无有机模板剂水热合成Co同晶取代的丝光沸石分子筛
王 琦 吴雅静 王 军,* 林 晓
(1南京工业大学化学化工学院,材料化学工程国家重点实验室,南京210009;2南京工业大学理学院,南京210009)
1 Introduction
Zeolite materials have been widely used as catalysts,ion-exchangers,and adsorbents due to their uniform micropores,high surface areas,adjustable acidities,and stable structures.Incorporation of transition metals like Fe,Ti,Ni,Co,or V into zeolites may cause new applications.1-5Particularly,Co-containing zeolites have attracted attention because they are efficient catalysts for reduction of NOx,6,7Fischer-Tropsch reaction,8,9and selective oxidation of alkanes.10,11However,in most of previous efforts,Co atoms were introduced into zeolites by impregnation and ion-exchange.In order to obtain single and highly dispersed Co sites without blocking the intrinsic micropores of zeolites,isomorphous substitution of Co ions into zeolite framework via a direct hydrothermal synthesis becomes an interesting topic.Nowadays,some framework-substituted Co-aluminophosphate(CoAPO)molecular sieves have been synthesized,12-14nevertheless,only a few Co-substituted aluminosilicate zeolites have been reported,such as MFI(mobil fifth), BEA(beta),ANA(analcime),and PHI(phillipsite)zeolites.15-17
Mordenite(MOR)has been widely applied in petroleum industry for isomerization,dewaxing,and alkylation,18,19but Co-mordenite has been scarcely reported.Kato et al.20successfully synthesized a Co-mordenite by a dynamic hydrothermal treatment with a cobalt-ethylenediamine-N-monoacetic acid complex as the cobalt source.On the other hand,synthesizing heteroatomic zeolites always employs organic templates as the structural directing agencies.21-26For catalytic utilizations,the organic templates have to be removed by a high-temperature calcination so as to obtain the prerequisite open micropores. Consequently,the high-temperature treatment results in environmental pollution,large energy consumption,and high cost.27,28In earlier studies,Fe-and Zn-containing mordenites were obtained by a conventional static hydrothermal synthesis using sodium silicate as the silica source in the presence of organic templates.29,30Moreover,an organotemplate-free synthesis could cause the produce of Fe-mordenite,still needing the dynamic hydrothermal condition.31Although Kato et al.20did not use an organic template in the synthesis of Co-mordenite,as mentioned above,the research on the organotemplate-free synthesis is insufficient due to the demand of dynamic operation and the use of the organic-containing cobalt source.
Herein,we report a direct static hydrothermal route for the incorporation of Co ions into the framework of mordenite with sodium silicate,aluminum sulfate,and cobalt nitrate as raw materials,and sodium hydroxide as the pH-adjusting agency,in the absence of any organic compounds,seeds,and pretreatments.The obtained products are complementarily characterized.
2 Experimental
2.1 Synthesis
The hydrothermal synthesis of framework-substituted Co-mordenite in the absence of organic templates and seeds was carried out with the following procedures.Under vigorous stirring,10.51 g of sodium silicate(w(SiO2)=56%,w(Na2O)= 20%,AR,Wako,Japan)was added and dissolved into the aqueous solution containing 0.44 g of sodium hydroxide (w(NaOH)=96.0%,AR,Wuxi Yasheng Chem.Ind.Com.Ltd.) and 40 g of deionized water to obtain solution A.Solution B was made by adding 1.65 g of aluminum sulfate(w(Al2(SO4)3· 18H2O)=99%,AR,Shantou Xilong Chem.Ind.Com.Ltd.)into 10 g of deionized water,and solution C was gotten by adding 0.29 g of cobaltous nitrate(w(Co(NO3)2·6H2O)=99%,AR, Shantou Xilong Chem.Ind.Com.Ltd.)into 10 g of deionized water.Then,solutions B and C were added successively into solution A under stirring to give the gel mixture with a molar composition n(Na2O):n(SiO2):n(Al2O3):n(Co(NO3)2):n(H2O)=0.4: 1:0.025:0.01:40,which was stirred further at ambient temperature for 2 h.Finally,the slurry was transferred to a Teflon-lined stainless steel autoclave and left to crystallize statically at 170°C for 3 d.The resultant solid from the synthesis was separated by centrifugation,followed by washing with deionized water and air-drying at 70°C for 12 h.
