芒柄花素保护BV2小胶质细胞糖氧剥夺再灌注损伤及其机制研究
【摘 要】目的:探讨芒柄花素(formononetin,FN)对糖氧剥夺再灌注(glucose and oxygen deprivation/reperfusion,OGD/R)刺激的BV2小胶质细胞多聚ADP核糖聚合酶1[poly(ADP-ribose) polymerase 1,PARP1]/聚(ADP-核糖)糖水解酶[poly(ADP-Ribose)glycohydrolase,PARG]信号通路及神经炎症的影响。方法:建立OGD/R BV2细胞模型,分为空白对照组、OGD/R组、10 μm FN组、OGD/R+10 μm FN处理组、OGD/R+抑制剂PJ34(10 μm)处理组、OGD/R+抑制剂Ethacridine lactate(7.5 μm)处理组。采用免疫荧光法(immunofluorescence method,IF)检测BV2细胞核因子-κB p65(Nuclear factor-kappa B p65,NF-κB p65)蛋白核转移及p53、凋亡诱导因子(apoptosis-inducing factor,AIF)、Toll样受体4(Toll-like receptor 4,TLR4)蛋白表达;免疫印迹法检测(Western blot,WB)PARP1/PARG通路相关蛋白表达;酶联免疫吸附试验(enzyme-linked immunosorbent assay,ELISA)检测血清γ干扰素(interferon-γ,IFN-γ)、白细胞介素-1β(interleukin-1β,IL-1β)以及肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)含量。结果:WB结果显示,与对照组相比,进行糖氧剥夺6 h后再进行复糖复氧培养1 h,细胞中PARP1、PARG表达明显升高(P= 0.000);与OGD/R组相比,在OGD/R后加入FN处理,BV2细胞中PARP1(P=0.000)和PARG(P=0.000)蛋白表达量明显下降,Iduna蛋白表达量明显上升(P=0.000);在OGD/R后加入PARP抑制剂PJ34处理后,PARP1蛋白表达量明显下降(P=0.000),但PARG和Iduna蛋白表达量无明显变化(P=0.061);在OGD/R后加入PARG抑制剂Ethacridine lactate处理后,BV2细胞中PARG蛋白表达量明显下降(P=0.000),但PARP1和Iduna蛋白表达量无明显变化(P=0.072)。IF结果显示,与OGD/R组相比,OGD/R后加入FN,细胞中p53、AIF、TLR4、NF-κB p65表达均明显下降,且NF-κB p65核转移明显减少;在OGD/R后加入PARP抑制剂PJ34或PARG抑制剂Ethacridine lactate,p53、AIF、TLR4表达同样下降,NF-κB p65核转移也明显减少。ELISA结果显示,与OGD/R组BV2细胞相比,在OGD/R后加入FN处理,细胞中IL-1β(P=0.004)和TNF-α(P=0.040)表达明显降低,但INF-γ水平无明显变化(P=0.056);在OGD/R后加入PARP抑制剂PJ34处理后,BV2细胞中促炎因子INF-γ(P=0.000)、IL-1β(P=0.021)和TNF-α(P=0.003)表达水平明显降低,或PARG抑制剂Ethacridine lactate处理后,BV2细胞中促炎因子INF-γ、IL-1β和TNF-α表达水平亦明显降低(P=0.000)。结论:FN可抑制糖氧剥夺BV2小胶质细胞中神经炎症,其机制涉及PARP1/PARG信号通路调控。
【关键词】芒柄花素;BV2小胶质细胞;糖氧剥夺再灌注;多聚ADP核糖聚合酶1抑制剂;聚(ADP-核糖)糖水解酶抑制剂
【中图分类号】R926【文献标志码】A【收稿日期】2024-03-25
基金项目:国家自然科学基金资助项目(编号:82160850);贵州省科技基金资助项目(编号:黔科合基础[2020]1Z071)。
Protective effects of formononetin against oxygen-glucose deprivation/ reperfusion injury in BV2 microglia and associated mechanism
Wei Dingling1,Wang Mei1,Wang Wenxiu2,Cao Liping2,He Qiansong1
(1. The First Clinical Medical College of Guizhou University of Traditional Chinese Medicine;2. Department of Neurology,The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine)
