Fig.2 Crystallization curves of a series of samples
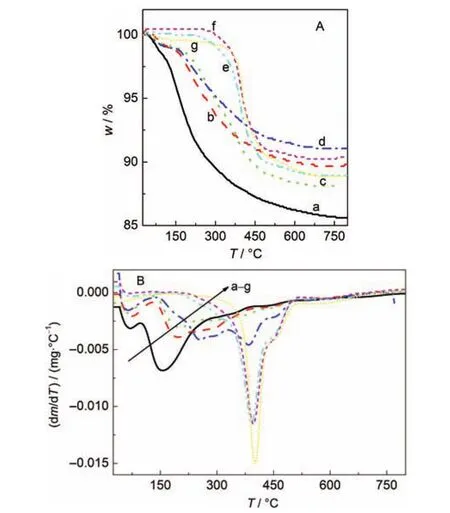
Fig.3 TG(A)and DTG(B)curves of the SZ-100 series of samples(a)SZ-100-0h,(b)SZ-100-30min,(c)SZ-100-50min,(d)SZ-100-70min, (e)SZ-100-90min,(f)SZ-100-2h,(g)SZ-100-24h
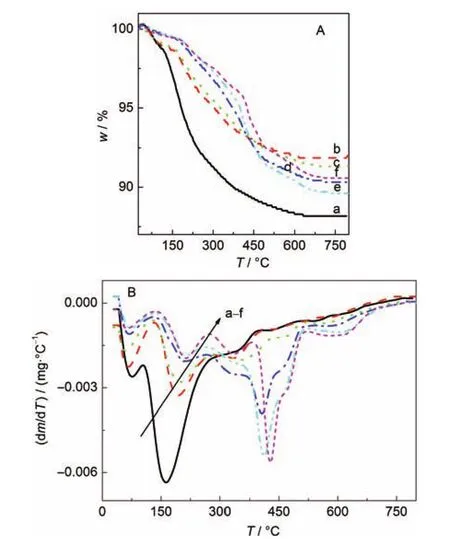
Fig.4 TG(A)and DTG(B)curves of the SZ-0 series of samples(a)SZ-0-0h,(b)SZ-0-50min,(c)SZ-0-2h,(d)SZ-0-150min, (e)SZ-0-5h,(f)SZ-0-24h
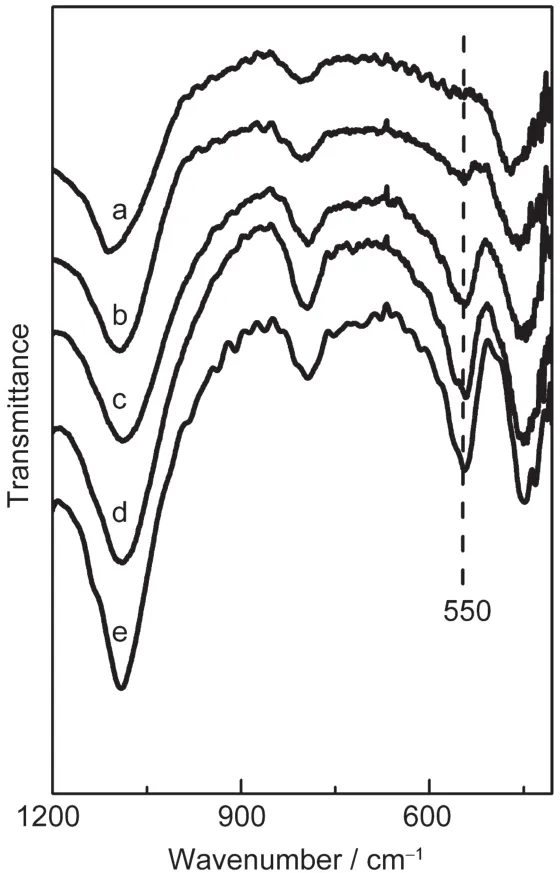
Fig.5 FTIR spectra of the SZ-100 series of samples(a)SZ-100-0h,(b)SZ-100-50min,(c)SZ-100-2h,(d)SZ-100-150min, (e)SZ-100-24h
Fig.5 shows the FTIR spectra of the calcined SZ-100 series of samples prepared with different crystallization times.Compared with the precursor(Fig.5a),the new band at 550 cm-1, characteristic of MFI zeolites with five-membered rings,appeared in four samples.The intensity ratio of the 550 and 450 cm-1bands had been used to assess the formation of MFI zeolite and has been termed the FTIR crystallinity.28,29As shown in Fig.5,the FTIR crystallinity of these samples increased rapidly after 2 h crystallization,which was in accordance with the results of XRD analysis.