In the synthesis gels,the contents of Al2(SO4)3and Co(NO3)2were changed(n(SiO2)/n(Al2O3)=20-100,n(Co)/n(SiO2)=0-0.05)to investigate the applicability of the present new synthesis route,with otherwise conditions unaltered.The pure mordenite sample,used as the reference material in this study with a supposed 100%crystallinity,was prepared according to the above procedures from a Co-free gel mixture with molar composition n(Na2O):n(SiO2):n(Al2O3):n(H2O)=0.4:1:0.025:40.The relative crystallinities of Co-mordenite samples were calculated by comparing the summed intensity of the X-ray diffraction (XRD)peaks featured for MOR at 2θ values of 6.5°,8.7°,9.7°, 13.5°,15.3°,19.6°,22.3°,25.7°,26.4°,and 27.6°with that of the reference material.For comparison,colloidal silica(HS-40, Aldrich)was also used as the silica source in the organotemplate-free synthesis.
2.2 Characterization
Powder X-ray diffraction(XRD)patterns were collected on the powder diffractometer(Bruker D8 Advance,Germany)using Ni-filtered Cu Kαradiation source(λ=0.1542 nm)at 40 kV and 40 mA with a scanning speed of 0.02(°)·s-1.The unit cell parameters of the synthesized zeolites were calculated by XRD curves.32Co,Al,and Si contents were determined by an inductively coupled plasma(ICP)spectrometer(PerkinElmer Jarrell-Ash 1100,America).BET surface areas were evaluated with nitrogen sorption isotherms using an automatism isothermal adsorption instrument(Micromeritics ASAP2010,America)at 77 K.Scanning electron microscopy(SEM)images were characterized on an instrument(ThermoNicolet Quanta 200 FEI,America).The UV-Vis spectra were obtained using a spectrophotometer(PerkinElmerPE Lambda 950,America) equipped with a diffuse reflectance attachment.Thermogravimetric(TG)analysis was carried out on an instrument (NETZSCH STA 409PC,Germany)using a Pt pan,with the heating temperature from 35 to 800°C at a heating rate of 10°C· min-1.
3 Results and discussion
3.1 Influence of n(Co)/n(SiO2)ratios
Fig.1 shows the XRD patterns for the Co-mordenite samples synthesized with various n(Co)/n(SiO2)ratios in starting gels, with otherwise similar conditions.The Co-free sample exhibited a set of diffraction peaks well assignable to the MOR structure,19without detecting other crystalline phases.Moreover,all the Co-containing samples gave the same peaks as those of the pure mordenite,while peak intensities decreased with the increase of n(Co)/n(SiO2)ratios.

Fig.1 XRD patterns for the Co-mordenites synthesized with various n(Co)/n(SiO2)ratios in starting gelsconditions:n(SiO2)/n(Al2O3)=40,n(H2O)/n(SiO2)=40,T=170°C,t=3 d; n(Co)/n(SiO2):(a)0,(b)0.01,(c)0.02,(d)0.03,(e)0.04,(f)0.05
Table 1 lists unit cell parameters,crystallinities,and BET surface areas for the samples synthesized with various n(Co)/ n(SiO2)ratios in starting gels.The Co loadings in final solid products were lower than the concentrations in starting gels,indicating that not all the cobalt species could be incorporated into the solid products.Meanwhile,the Co loadings in the solids increased gradually with the rise of n(Co)/n(SiO2)ratios in starting gels up to 0.04(entries 1-5).More importantly,the unit cell volumes increased monotonically with the increase of n(Co)/n(SiO2)up to 0.04,which is indicative of the insertion of Co ions into the TO4(T denotes tetrahedrally bonded cation) framework sites of MOR structure.This consists with the much larger radii of Co ions(0.0745 nm)than that of Si4+(0.040 nm).The increase of Co contents causes a gradual decrease in crystallinities,which is probably caused by the distortion of TO4tetrahedron due to the insertion of Co ions.At a very high n(Co)/n(SiO2)ratio of 0.05,the unit cell volume decreased obviously(entry 6),still corresponding to the drop of the n(Co)/n(SiO2)ratio in solid product.It is thus drawn that the molar ratio 0.04 for n(Co)/n(SiO2)in the starting gel is an upper limit to produce Co-mordenite.