【Abstract】Objective:To investigate the effects of formononetin on the PARP1/PARG signaling pathway and neuroinflammation in BV2 microglia activated by oxygen-glucose deprivation/reperfusion(OGD/R). Methods:An OGD/R model was established with BV2 cells. These microglia were divided into blank control group,OGD/R group,formononetin(10 μm) group,OGD/R+formononetin(10 μm) group,OGD/R+PJ34(PARP1 inhibitor,10 μm) group,and OGD/R+ethacridine lactate(PARG inhibitor,7.5 μm) group. We measured the nuclear translocation of nuclear factor-kappa B(NF-κB) p65 and the expression of p53,apoptosis-inducing factor(AIF),and Toll-like receptor 4(TLR4) proteins in BV2 cells by immunofluorescence assay;measured the expression of PARP1/ PARG pathway-related proteins by Western blot;and determined the content of interferon(INF)-γ,interleukin(IL)-1β,and tumor necrosis factor-α(TNF-α) by enzyme-linked immunosorbent assay(ELISA). Results:Western blot results showed that compared with the control group,the expression of PARP1 and PARG in cells was significantly increased after 6-hour oxygen-glucose deprivation followed by 1-hour reperfusion(P<0.05); compared with the OGD/R group,the OGD/R+formononetin group had significantly decreased expression of PARP1 and PARG proteins(P<0.05) and signifi? cantly increased expression of Iduna protein(P<0.05);the OGD/R+PJ34 group had significantly decreased expression of PARP1 pro? tein(P<0.05),but the expression of PARG and Iduna proteins had no significant changes(P>0.05);the OGD/R+ethacridine lactate group had significantly decreased expression of PARG protein(P<0.05),but had no significant changes in the expression of PARP1 and Iduna proteins(P>0.05). Immunofluorescence assay results showed that compared with the OGD/R group,formononetin signifi? cantly reduced the expression of p53,AIF,TLR4,and NF-κB p65 and the nuclear translocation of NF-κB p65 in OGD/R cells; and both PJ34 and ethacridine lactate significantly reduced the expression of p53,AIF,and TLR4 and the nuclear translocation of NF-κB p65 in OGD/R cells. ELISA results showed that compared with the OGD/R group,formononetin significantly decreased the expression of IL-1β and TNF-α(P<0.05),but did not significantly affect INF-γ levels in OGD/R cells (P>0.05); and both PJ34 and ethacridine lactate significantly decreased the expression levels of the proinflammatory factors INF-γ,IL-1β,and TNF-α in OGD/R cells (P< 0.05). Conclusion:Formononetin can inhibit neuroinflammation in BV2 microglia deprived of glucose and oxygen,through regulating the PARP1/PARG signaling pathway.