The nitrogen sorption isotherms and pore size distributions of these samples are shown in Fig.6.Before steam heating,a type IV isotherm is observed for shaped precursor(SZ-100-0h) with pore distribution around 10 nm(Fig.6B(a)).After steam heating treatment for 24 h,the mesoporosity of the obtained samples(SZ-100-24h)is still retained.However,the pore volume declines from 0.68 to 0.38 cm3·g-1and the pore size decreases from 10 to 2 nm.The reason can be ascribed to micropore creation and mesopore shrinkage in the crystallization process.The SZ-100-24h sample displays a slight uptake of nitrogen and a small hysteresis loop at higher relative pressure range of p/p0=0.50-0.90,indicating the existence of some mesopores.Furthermore,this H4 type horizontal hysteresis loop could be related to the presence of large mesopores embedded in a matrix with pore mouths of a much smaller size,which means that there are secondary pores in contact with the exterior by the zeolite channels.30As shown from the PSD curves, the secondary pores are about 2 nm for this SZ-100-24h sample(Fig.6B(b)),which suggests the presence of mesopores and micropores in such a hierarchical structure.The BET specific surface area and micropore volume of the sample(SZ-100-24h, 363 m2·g-1and 0.10 cm3·g-1)are much larger than the precursor(SZ-100-0h,320 m2·g-1and 0.01 cm3·g-1),further proving the formation of the microporous ZSM-5 zeolite.Especially, SZ-100-24h sample possessed a high mesopore volume(0.28 cm3·g-1).
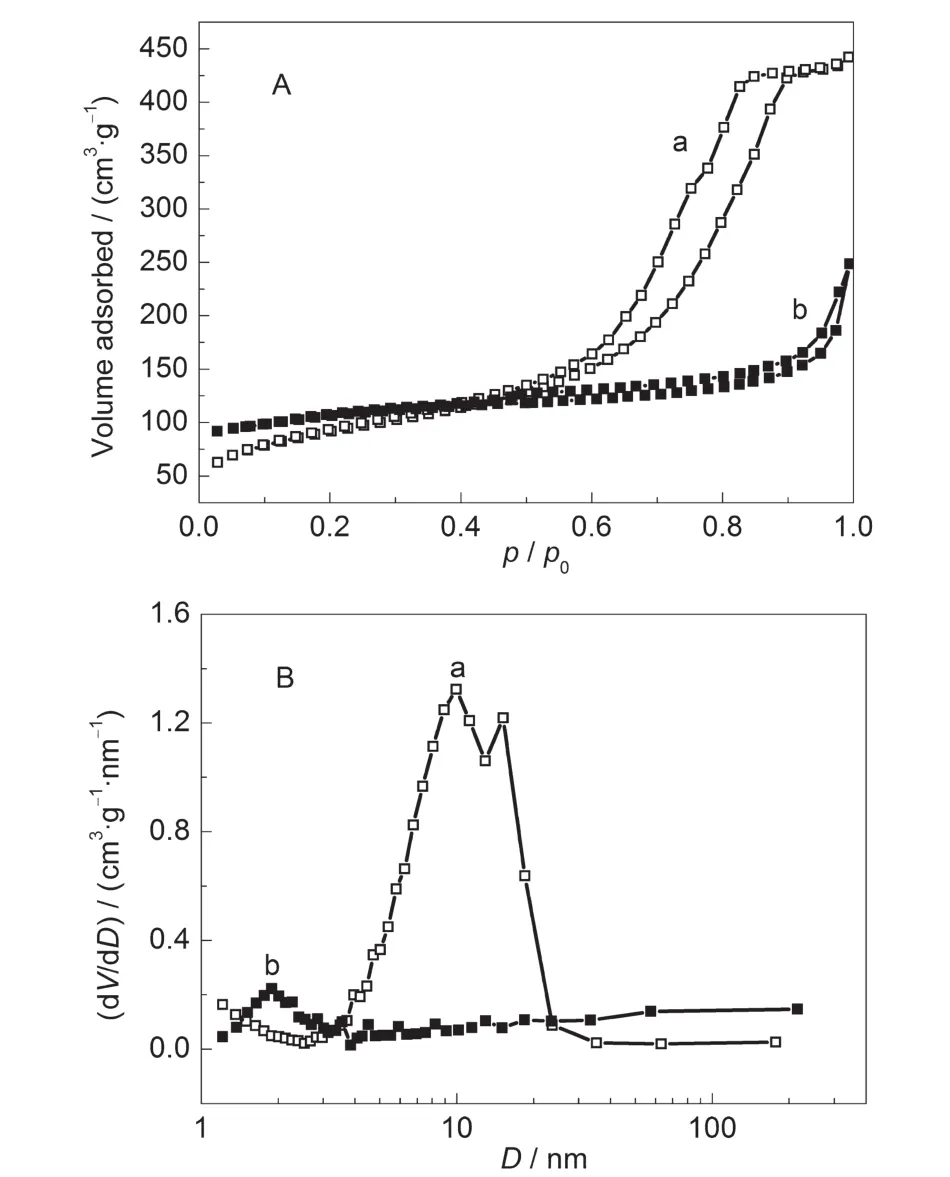
Fig.6 Nitrogen adsorption-desorption isotherms(A)and pore size distributions(B)of the SZ-100 series of samples(a)SZ-100-0h,(b)SZ-100-24h

Fig.7 Photograph(A),SEM(B),and TEM(C)images of the SZ-100-24h sample
As shown in the SEM image(Fig.7B),the SZ-100-24h sample is composed of zeolite nanocrystals which overlap but do not aggregate to microspheres.Fig.7C gives the TEM image of the SZ-100-24h sample after grinding to powder,which also proves that the zeolite crystals are around 200 nm in this sample.The reason is ascribed to the addition of the seed gel.The crystallization of zeolite was carried out on the surface of seed and the amorphous aluminosilicate gel converted into nanosized zeolite in situ.In addition,the initial cylindrical shape of the aluminosilicate extrudates was well maintained during the crystallization process(Fig.7A),and the final products possessed excellent mechanical strength.This photograph demonstrated that the amorphous aluminosilicate precursor could be converted into ZSM-5 zeolite and the morphology retained during the DGC process.