The typical sample of entry 4 was ion exchanged with aqueous solution of NH4Cl(2 mol·L-1)at 90°C for 3 h and then calcined at 550°C for 3 h.This procedure was repeated three times,and the resultant sample was analyzed by ICP.The treated sample gave n(SiO2)/n(Al2O3)=24.1 and n(Co)/n(SiO2)= 0.021,very close to those for the untreated one,strongly indicating that the Co ions in MOR framework are rather stable.
Ratios of n(SiO2)/n(Al2O3)are also listed in Table 1.Much lower n(SiO2)/n(Al2O3)ratios of around 20 were observed for the final solid products compared with the constant value of 40 in starting gels,i.e.,the increase of Co loadings did not significantly influence the amount of Al ions incorporated in MOR framework.This result may be relative to the condition of n(Na2O)/n(SiO2)=0.4,a high alkalinity used for the hydrothermal synthesis.
BET surface areas and micropore volumes in Table 1 show that the pure mordenite had a high surface area of 434 m2·g-1, and the Co-mordenites also possessed considerably high surface areas of around 350 m2·g-1.This strongly demonstrates the existing of open micropores for these organotemplate-free synthesized samples.The micropore volumes for Co-mordenite samples were only slightly lower than that of the pure mordenite,which may arise from the lower crystallinities of Co-mordenites due to the isomorphous substitution of the large Co ions in the MOR framework.
The thermal properties for the pure mordenite and the selected Co-mordenite sample(n(Co)/n(SiO2)=0.03)were tested by the TG analysis,as shown in Fig.2.The gradual mass loss up to 350°C for the two samples due to water desorption is verydistinguished from the drastic decease at 420-520°C due to the decomposition of the involved organic templates for the template-synthesized mordenite.33This confirms that the samples prepared herein do not bear any organic species,needless to be subjected to a high-temperature calcination.

Table 1 Textural properties for the Co-mordenites synthesized with various n(Co)/n(SiO2)ratios in starting gels
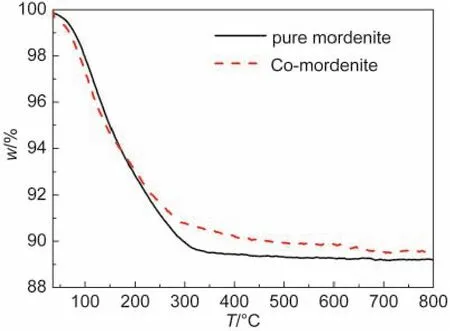
Fig.2 TG curves for the organotemplate-free synthesized pure mordenite and the Co-mordenite with a n(Co)/n(SiO2) ratio of 0.03 in the starting gel

Fig.3 UV-Vis spectra for the Co-mordenites synthesized with various n(Co)/n(SiO2)ratios in starting gelsconditions:n(SiO2)/n(Al2O3)=40,n(H2O)/n(SiO2)=40,T=170°C,t=3 d; n(Co)/n(SiO2):(a)0,(b)0.01,(c)0.02,(d)0.03,(e)0.04,(f)0.05
UV-Vis spectra provide convincing evidences for the isomorphous substitution of metal ions for Si4+in zeolite crystal lattice.34Fig.3 shows the UV-Vis spectra for the Co-mordenite samples synthesized with various n(Co)/n(SiO2)ratios in starting gels.All the Co-mordenites presented well-resolved triplet bands in the wavelength range of 500-650 nm,attributable to4A2→4T1(4P),4A2→4T1(4F),and4A2→4T2transitions of high-spin Co2+in tetrahedral coordination sites.17,35-41In contrast,the triplet band could not be found for the pure mordenite.Furthermore,except for the sample with a very high n(Co)/n(SiO2)ratio of 0.05,the relative intensities of the triplet bands increased with Co contents in initial gels,which again suggests that the more Co2+introduced into initial gels,the more Co2+incorporated in MOR frameworks.12,15The absence of the band at ca 300 nm featured for cobalt oxides excludes the possible existence of extra-framework Co species.42-44

