SDF-1/CXCR4轴通过NF-κb信号通路调控炎症反应减轻动脉粥样硬化的形成
【摘 要】目的:确定基质细胞衍生因子-1/趋化因子受体4(stromal cell-derived factor-1/C-X-C chemokine receptor 4,SDF-1/ CXCR4)信号轴如何在动脉粥样硬化中发挥作用,并探讨其相关的分子机制。方法:将40只载脂蛋白E-/-小鼠分为5组:对照组(control group,CON)、高脂饲料组(high-fat diet,HFD)、空载病毒组(adeno-associated virus 9 enhanced green fluorescent pro? tein,AAV9-eGFP)、病毒敲减组(adeno-associated virus 9-CXCR4-small interfering RNA,AAV9-CXCR4-siRNA)和吡咯烷二硫代氨基甲酸酯(pyrrolidine dithiocarbamate,PDTC)组。CON组普通饲料喂养,其余4 组高脂饲料喂养16周。PDTC组从第五周开始腹腔注射60 mg/kg PDTC,2次/周。12周时,AAV9-CXCR4-siRNA组和AAV9-eGFP组分别接受尾静脉注射rAAV9-CXCR4-RNAi和阴性对照病毒,HFD组注射等量生理盐水。使用共聚焦荧光显微镜测定增强型绿色荧光蛋白(enhanced green fluorescent protein,eGFP)的表达,采用苏木精-伊红染色(hematoxylin-eosin,HE)染色法显示动脉粥样硬化斑块的面积,免疫组织化学(immunohistochemistry,IHC)染色和免疫印迹法(Western blot,WB)检测CXCR4、核因子-κB p65(nuclear factor kappaB p65,NF-κB p65)、磷酸化核因子-κB p65(nuclear factor kappaB p-p65,NF-κB p-p65)、白细胞介素-1β(interleukin-1β,IL-1β)和肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)。结果:HE染色显示,与CON组相比,各组均出现动脉粥样硬化斑块,AAV9-CXCR4-siRNA组的斑块小于AAV9-eGFP组。与HFD组相比,PDTC组的斑块更小。此外,与HFD组相比,PDTC组的血清中SDF-1、IL-1β和TNF-α水平较低;与AAV9-eGFP组相比,PDTC组的SDF-1、IL-1β和TNF-α血清水平更低。IHC结果显示,与CON组相比,CXCR4和SDF-1在HFD组和AAV9-eGFP组中高表达。然而,AAV9-CXCR4-siRNA组与AAV9-eGFP组相比,斑块区域的CXCR4(F=9.621,P=0.000)和SDF-1(F=20.102,P=0.000)表达减少。此外,WB实验表明,与HFD组相比,PDTC 组的 SDF-1(F=54.093,P=0.000)和CXCR4(F=28.485,P=0.000)表达降低。与AAV9-eGFP组相比,AAV9-CXCR4-siRNA组的SDF-1和CXCR4表达量较低(F=9.621,P=0.000;F=20.102,P=0.000)。Pearson相关分析表明,CXCR4与NF-κb p65(r=0.762,P=0.000)、NF-κb p-p65(r=0.795,P=0.000)、白细胞介素-1(interleukin-1,IL-1)(r=0.786,P=0.000)、TNF-α(r=0.844,P=0.000)和SDF-1(r=0.815,P=0.000)蛋白水平呈正相关。结论:抑制SDF-1/CXCR4轴可通过NF-κb信号通路降低炎症反应,从而减轻动脉粥样硬化的发生发展。
【关键词】趋化因子配体12/趋化因子受体4信号轴;动脉粥样硬化;核因子-κB信号通路;炎症反应
【中图分类号】R543.5;R363.2【文献标志码】A【收稿日期】2024-04-07
基金项目:国家自然科学基金资助项目(编号:82060340);贵州省科技计划资助项目(编号:黔科合基础-ZK[2021]一般487);贵州省基础研究计划项目(编号:黔科合基础-ZK[2023]一般330);贵州省普通高等学校青年科技人才成长项目(编号:黔教合KY字[2021]157)。