NH3-TPD curves of the SZ-100-24h and commercial HZSM-5(with same SiO2/Al2O3molar ratio of 90 as SZ-100-24h)samples are shown in Fig.8.These two samples gave similar TPD curves with two desorption peaks at 170 and 360 °C,indicating weak and strong acid sites,respectively.The acid strength of the SZ-100-24h sample was similar to that of the commercial H-ZSM-5 zeolite.These results mean that the SZ-100-24h sample displays a similar acidity to that of the commercial H-ZSM-5 catalyst,which would be used as an industrial zeolite catalyst.
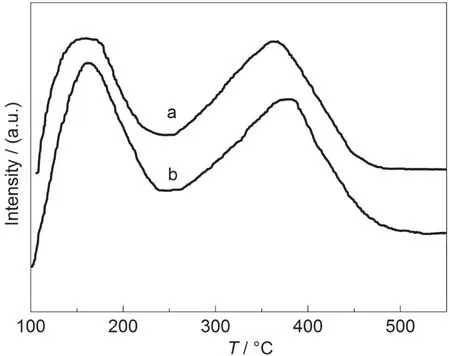
Fig.8 NH3-TPD curves of two samples(a)SZ-100-24h,(b)commercial H-ZSM-5
4 Conclusions
Column shaped ZSM-5,composed of nanocrystalline zeolites,with mesoporous structure has been successfully synthesized by the DGC method.The formation of nanocrystalline zeolite is contributed by the addition of seed gel and involved surface crystallization on the seed.Moreover,the seed gel not only acts as a seed but also serves as a binder to shape the zeolite. The obtained shaped ZSM-5 possesses a significant textual porosity and exhibits an acidity comparable with that found in a commercial powder ZSM-5.
(1) Holma,M.S.;Taarninga,E.;Egeblada,K.;Christensen,C.H. Catal.Today 2011,168,3.doi:10.1016/j.cattod.2011.01.007
(2) Jones,C.W.;Tsuji,K.;Davis,M.E.Nature 1998,393,52.doi: 10.1038/29959
(3)Zhai,S.R.;Wei,L.;Yang,D.J.;Wu,D.;Sun,Y.H.Prog.Chem. 2006,18,1330.[翟尚儒,魏 莉,杨东江,吴 东,孙予罕.化学进展,2006,18,1330.]