Fig.4 SEM images for the Co-mordenites synthesized with various n(Co)/n(SiO2)ratios in starting gelsconditions:n(SiO2)/n(Al2O3)=40,n(H2O)/n(SiO2)=40,T=170°C,t=3 d;n(Co)/n(SiO2):(a)0,(b)0.01,(c)0.02,(d)0.03,(e)0.04,(f)0.05
The SEM images for the samples with various n(Co)/n(SiO2) ratios are illustrated in Fig.4.Ellipsoidal crystal particles were observed for Co-mordenites,similar to the pure mordenite and the previously reported result.19Also,the particle sizes for Co-mordenites generally increased with the Co contents in synthesis gels.Obviously,amorphous phases occurred for Co-mordenites at high Co content,which is responsible for the lowered crystallinities,as already shown in Fig.1 and Table 1.The color of the obtained Co-mordenite was violescent,suggesting that the Co ions do not exist in the pore of the MOR structure.20
3.2 Influence of crystallization time
The crystallization process for Co-mordenite was investigated by changing the crystallization time from 0 to 7 d on the sample with n(Co)/n(SiO2)=0.03 in the starting gel.The crystallization curve is plotted in Fig.5.The relative crystallinities increased quickly at the early stage of the hydrothermal treatment without observing an induction period.The increasing rate became slow at the time beyond 3 d.A long hydrothermal period of 7 d was needed to obtain a quite high crystallinity of 81%.In comparison,a short time of 3 d could result in a considerably high crystallinity 78%with a n(Co)/n(SiO2)ratio of 0.02(entry 3,Table 1).These results indicate that the obtained well crystallized Co-mordenite with a higher Co content requires longer crystallization time.
3.3 Influence of n(SiO2)/n(Al2O3)ratios
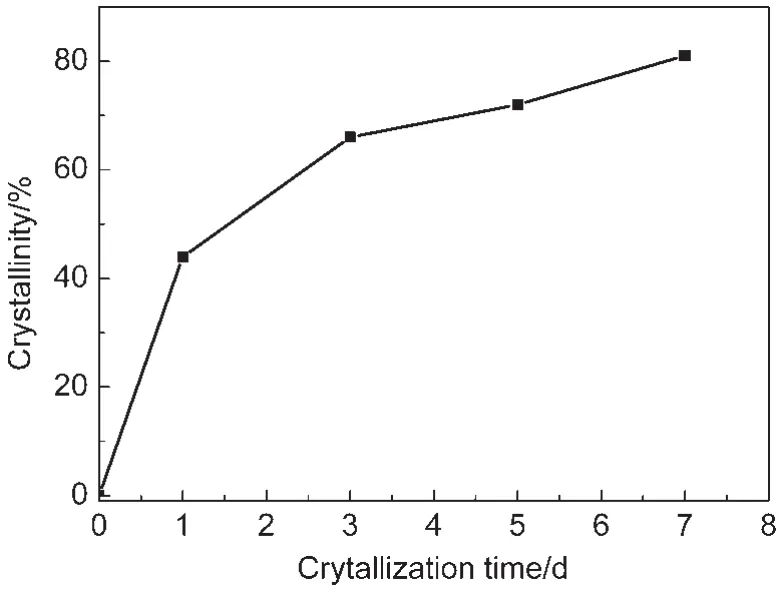
Fig.5 Crystallization curve for Co-mordenite synthesized at n(Co)/n(SiO2)=0.03,n(SiO2)/n(Al2O3)=40, n(H2O)/n(SiO2)=40,and T=170°C
n(SiO2)/n(Al2O3)ratios in starting gels are known to affect significantly the syntheses of heteroatomic zeolites.25The aforesaid syntheses focused on a certain n(SiO2)/n(Al2O3)of 40;furthermore,Table 2 presents the results for the Co-mordenites at n(SiO2)/n(Al2O3)ratios of 20 and 50.It can be seen that whatever the n(SiO2)/n(Al2O3)ratios,the Co contents in solid products raised with the increase of n(Co)/n(SiO2)ratios in starting gels except for the high n(Co)/n(SiO2)ratio of 0.05.The unit cell volumes also increased with the Co loadings in the final products with simultaneously decreased crystallinities.These observations,together with the tendency of n(SiO2)/n(Al2O3)ratios for final products,are consistent with those in Table 1 at n(SiO2)/n(Al2O3)=40.It therefore seems that the present organotemplate-free method is applicable to a broad range of n(SiO2)/ n(Al2O3)ratios in starting gels;nevertheless,n(SiO2)/n(Al2O3) ratios for final products have not been significantly enhanced by a higher n(SiO2)/n(Al2O3)ratio in the starting gel.