SDF-1/CXCR4 axis regulates inflammatory responses to attenuate atherosclerosis via the NF-κb signaling pathway
Zhou Ming1,Wang Jiawen2,Lu Yanlin2,Peng Jin2,Ding Jiuyang2,Le Cuiyun2,Li Fangqin2,Wang Jie2,Liu Yubo2,Xia Bing2
(1. Department of Forensic Medicine,Zunyi Medical University;2. Department of Forensic Medicine,Guizhou Medical University)
【Abstract】Objective:To determine the role of stromal cell-derived factor-1/C-X-C chemokine receptor 4(SDF-1/CXCR4) signaling axis in atherosclerosis and to investigate its associated molecular mechanisms.Methods:Forty ApoE-/- mice were divided into five groups:control(CON) group,high-fat diet(HFD) group,empty vi? rus(adeno-associated virus 9 enhanced green fluorescent protein,AAV9-eGFP) group,virus knockdown(adeno-associated virus 9-CXCR4-small interfering RNA,AAV9-CXCR4-siRNA) group,and pyrrolidine dithiocarbamate(PDTC) group. The CON group was fed normal chow and the remaining four groups were fed high-fat chow for 16 weeks. The PDTC group received intraperitoneal injec? tions of 60 mg/kg PDTC twice/week starting from the fifth week. At 12 weeks,the AAV9-CXCR4-siRNA group and the AAV9-eGFP group received tail-vein injection of rAAV9-CXCR4-RNAi and negative control viruses,respectively,while the HFD group was in? jected with an equal amount of physiologic saline. The expression of enhanced green fluorescent protein(eGFP) was determined using confocal fluorescence microscopy. The area of atherosclerotic plaques was visualized by hematoxylin and eosin staining. Immunohisto? chemical staining and Western blot were used to detect the expression of CXCR4,nuclear factor kappa B p65 (NF-κB p65),phos? phorylated nuclear factor-κB p65(NF-κB p-p65),interleukin-1β(IL-1β),and tumor necrosis factor-α(TNF-α). Results:Hema? toxylin and eosin staining showed that atherosclerotic plaques were clearly present in all groups except the CON group,and plaques in the AAV9-CXCR4-siRNA