【Key words】formononetin;BV2 microglia;oxygen-glucose deprivation/reperfusion;PARP1 inhibitor;PARG inhibitor
缺血性中风作为全球死亡和致残的主要原因之一[1],目前其治疗选择有限,静脉溶栓和介入取栓治疗法虽然可以恢复血流,但也可能导致炎症反应、细胞凋亡、氧化应激等,并进而引起脑缺血再灌注损伤[2-3]。聚(ADP-核糖)糖水解酶[poly(ADPRibose) glycohydrolase,PARG]是一种内切和外切糖水解酶,可快速降解多聚ADP核糖聚合酶1[poly(ADP-ribose) polymerase 1,PARP1]生成的聚合物(腺苷二磷酸核糖)[poly(ADP-ribose),PAR][4]。PARP1是一种由DNA链断裂激活的核酶,在应激条件下,PARP1的激活会消耗NAD+和腺苷三磷酸(adenosine triphosphate,ATP),最终导致细胞死亡[5-6]。此外,PARP1的激活会触发线粒体释放凋亡诱导因子(apoptosis-inducing factor,AIF)[7],以及参与核因子-κB(nuclear factor-kappa B,NF-κB)转录的调控[8]。PARP1/PARG信号通路可为缺血性中风的治疗提供新的途径和靶点。
小胶质细胞作为中枢神经系统中的免疫细胞,在神经炎症的调节中发挥着关键作用[9-10]。脑缺血后,静息状态的小胶质细胞会转变为活化状态,产生血清γ干扰素(interferon-γ,IFN-γ)、白细胞介素-1β(interleukin-1β,IL-1β)以及肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)等促炎因子,从而加重神经细胞的坏死和凋亡[11]。芒柄花素(formononetin,FN)是从阳雀花根(植物锦鸡儿根)中提取的一种异黄酮成分,可用于治疗缺血性脑卒中后遗留的神经功能缺损[12]。项目前期研究表明,FN对大鼠脑缺血/再灌注(ischemia/reperfusion,I/R)损伤具有有效的神经保护作用,并发现其可以减少脑梗面积和神经功能评分[13]。然而,FN是否可以通过调节小胶质细胞的状态参与脑梗死急性期脑组织的炎症反应,以及PARP信号通路是否参与了这一调节过程,尚待深入研究。因此,本研究建立了体外BV2细胞糖氧剥夺再灌注(glucose and oxygen deprivation/reperfusion,OGD/R)模型,以探讨FN对小胶质细胞介导的神经保护作用,从而为缺血性中风的新治疗靶点和方法提供科学依据。
1 材料与方法
1.1 实验材料
1.1.1 细胞 小鼠小胶质细胞BV2来源于武汉普诺赛生命科技有限公司,目录号为CL-0697,已传至第4代。