Comparing the samples at n(Co)/n(SiO2)=0.03 with different n(SiO2)/n(Al2O3)ratios of 20,40,and 50(entries 3 and 6 in Table 2,and entry 4 in Table 1),it is found that both the crystallinities and Co loadings in final products are enhanced with the raise of n(SiO2)/n(Al2O3)ratios in starting gels.Thus,a high n(SiO2)/n(Al2O3)ratio in gel mixture seems favorable to the insertion of Co ions into MOR framework.However,synthesizing an extremely high silica mordenite is still known as a challenging topic.Therefore,when a separate experiment for the very high n(SiO2)/n(Al2O3)ratio of 100 in the starting gel was conducted,it is not surprising that only a very low crystallinity of 38%for the resultant solid product was obtained.
3.4 Preliminaryunderstandingoftheorganotemplatefree synthesis
The present organotemplate-free synthesis uses sodium silicate as the Si source and NaOH as the alkalinity-adjusting agency.It is known that Na+may play a role as an inorganic template instead of organic ones that leads to nucleation for the growth of zeolite crystals.45-47It is thus proposed that in the present approach,the Na+ion in starting gels acts as a central body in its positive tetrahedral model,around which the dispersed silicate ions and Co ions would have condensed to form the Si-O-Co linkages,the primary building blocks of nuclei for mordenite crystals.
To understand this proposal,several control tests were further carried out.If silica sol,rather than sodium silicate,was used as the silica source under the same synthesis conditions asthose in entry 2(Table 1),only an amorphous solid product was obtained.It is therefore supposed that in the gel stage,the highly condensed silica species could not react with Co ions to form the nuclei precursor of Si-O-Co linkage,which would fail to cause the creation of zeolite phases,even in the presence of Na+ions.However,when the 0.1%(w)mordenite crystalline seed was added into the above silica sol-based system,the pure phase of Co-mordenite was obtained(entry 7,Table 1),wherein the so-called“initial-bred nuclei”from the seed-amorphous interfacial layers in a seeded hydrothermal synthesis could be assumed.48Interestingly,when KOH was used as the alkalinityadjusting agency for the seeded silica sol-based system to form a sodium-free system,Co-mordenite could not be produced any more.These comparisons demonstrate the template role of Na+ions for the creation of MOR structure.As a result,without aid of a mordenite crystalline seed,sodium silicate is a necessary silica source for synthesizing Co-mordenite by the present organotemplate-free approach.

Table 2 Textural properties for Co-mordenites synthesized with various n(Co)/n(SiO2)ratios in starting gels
4 Conclusions
We develop a facile organotemplate-free hydrothermal approach for synthesizing framework-substituted Co-mordenites. The synthesis simply uses sodium silicate,aluminum sulfate, and cobalt nitrate as Si,Al,and Co sources,with NaOH as the only additive to adjusting the alkalinity of starting gels. Co-mordenites are obtained with broad ranges of Al and Co contents in synthesis gels.Na+ions are proposed to serve as the inorganic structure-directing template and sodium silicate is the necessary silica source for this organotemplate-free method.The as-synthesized products have open micropores needless to use a high-temperature calcination,which is more environmentally benign and less energy consumption.
(1) Wichterlova,B.;Dedecek,J.;Sobalik,Z.;Vondrova,A.;Klier, K.J.Catal.1997,169,194.doi:10.1006/jcat.1997.1687
(2)Li,Y.;Armor,J.N.J.Chem.Soc.Chem.Commun.1997,2013.
(3) Chen,H.H.;Shen,S.C.;Chen,X.;Kawi,S.Appl.Catal.B: Environ.2004,50,37.doi:10.1016/j.apcatb.2003.10.005
(4) Schwidder,M.;Heikens,S.;De Toni,A.;Geisler,S.;Berndt, M.;Brueckner,A.;Gruenert,W.J.Catal.2008,259,96.doi: 10.1016/j.jcat.2008.07.014
(5) Ratnasamy,P.;Kumar,R.;Catal.Lett.1993,22,227.doi: 10.1007/BF00810369
(6)Wang,X.;Chen,H.Y.;Sachtler,W.M.H.J.Catal.2001,19, 281.