group were significantly smaller than those in the AAV9-eGFP group. Plaques were significantly smaller in the PDTC group compared with the HFD group. In addition,the serum levels of SDF-1,IL-1β,and TNF-α were lower in the PDTC group compared with the HFD group. The serum levels of SDF-1,IL-1β,and TNF-α were lower in the PDTC group compared with the AAV9-eGFP group. Immunohistochemical staining showed that the expression levels of CXCR4 and SDF-1 were higher in the HFD and AAV9-eGFP groups than in the CON group. However,the expression levels of CXCR4(F=9.621,P=0.000) and SDF-1(F= 20.102,P=0.000) were significantly reduced in the plaque region in the AAV9-CXCR4-siRNA group compared with the AAV9-eGFP group. In addition,Western blot showed that the expression levels of SDF-1(F=54.093,P=0.000) and CXCR4(F=28.485,P= 0.000) were significantly reduced in the PDTC group compared with the HFD group. SDF-1 and CXCR4 expression levels were signifi? cantly lower in the AAV9-CXCR4-siRNA group compared with the AAV9-eGFP group(F=9.621,P=0.000;F=20.102,P=0.000). Pearson correlation analysis showed that CXCR4 was positively correlated with the protein levels of NF-κb p65(r=0.762,P=0.000),NF-κb p-p65(r=0.795,P=0.000),IL-1(r=0.786,P=0.000),TNF-α(r=0.844,P=0.000),and SDF-1(r=0.815,P=0.000). Conclusion:Inhibition of the SDF-1/CXCR4 axis reduces the inflammatory response through the NF-κb signaling pathway,thereby attenuating the development and progression of atherosclerosis.
【Key words】C-X-C motif chemokine ligand 12/C-X-C chemokine receptor 4 axis;atherosclerosis;NF-κb signaling pathway;inflam? matory response
动脉粥样硬化是由动脉血管内皮损伤和低密度脂蛋白滞留引起的一种慢性炎症性疾病。近年来,动脉粥样硬化的诊断和治疗取得了重大进展。然而,动脉粥样硬化在普通人群中仍然非常普遍。例如,一项对未确诊为动脉粥样硬化的普通人群进行的大型随机抽样动脉粥样硬化研究显示,无症状冠状动脉粥样硬化非常普遍[1]。动脉粥样硬化与冠心病和其他心血管疾病有关。此外,2019年与动脉粥样硬化相关的死亡人数约为1 860万[2]。动脉粥样硬化斑块突然破裂导致管腔闭塞和随后的心肌缺氧缺血性损伤并不总是发生在严重狭窄病变中[3-4]。因此,有必要探索无症状冠状动脉粥样硬化背后的分子机制。