(4)Tao,Y.;Kanoh,H.;Abrams,L.;Kaneko,K.Chem.Rev.2006, 106,896.doi:10.1021/cr040204o
(5) Perez-Ramirez,J.;Christensen,C.H.;Egeblad,K.;Groen,J.C. Chem.Soc.Rev.2008,37,2530.doi:10.1039/b809030k
(6) Egeblad,K.;Christensen,C.H.;Kustova,M.Chem.Mater. 2008,20,946.doi:10.1021/cm702224p
(7) Ma,Y.H.;Zhao,H.L.;Tang,S.J.;Hu,J.;Liu,H.L.Acta Phys.-Chim.Sin.2011,27,689.[马燕辉,赵会玲,唐圣杰,胡 军,刘洪来.物理化学学报,2011,27,689.]doi:10.3866/ PKU.WHXB20110335
(8) Choi,M.;Cho,H.S.;Srivastava,R.;Venkatesan,C.;Choi,D. H.;Ryoo,R.Nat.Mater.2006,5,718.doi:10.1038/nmat1705
(9)Zhang,Z.T.;Han,Y.;Zhu,L.;Wang,R.W.;Yu,Y.;Qiu,S.L.; Zhao,D.Y.;Xiao,F.S.Angew.Chem.Int.Edit.2001,40,1258. doi:10.1002/1521-3773(20010401)40:7<1258::AIDANIE1258>3.0.CO;2-C
(10)Hu,G.;Ma,D.;Liu,L.;Cheng,M.J.;Bao,X.H.Angew.Chem. Int.Edit.2004,43,3452.doi:10.1002/anie.200453777
(11) Li,W.C.;Lu,A.H.;Palkovits,R.;Schmidt,W.;Spliethoff,B.; Schuth,F.J.Am.Chem.Soc.2005,127,12595.doi:10.1021/ ja052693v
(12)Yoo,W.C.;Kumar,S.;Penn,R.L.;Tsapatsis,M.;Stein,A. J.Am.Chem.Soc.2009,131,12377.doi:10.1021/ja904466v
(13)Tong,Y.;Zhao,T.;Li,F.;Wang,Y.Chem.Mater.2006,18, 4218.doi:10.1021/cm060035j
(14) Zhao,T.;Xu,X.;Tong,Y.;Lei,Q.;Li,F.;Zhang,L.Catal.Lett. 2010,136,266.doi:10.1007/s10562-009-0131-8
(15)Dong,A.G.;Wang,Y.J.;Tang,Y.;Zhang,Y.H.;Ren,N.;Gao, Z.Adv.Mater.2002,14,1506.doi:10.1002/1521-4095 (20021016)14:20<1506::AID-ADMA1506>3.0.CO;2-Z
(16)Lee,Y.J.;Kim,Y.W.;Jun,K.W.;Viswanadham,N.;Bae,J.W.; Park,H.S.Catal.Lett.2009,129,408.doi:10.1007/ s10562-008-9811-z
(17) Saini,V.K.;Pinto,M.L.;Pires,J.Colloids Surf.A 2011,373, 158.doi:10.1016/j.colsurfa.2010.10.047
(18) Huang,Y.;Dong,D.;Yao,J.;He,L.;Ho,J.;Kong,C.;Hill,A. J.;Wang,H.Chem.Mater.2010,22,5271.doi:10.1021/ cm101408n
(19) Wang,D.J.;Liu,Z.N.;Xie,Z.K.J.Inorg.Mater.2008,23(3), 592. [王德举,刘仲能,谢在库.无机材料学报,2008,23(3), 592.]doi:10.3724/SP.J.1077.2008.00592
(20) Tokudome,Y.;Nakanishi,K.;Kosaka,S.;Kariya,A.;Kaji,H.; Hanada,T.Microporous Mesoporous Mat.2010,132,538.doi: 10.1016/j.micromeso.2010.04.005
(21) Möller,K.;Yilmaz,B.;Jacubinas,R.M.;Müller,U.;Bein,T. J.Am.Chem.Soc.2011,133,5284.doi:10.1021/ja108698s
(22) Shi,J.;Ren,N.;Zhang,Y.H.;Tang,Y.Microporous Mesoporous Mat.2010,132,181.doi:10.1016/j.micromeso. 2010.02.018
(23) Wang,D.J.;Liu,Z.N.;Wang,H.;Xie,Z.K.;Tang,Y. Microporous Mesoporous Mat.2010,132,428.doi:10.1016/ j.micromeso.2010.03.023
(24) Lei,Q.;Zhao,T.;Li,F.;Wang,Y.F.;Hou,L.J.Porous Mater. 2008,15,643.doi:10.1007/s10934-007-9144-0
(25) Xu,X.;Zhao,T.;Qi,J.;Guo,Y.;Miao,C.;Li,F.;Liang,M. Mater.Lett.2010,64,1660.doi:10.1016/j.matlet.2010.04.057
(26) Schmidt,I.;Madsen,C.;Jacobsen,C.J.H.Inorg.Chem.2000, 39,2279.doi:10.1021/ic991280q
(27) Ren,N.;Yang,Z.J.;Liu,X.C.;Shi,J.;Zhang,Y.H.;Tang,Y. Microporous Mesoporous Mat.2010,131,103.doi:10.1016/ j.micromeso.2009.12.009
(28) Jacobs,P.A.;Beyer,H.K.;Valyon,J.Zeolites 1981,1,161.doi: 10.1016/S0144-2449(81)80006-1
(29) Jansen,J.C.;Vander-Gaag,F.J.;Van-Bekkum,H.Zeolites 1984,4,369.doi:10.1016/0144-2449(84)90013-7
(30) Kruk,M.;Jaroniec,M.Chem.Mater.2001,13,3169.doi: 10.1021/cm0101069