(7)Hadjiivanov,K.;Mihaylov,M.Chem.Commun.2004,2200.
(8) Fierro,G.;Eberhardt,M.A.;Houalla,M.;Hercules,D.M.; Hall,W.K.J.Phys.Chem.1996,100,8468.doi:10.1021/ jp960121e
(9) Čapek,L.;Dĕdeček,J.;Wichterlová,B.J.Catal.2004,227, 352.doi:10.1016/j.jcat.2004.08.001
(10)Zhai,Z.B.;Miao,Y.C.;Sun,Q.L.;Tao,H.W.;Wang,W.; Wang,J.Q.Catal.Lett.2009,131,538.doi:10.1007/ s10562-009-9961-7
(11)Zhao,R.H.;Dong,M.;Qin,Z.F.;Ding,J.F.;Guo,X.C.; Wang,J.G.Acta Phys.-Chim.Sin.2008,24,2304.[赵瑞花,董 梅,秦张峰,丁建飞,郭星翠,王建国.物理化学学报, 2008,24,2304.]doi:10.3866/PKU.WHXB20081226
(12)Gao,Q.;Weckhuysen,B.M.;Schoonheydt,R.A.Microporous Mesoporous Mat.1999,27,75.doi:10.1016/S1387-1811(98) 00274-1
(13)Fan,W.;Li,R.;Dou,T.;Tatsumi,T.;Weckhuysen,B.M. Microporous Mesoporous Mat.2005,84,116.doi:10.1016/ j.micromeso.2005.04.025
(14) Duan,F.;Li,J.;Chen,P.;Yu,J.;Xu,R.Microporous Mesoporous Mat.2009,126,26.doi:10.1016/j.micromeso. 2009.05.015
(15) Janas,J.;Shishido,T.;Che,M.;Dzwigaj,S.Appl.Catal.B: Environ.2009,89,196.doi:10.1016/j.apcatb.2008.11.028
(16) Pai,S.;Newalkar,B.L.;Choudary,N.V.Microporous Mesoporous Mat.2006,96,135.doi:10.1016/j.micromeso. 2006.06.027
(17) Nagase,T.;Chatterjee,A.;Tanaka,A.P.;Tanco,M.L.;Tazaki, K.Chem.Lett.2004,33,1416.doi:10.1246/cl.2004.1416
(18) Meier,W.M.Z.Kristallogr.1961,115,439.doi:10.1524/ zkri.1961.115.5-6.439
(19) Degnan,T.F.,Jr.J.Catal.2003,216,32.doi:10.1016/ S0021-9517(02)00105-7
(20) Kato,M.;Ikeda,T.;Kodaira,T.;Takahashi,S.Microporous Mesoporous Mat.2011,142,444.doi:10.1016/j.micromeso. 2010.12.030
(21) Qiao,K.;Zhang,F.M.;Pan,D.L.;Zhang,N.F.;Jian,P.M. Chin.J.Inorg.Chem.2008,24,748.[乔 亏,张富民,潘多丽,张鸟飞,菅盘铭.无机化学学报,2008,24,748.]