基质细胞衍生因子-1(stromal cell-derived fac? tor-1,SDF-1)又称C-X-C趋化因子配体12(C-XC motif chemokine ligand 12,CXCL12),是C-X-C趋化因子受体4(C-X-C chemokine receptor 4,CXCR4)的配体。SDF-1由位于人类染色体 10q上的基因编码,含有68个氨基酸残基。另一方面,CXCR4是一种由352个氨基酸组成的类视黄醇G蛋白偶联受体(G protein-coupled receptor,GPCR),含有1个细胞外N端结构域、7个跨膜螺旋、3个细胞外环、3个细胞内环和1个细胞内C端结构域[5]。SDF-1/CXCR4轴介导涉及动脉粥样硬化的多种反应,包括炎症、免疫和自噬。CXCR4进展中的动脉粥样硬化斑块的内皮中高度表达,尤其是在血管通透性增加和单核细胞招募增加的增生内皮上[6]。募集的单核细胞吞噬内皮下残留的脂质,形成泡沫细胞,并通过释放SDF-1促进动脉粥样硬化的发生[7]。先前的1项研究表明,缺乏SDF-1的高脂血症小鼠动脉粥样硬化病变面积缩小[8]。此外,在小鼠心肌细胞中过表达CXCR4与梗死面积增大、心脏功能降低和炎症细胞招募增加有关[9]。相反,使用AMD3100(Plerixafor或 Mozobil)抑制CXCR4的活化可减少炎症介质[包括白细胞介素-1β(interleukin-1β,IL-1β)]的表达,并与炎症反应的减少有关[10]。然而,SDF-1/CXCR4轴调节动脉粥样硬化的确切机制尚不清楚。
本研究通过给载脂蛋白E-/-小鼠喂食高脂饲料建立了动脉粥样硬化模型。然后,使用腺相关病毒9型(adeno-associated virus 9,AAV9)敲除目标基因CXCR4,PDTC用于抑制核因子κb(nuclear factor kappa-B,NF-κb)信号通路。此外,还对小鼠的斑块面积大小、NF-κb信号蛋白[包括白细胞介素-1(interleukin-1,IL-1)、肿瘤坏死因子-α(tumor necro? sis factor-α,TNF-α)和SDF-1]的表达进行了评估。
1 材料与方法
1.1 实验动物和分组
采购40只6周龄雄性载脂蛋白E基因敲除(apolipopro?tein E-/-,ApoE-/-)小鼠(由北京维通利华实验动物技术有限公司提供,生产合格证号:SCXK(京)2016-0006),适应性饲养1周后分为CON组,HFD组,AAV9-eGFP组,AAV9-CXCR4-siRNA组,PDTC组,每组8只。CON组给予普通饲料喂养,其余4组高脂饲料(饲料配方:3%胆固醇,0.5%胆酸钠,0.2%丙基硫氧嘧啶,5%白糖,10%猪油,81.3%基础饲料)喂养;其中,PDTC组在高脂饲料喂养至第5周时,进行腹腔注射PDTC 60 mg/kg,每周2次[11],HFD组注射等量生理盐水。AAV9-eGFP组及AAV9-CXCR4-siRNA组在12周时分别注射AAV9-eGFP病毒与AAV9-CXCR4-RNAi病毒。所有小鼠喂养至16周后麻醉处死[12],非抗凝采血管取血,高速离心机3 500 r/min离心10 min,取上清液-80 ℃留存;取小鼠主动脉,部分4%多聚甲醛固定,部分液氮保存。
1.2 荧光显微镜检测
小鼠主动脉冷冻切片在磷酸盐缓冲盐水(phosphate buffered saline,PBS)中洗涤3次,每次5 min。HFD组和AAV9-eGFP组用0.5%滂胺天蓝处理,以掩盖组织自发荧光。4’,6-二脒基-2-苯基吲哚(4’,6-diamidino-2-phenylin? dole ,DAPI)染色后,用抗荧光猝灭密封剂密封切片,并在荧光显微镜下观察。
1.3 ELISA实验
冰箱取出SDF-1、IL-1β及TNF-α试剂盒;酶标包被板上设置标准品孔各加不同浓度的标准品50 μL、空白孔(空白对照孔不加样品及酶标试剂,其余各步操作相同)、待测样品孔。在待测样品孔中先加样品稀释液40 μL,然后再加待测样品10 μL(样品最终稀释度为5倍)。每孔加入酶标试剂100 μL,空白孔除外。用封板膜封板后置37 ℃温育60 min。将20倍浓缩洗涤液用蒸馏水20倍稀释后备用。揭掉封板膜,弃去液体,甩干,每孔加满洗涤液,静置30 s后弃去,如此重复5次。每孔先加入显色剂A 50 μL,再加入显色剂B 50 μL,震荡混匀后37 ℃避光显色15 min。每孔加终止液50 μL,终止反应(此时蓝色立转黄色)。以空白孔调零,450 nm波长依序测量各孔的吸光度(absorbance,A值)。测定应在加终止液后15 min以内进行。