1.1.2 药品和试剂 FN(MedChe-mExpress,美国,HYN0183);PARP抑制剂PJ34(MedChe-mExpress,美国,HY-13688A);PARG抑制剂Ethacridine lactate(MedChe-mExpress,美国,HY-B2714);细胞裂解液放射免疫沉淀法裂解缓冲液(radio immunoprecipitation assay buffer,RIPA)(北京普利莱基因技术有限公司,C1053);BCA蛋白定量试剂盒(BCA protein assay kit)(武汉伊莱瑞特,E-BC-K318-M);P53抗体(江苏亲科生物研究中心有限公司,AF0879);AIF抗体(武汉三鹰,17984-1-AP);β-Actin抗体(北京全式金,HC201);PARP1抗体(武汉三鹰生物技术有限公司,13371-1AP);PARG抗体(江苏亲科,DF13517);Iduna抗体(北京博奥森,bs-11669R);Cy3Goat Anti-Mouse IgG(H+L)(武汉爱博泰克,AS008)。IFN-γ试剂盒(江苏酶免,MM-182M1);IL-1β试剂盒(江苏酶免,MM0-0040M1);TNF-α试剂盒(江苏酶免,MM-0132M1)。
1.1.3 仪器 高速冷冻离心机(上海卢湘仪,TGL-16.5M);低温高速离心机(Eppendorf,5424R);全自动酶标仪(上海闪普,SuPerMax 3100);蛋白垂直电泳仪(北京市六一,DYY-6C);全自动样品快速研磨仪(上海净信实业发展有限公司,Tiss-12);超高灵敏度化学发光成像系统(上海伯乐,Chemi DocTM XRS+);CO2培养箱(上海一恒,BPN-80CW);倒置荧光显微镜(广州明美,MF53);洁净工作台(BIOBASEB,BSSDC);医用离心机(长沙英泰,TD4A);三气培养箱(长沙华曦,YCP-10S)。
1.2 实验方法
1.2.1 细胞培养与分组 本实验利用经过鉴定的小鼠小胶质细胞BV2(来源于武汉普诺赛生命科技有限公司,货号:CL-0493)。细胞在37℃、5% CO2培养条件下采用含有1%双抗和10%胎牛血清的DMEM培养基培养,每3 d更换1次培养液,直至细胞达到对数生长期以备后用。BV2细胞接种后24 h进行缺氧-缺糖(OGD/R)实验。弃去BV2细胞原培养基,换成无糖培养基之前用无糖培养基洗涤数次,之后,置于37 ℃、1%O2、94%N2、5%CO2的条件下培养,进行6 h糖氧剥夺。随后,在95%的空气和5%的CO2条件下,细胞被转入到正常培养基中,进行1、2、3、24 h的复氧复糖试验。复氧不同时间点(1、2、3、24 h)细胞内PARP1和PARG蛋白的表达情况采用蛋白质印迹法(Western blot,WB)检测,为后续实验确定条件。OGD/R的时间点确认后,分为对照组、对照组+FN组、OGD/R组、OGD/R+FN(10 mol/L)组、OGD/R+PJ34(10 mol/L)组和OGD/R+Ethacridine lactate(7.5 mol/L)组6个实验组。其中,不进行OGD/R处理,正常培养的BV2细胞,被设为对照组。OGD/R+FN(10 mol/L)、OGD/R+PJ34(10 mol/L)和OGD/R+Ethacridine lactate(7.5 mol/L)处理组在OGD/R后放入对应浓度的药物进行孵育处理。