(22)Watanabe,K.;Ogura,M.Microporous Mesoporous Mat.2008, 114,229.doi:10.1016/j.micromeso.2008.01.008
(23) Chen,B.;Huang,Y.Microporous Mesoporous Mat.2009,123, 71.doi:10.1016/j.micromeso.2009.03.025
(24) Niphadkar,P.S.;Kotwal,M.S.;Deshpande,S.S.;Bokade,V. V.;Joshi,P.N.Mater.Chem.Phys.2009,114,344.doi:10.1016/ j.matchemphys.2008.09.026
(25) Xia,Q.H.;Tatsumi,T.Mater.Chem.Phys.2005,89,89.doi: 10.1016/j.matchemphys.2004.08.034
(26)Yang,D.H.;Zhao,J.F.;Zhang,J.L.;Dou,T.;Wu,Z.H.;Chen, Z.J.Acta Phys.-Chim.Sin.2012,28,720. [杨冬花,赵君芙,张军亮,窦 涛,吴忠华,陈中军.物理化学学报,2012,28, 720.]doi:10.3866/PKU.WHXB201201031
(27) Song,J.W.;Dai,L.;Ji,Y.Y.;Xiao,F.S.Chem.Mater.2006,18, 2775.doi:10.1021/cm052593o
(28) Zhang,W.;Wu,Y.J.;Gu,J.;Zhou,H.L.;Wang,J.Mater.Res. Bull.2011,46,1451.doi:10.1016/j.materresbull.2011.05.006
(29) Chandwadkar,A.J.;Bhat,R.N.;Ratnasamy,P.Zeolites 1991, 11,42.doi:10.1016/0144-2449(91)80354-3
(30)Dong,M.;Wang,J.G.;Sun,Y.H.Microporous Mesoporous Mat.2001,43,237.doi:10.1016/S1387-1811(01)00211-6
(31)Wu,P.;Komastsu,T.;Yashima,T.Microporous Mesoporous Mat.1998,20,139.doi:10.1016/S1387-1811(97)00005-X
(32) Treacy,M.M.J.;Higgins,J.B.Collection of Simulated XRD Powder Patterns for Zeolites,5th ed.;Elsevier:Netherlands, 2007;p 284.
(33)Mohamed,M.M.;Salama,T.M.;Othman,I.;Ellah,I.A. Microporous Mesoporous Mat.2005,84,84.doi:10.1016/ j.micromeso.2005.05.017
(34) Hunger,M.;Weitkamp,J.Angew.Chem.Int.Edit.2001,40, 2954.doi:10.1002/1521-3773(20010817)40:16<2954:: AID-ANIE2954>3.0.CO;2-#
(35) Duran,A.;Fernandez Navarro,J.M.;Casariego,P.;Joglar,A. J.Non-Cryst.Solids 1986,812,391.
(36) Fujii,Y.;Kyuno,E.;Tsuchiya,R.Bull.Chem.Soc.Jpn.1970, 43,786.doi:10.1246/bcsj.43.786
(37)Weckhuysen,B.M.;Verberckmoes,A.A.;Uytterhoeven,M.G.; Mabbs,F.E.;Collison,D.;de Boer,E.;Schoonheydt,R.A. J.Phys.Chem.B.2000,104,37.doi:10.1021/jp991762n
(38)Weckhuysen,B.M.;Rao,R.R.;Martens,J.A.;Schoonheydt,R. A.Eur.J.Inorg.Chem.1999,565.
(39) Hartmann,M.;Kevan,L.Chem.Rev.1999,99,635.doi: 10.1021/cr9600971
(40)Verberckmoes,A.A.;Weckhuysen,B.M.;Schoonheydt,R.A. Microporous Mesoporous Mat.1998,22,165.doi:10.1016/ S1387-1811(98)00091-2
(41)Fan,W.;Weckhuysen,B.M.;Schoonheydt,R.A.Phys.Chem. Chem.Phys.2001,3,3240.
(42) Dzwigaj,S.;Che,M.J.Phys.Chem.B 2006,110,12490.doi: 10.1021/jp0623387
(43)Thomson,S.;Luca,V.;Howe,R.F.Phys.Chem.Chem.Phys. 1999,1,615.
(44)Eldewik,A.;Howe,R.F.Microporous Mesoporous Mat.2001, 48,65.doi:10.1016/S1387-1811(01)00331-6
(45) Persson,A.E.;Schoeman,B.J.;Sterte,J.Zeolites 1995,15, 611.doi:10.1016/0144-2449(95)00070-M
(46) Cheng,Z.L.;Chao,Z.S.;Lin,H.Q.;Wan,H.L.Chin.J.Inorg. Chem.2003,19,396.[程志林,晁自胜,林海强,万惠霖.无机化学学报,2003,19,396.]
(47) Shin,D.K.;Shi,H.N.;Kyeong,H.S.;Wha,J.K.Microporous Mesoporous Mat.2004,72,185.doi:10.1016/j.micromeso. 2004.04.024
(48) Jansen,J.C.;Stocker,M.;Kargc,H.G.;Weilkamp,J.Stud. Surf.Sci.Catal.1994,85,43.doi:10.1016/S0167-2991(08) 60764-8