1.4 免疫组织化学染色
取常温保存的石蜡切片,60 ℃烤片,依次入缸脱蜡、水合,PBS缓冲液漂洗后甩干,滴加内源性过氧化物酶阻断剂后放入湿盒避光孵育;再次漂洗后EDTA进行抗原修复,山羊血清封闭,甩干封闭液后直接滴加第一抗(SDF-1抗体浓度:1∶600、CXCR4抗体浓度:1∶400),滴加PBS缓冲液为阴性对照,4 ℃冰箱孵育过夜;次日滴加酶标山羊抗小鼠/兔IgG聚合物,37 ℃恒温孵育40 min,漂洗后滴加DAB显色,自来水冲洗终止反应,苏木素复染后置于流水返蓝,逆梯度酒精脱水后二甲苯透明,中性树胶封片。
1.5 蛋白印迹分析
将小鼠主动脉在研钵中碾碎,在蛋白溶液中裂解,然后离心取上清液。用分光光度法测量每个样品中的蛋白质浓度。加入5×蛋白质样品上样缓冲液。然后用以下一抗在4 ℃孵育过夜:微管蛋白兔多克隆抗体1∶2 000、CXCR4兔单克隆抗体1∶2 000、SDF-1兔多克隆抗体1∶2 000、NF-κb p65兔多克隆抗体1∶2 000、NF-kb p-p65兔多克隆抗体1∶2 000、 IL-1β兔多克隆抗体1:2 000)。次日,洗膜后用辣根过氧化物酶(HRP)山羊抗兔二抗(1∶5 000)在室温下孵育1 h,使用增强化学发光液显现蛋白条带。
1.6 统计学方法
所有数据均使用统计学软件SPSS 22.0进行统计分析,并使用GraphPad Prism 7.0 绘制图表,计量资料以均数±标准差(x±s)表示,组间比较采用单因素方差分析,相关性检验采用Pearson相关分析。检验水准α=0.05。
2 结 果
2.1 AAV病毒导入小鼠体内情况
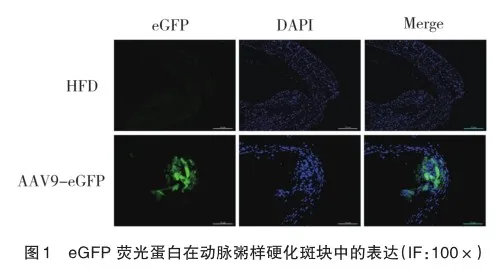
通过共聚焦荧光显微镜观察eGFP在AAV9-eGFP组中的表达。eGFP用于确定病毒是否成功导入并在小鼠中表达(图1),结果显示,病毒已成功导入小鼠体内并获得表达。
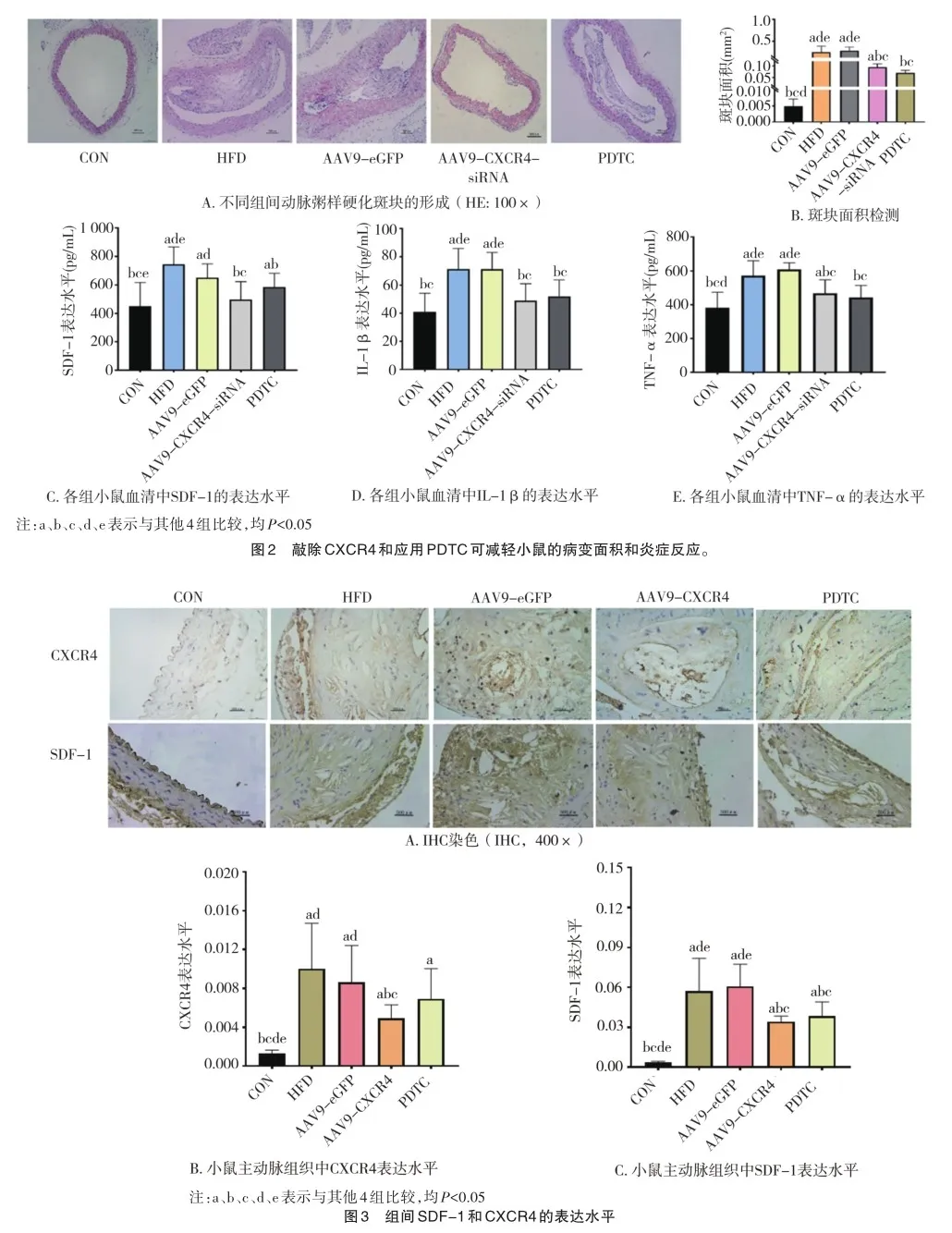
2.2 敲减CXCR4与运用PDTC对小鼠斑块面积及对血清中炎性因子的影响
HE染色结果显示,与CON组相比,其余4组的动脉粥样硬化斑块增大(F=20.987,P=0.000)(图2A、B)。然而,与AAV9-eGFP组相比,AAV9-CXCR4-siRNA组斑块减少(P= 0.000)。此外,与HFD组相比,PDTC组斑块面积减少(P= 0.000)。对血清中炎性因子的影响方面,与HFD组相比,PDTC组的血清SDF-1(F=7.129,P=0.000)、IL-1β(F=9.292,P=0.000)和TNF-α(F=11.872,P=0.000)水平均较低(图2C、E)。此外,与AAV9-eGFP组相比,AAV9-CXCR4-siRNA组血清中SDF-1、IL-1β和TNF-α水平均降低(P=0.000)。
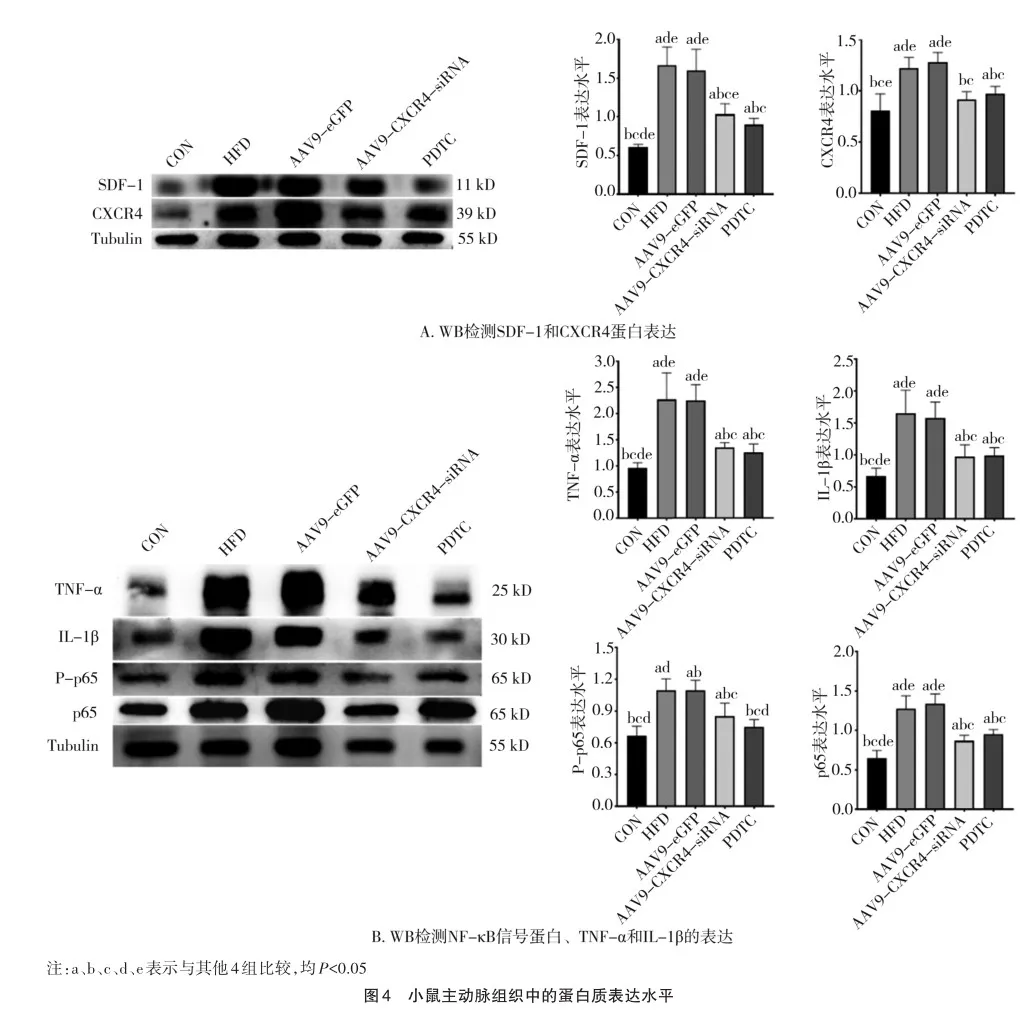
2.3 动脉粥样硬化斑块内CXCR4及SDF-1蛋白表达的变化
免疫组织化学染色分析显示,与CON组相比,HFD和AAV9-eGFP组的动脉粥样硬化斑块中SDF-1和CXCR4蛋白高表达(图3A)。然而,与AAV9-eGFP组相比,AAV9-CXCR4-siRNA组斑块中CXCR4(F=9.621,P=0.000)和SDF-1(F=20.102,P=0.000)蛋白低表达(图3B、C)。
2.4 小鼠主动脉内SDF-1/CXCR4轴蛋白及下游炎性蛋白的表达变化
用吡咯烷二硫代氨基甲酸酯(PDTC)抑制NF-κb信号。PDTC组的SDF-1水平显著低于HFD组(P<0.05)。此外,与AAV9-eGFP组相比,AAV9-CXCR4-siRNA组的SDF-1水平降低(P<0.05)。与HFD组相比,PDTC组NF-κb信号通路下游分子IL-1、TNF-α降低(P<0.05)。此外,与AAV9-eGFP组相比,AAV9-CXCR4-siRNA组IL-1、TNF-α和其他炎症介质水平显著降低(P<0.05)(图4A,B)。这些发现表明,PDTC敲低CXCR4和抑制NF-κb信号通路可以有效降低炎症介质的表达。Pearson相关分析显示,CXCR4与TNF-α(r= 0.786,P=0.000)、IL-1(r=0.762,P=0.000)、NF-κb P-p65(r= 0.795,P=0.000)、NF-κb p65(r=0.844,P=0.000)、SDF-1(r= 0.815,P=0.000)蛋白水平呈正相关(图5)。
3 讨 论
本研究探讨了在敲除CXCR4和抑制NF-κb信号传导后,SDF-1/CXCR4轴对动脉粥样硬化的影响。结果发现,在载脂蛋白E-/-小鼠体内敲除CXCR4并阻断NF-κb信号传导后,动脉粥样硬化斑块显著减少,小鼠血清中的SDF-1、IL-1β和TNF-α水平也有所降低。此外,免疫组化分析发现动脉粥样硬化斑块的SDF-1和CXCR4表达减少,免疫印迹技术显示,SDF-1、CXCR4和NF-κb信号蛋白(包括p65、IL-1β和TNF-α)的表达均减少,且与CXCR4呈正相关。因此,这些研究结果表明,SDF-1/ CXCR4轴可能通过NF-κb信号通路减轻动脉粥样硬化。
趋化因子及其受体作为一类细胞分泌的“小细胞因子”或信号蛋白,诱导附近反应细胞定向趋化以及控制细胞定向迁移,参与动脉粥样硬化炎症的静止、增殖、归巢和滞留过程[13]。SDF-1具有强大的细胞趋化作用,通过与CXCR4或CXCR7结合来调节细胞活性,这两种受体蛋白位于细胞膜上,导致多种细胞内信号通路的激活调控炎性反应[14-15]。近年来,研究人员对减少炎症反应以解决动脉粥样硬化问题越来越感兴趣。卡拉单抗抗炎血栓形成结果研究(Canakinumab Antiinflammatory Thrombosis Outcome Study,CANTOS)显示,白细胞介素-1β抗体可减轻动脉粥样硬化引起的心脏事件和死亡[16]。
此外,多项研究表明,SDF-1可促进动脉粥样硬化的进展[17-18],并可作为动脉粥样硬化相关心血管疾病的诊断和预后标志物[19]。先前的1项研究显示,与进展斑块的内皮区域相比,非斑块区域的CXCR4表达较低,尤其是在增生的内皮区域,血管通透性增加,单核细胞招募增多,与本研究结果一致。这些结果表明,SDF-1/CXCR4轴在动脉粥样硬化中发挥着重要的调节功能。

NF-κb是炎症基因的关键转录调节因子。它能诱导多种参与炎症、组织损伤和免疫反应的基因表达[20]。动脉粥样硬化的特点是动脉内膜增厚导致管腔狭窄,从而引发心肌缺血和缺氧后的心血管事件。抑制NF-κb信号通路可抑制内皮细胞的增殖[21-23]。NF-κb由RelA(p65)、RelB、p50、p52和C- rel等五个不同的亚基二聚形成。在静息状态下,NF-κb与细胞质中的IκB结合,形成转录不活跃的复合物[24]。然而,在炎症因子的刺激下,IκB激酶会将IκB磷酸化,使其降解并泛素化。IκB降解后,p50/p65异源二聚体转运至细胞核。磷酸化的p65亚基会识别并结合到参与靶基因转录的特定DNA序列上[25-26]。吡咯烷二硫代氨基甲酸酯是一种NF-κb通路抑制剂,可减轻不同组织和器官的炎症反应[27]。以往的研究表明,通过抑制SDF-1/CXCR4/ NF-κB信号通路,可以缓解关节炎、前列腺炎和小胶质细胞炎症等炎症性疾病[28-30]。此外,研究还表明,敲除CXCR4可通过抑制MAPK和NF-κB信号通路的激活来抑制巨噬细胞中炎性细胞因子的表达[31]。本研究表明,敲除CXCR4后,SDF-1、p65、pp65、IL-1β和TNF-α的表达均有所下降,这表明SDF-1/CXCR轴在NF-κb信号通路中起着关键的调节作用。
总之,抑制SDF-1/CXCR4轴可减轻动脉粥样硬化。本研究的发现为动脉粥样硬化的潜在治疗靶点提供了宝贵的见解。
参 考 文 献
[1] Bergstr?m G,Persson M,Adiels M,et al. Prevalence of Subclinical Coronary Artery Atherosclerosis in the General Population[J]. Circula? tion. 2021,144(12):916-929.
[2] Roth GA,Mensah GA,Johnson CO,et al. Global burden of cardio? vascular diseases and risk factors,1990-2019:update from the GBD 2019 study[J]. J Am Coll Cardiol,2020,76(25):2982-3021.
[3] Little WC,Constantinescu M,Applegate RJ,et al. Can coronary an? giography predict the site of a subsequent myocardial infarction in pa? tients with mild-to-moderate coronary artery disease?[J]. Circulation,1988,78(5 Pt 1):1157-1166.
[4] Ambrose JA,Tannenbaum MA,Alexopoulos D,et al. Angiographic progression of coronary artery disease and the development of myocar? dial infarction[J]. J Am Coll Cardiol,1988,12(1):56-62.
[5] Wu BL,Chien EY,Mol CD,et al. Structures of the CXCR4 chemo? kine GPCR with small-molecule and cyclic peptide antagonists[J]. Sci? ence,2010,330(6007):1066-1071.
[6] Baba O,Huang LH,Elvington A,et al. CXCR4-binding positron emission tomography tracers link monocyte recruitment and endothelial injury in murine atherosclerosis[J]. Arterioscler Thromb Vasc Biol,2021,41(2):822-836.
[7] Li LX,Du ZL,Rong B,et al. Foam cells promote atherosclerosis progression by releasing CXCL12[J]. Biosci Rep,2020,40(1):BSR2019 3267.
[8] D?ring Y,van der Vorst EPC,Duchene J,et al. CXCL12 derived from endothelial cells promotes atherosclerosis to drive coronary artery disease[J]. Circulation,2019,139(10):1338-1340.
[9] Chen JQ,Chemaly E,Liang LF,et al. Effects of CXCR4 gene trans? fer on cardiac function after ischemia-reperfusion injury[J]. Am J Pathol,2010,176(4):1705-1715.
[10] Hou JQ,Wang C,Ma D,et al. The cardioprotective and anxiolytic effects of Chaihujialonggumuli Granule on rats with anxiety after acute myocardial infarction is partly mediated by suppression of CXCR4/NF-κB/GSDMD pathway[J]. Biomedecine Pharmacother,2021,133:111015.
[11] Zhai JX,Zhang ZX,Feng YJ,et al. PDTC attenuate LPS-induced kidney injury in systemic lupus erythematosus-prone MRL/lpr mice[J]. Mol Biol Rep,2012,39(6):6763-6771.
[12] van Kuijk K,Demandt JAF,Perales-Patón J,et al. Deficiency of myeloid PHD proteins aggravates atherogenesis via macrophage apopto? sis and paracrine fibrotic signalling[J]. Cardiovasc Res,2022,118(5):1232-1246.
[13] Gavriel Y,Rabinovich-Nikitin I,Ezra A,et al. Subcutaneous ad? ministration of AMD3100 into mice models of Alzheimer’s disease ame? liorated cognitive impairment,reduced neuroinflammation,and im? proved pathophysiological markers[J]. J Alzheimers Dis,2020,78(2):653-671.
[14] Liang DS,Huang AR,Lin MM,et al. Pyrrolidine dithiocarbamate and dexamethasone are novel treatments of Acute Exogenous Lipoid Pneumonia[J]. Cytokine,2020,133:155122.
[15] Song ZY,Wang F,Cui SX,et al. Knockdown of CXCR4 inhibits CXCL12-induced angiogenesis in HUVECs through downregulation of the MAPK/ERK and PI3K/AKT and the Wnt/β-catenin pathways[J]. Cancer Invest,2018,36(1):10-18.
[16] Ridker PM,Everett BM,Thuren T,et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease[J]. N Engl J Med,2017,377(12):1119-1131.
[17] Gao JH,He LH,Yu XH,et al. CXCL12 promotes atherosclerosis by downregulating ABCA1 expression via the CXCR4/GSK3β/β-cateninT120/TCF21 pathway[J]. J Lipid Res,2019,60(12):2020-2033.
[18] Gencer S,D?ring Y,Jansen Y,et al. Endothelial ACKR3 drives atherosclerosis by promoting immune cell adhesion to vascular endothe? lium[J]. Basic Res Cardiol,2022,117(1):30.
[19] Gao JH,Yu XH,Tang CK. CXC chemokine ligand 12 (CXCL12)in atherosclerosis:an underlying therapeutic target[J]. Clin Chim Acta,2019,495:538-544.
[20] Liu T,Zhang LY,Joo D,et al. NF-κB signaling in inflammation[J]. Signal Transduct Target Ther,2017,2:17023.
[21] Li YQ,Wang JY,Qian ZQ,et al. Osthole inhibits intimal hyper? plasia by regulating the NF-κB and TGF-β1/Smad2 signalling path? ways in the rat carotid artery after balloon injury[J]. Eur J Pharmacol,2017,811:232-239.
[22] Yao JT,Zhao XZ,Tan FC,et al. Early modulation of macrophage ROS-PPARγ-NF-κB signalling by sonodynamic therapy attenuates neointimal hyperplasia in rabbits[J]. Sci Rep,2020,10(1):11638.
[23] Zhang J,Chen J,Yang J,et al. Resveratrol attenuates oxidative stress induced by balloon injury in the rat carotid artery through actions on the ERK1/2 and NF-kappa B pathway[J]. Cell Physiol Biochem,2013,31(2-3):230-241.
[24] Sun SC. The non-canonical NF-κB pathway in immunity and in? flammation[J]. Nat Rev Immunol,2017,17(9):545-558.
[25] Williams LM,Gilmore TD. Looking down on nf-κb[J]. Mol Cell Biol,2020,40(15):104-120.
[26] van Essen D,Engist B,Natoli G,et al. Two modes of transcrip? tional activation at native promoters by NF-kappaB p65[J]. PLoS Biol,2009,7(3):e73.
[27] He CY,Jiang LP,Wang CY,et al. Inhibition of NF-κB by pyrro? lidine dithiocarbamate prevents the inflammatory response in a ligatureinduced peri-implantitis model:a canine study[J]. Cell Physiol Bio? chem,2018,49(2):610-625.
[28] Si M,Ma Z,Zhang J,et al. Qingluoyin granules protect against adjuvant-induced arthritis in rats via downregulating the CXCL12/ CXCR4-NF-κB signalling pathway[J]. Pharm Biol,2021,59(1):1441-1451.
[29] Zhang M,Liu Y,Chen J,et al. Targeting CXCL12/CXCR4 signal? ing with AMD3100 might selectively suppress CXCR4+ T-cell chemo? taxis leading to the alleviation of chronic prostatitis[J]. J Inflamm Res,2022,15:2551-2566.
[30] Lee HH,Jeong JW,Hong SH,et al. Diallyl trisulfide suppresses the production of lipopolysaccharide-induced inflammatory mediators in BV2 microglia by decreasing the NF-κB pathway activity associated with toll-like receptor 4 and CXCL12/CXCR4 pathway blockade[J]. J Cancer Prev,2018,23(3):134-140.
[31] Tian X,Xie GG,Xiao H,et al. CXCR4 knockdown prevents in? flammatory cytokine expression in macrophages by suppressing activa? tion of MAPK and NF-κB signaling pathways[J]. Cell Biosci,2019,9:55.
(责任编辑:周一青)
本文引用格式:
周 明,汪家文,路艳林,等. SDF-1/CXCR4轴通过NF-κb信号通路调控炎症反应减轻动脉粥样硬化的形成[J]. 重庆医科大学学报,2025,50(2):237-243.