1.2.2 免疫荧光法(immunofluorescence method,IF) 用胰酶消化各组BV2细胞后制作爬片。24 h后用磷酸缓冲盐溶液(phosphate buffered saline,PBS)在培养板中浸洗爬片3次,使用4%多聚甲醛固定后,用5%胎牛血清白蛋白(bovine serum albumin,BSA),37°封闭30 min;丢弃封闭液后,加入稀释好的一抗p53抗体、AIF抗体、Toll样受体4(Toll-like receptor 4,TLR4)抗体和核因子-κB p65(nuclear factorkappa B p65,NF-κB p65)抗体,并按照1∶200比 例进行4 ℃孵育过夜;PBS洗涤后,加入稀释至1∶200的荧光二抗Cy3,在37 ℃下孵育30 min。随后采用4’,6-二脒基-2-苯基吲哚(DAPI)对细胞核进行染色,封片利用含抗荧光淬灭剂的封片液进行,最后观察和采集图像在荧光显微镜下进行。
1.2.3 WB检测 离心去上清的各组BV2细胞用蛋白裂解液裂解30 min后,再次离心5 min,取上清液,用BCA法检测蛋白浓度。变性后的蛋白样品,利用十二烷基硫酸钠聚丙烯酰胺凝胶电泳(SDS-PAGE)1.5 h后转膜1 h。聚偏氟乙烯膜用脱脂奶粉封闭后,与一抗(PARP1、PARG、Iduna)在4 ℃下孵育过夜,Actin抗体作为内参。第2天,聚偏氟乙烯膜用发光液浸湿之前,在室温下孵育二抗2 h,并用超高灵敏度化学发光成像系统进行显影。随后使用Image J软件对蛋白条带强度展开定量分析,算出目标蛋白与内参蛋白灰度值的比值,以进行半定量统计分析。
1.2.4 酶联免疫吸附试验(enzyme linked immunosorbent assay,ELISA) 分别使用INF-γ、IL-1β和TNF-α ELISA试剂盒,严格按照试剂盒测定说明书进行实验操作,用标准曲线计算每个样品中活化小胶质细胞上清中释放INF-γ、IL-1β和TNF-α的量。
1.3 统计学方法
应用GraphPad Prism 8.0.1软件进行统计分析并作图。所有实验重复3次,计量资料采用均数±标准差(x±s)表示,多组之间定量数值比较采用单因素方差分析。检验水准α=0.05。
2 结 果

2.1 OGD/R模型对BV2细胞中PARP1/PARG通路的影响
如图1A所示,复氧不同时间(1、2、 3、24 h)后的BV2细胞中PARP1和PARG蛋白表达情况使用Western blot检测,确定OGD/R对BV2细胞PARP1/PARG通路激活的最佳影响时间。在小鼠小胶质BV2细胞中,OGD/R组与对照组相比,PARP1蛋白进行糖氧剥夺6 h后再进行复糖复氧培养1 h(图1B,1.518±0.025)及2 h(图1B,1.422±0.031),表达较对照组(Control,CON)明显升高(P=0.000),OGD/R组与对照组相比,PARG蛋白进行糖氧剥夺6 h后再进行复糖复氧培养1 h(图1C,1.255±0.036)后表达较CON组(0.830±0.0320)明显升高(P=0.000)。随着复糖复氧时间延长,PARP1和PARG表达逐渐下降。得结论为,糖氧剥夺再灌注1 h后,BV2细胞中激活最明显的为PARP-1/PARG通路,因此,选用糖氧剥夺再灌注1 h构建OGD/R模型展开后续实验。
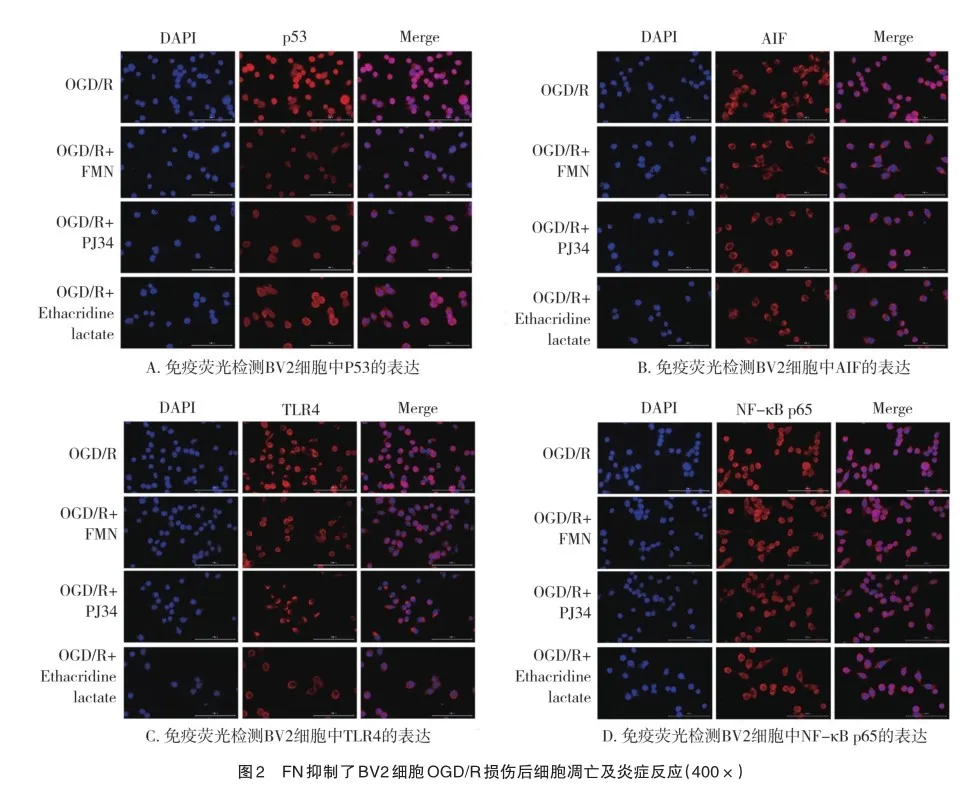
2.2 FN对BV2细胞OGD/R损伤后p53、AIF、TLR4的表达及NF-κB p65核转移的影响
如图2所示,免疫荧光检测结果显示,与OGD/R组细胞相比,OGD/R后加入FN,细胞中p53、AIF、TLR4、NF-κB p65表达均明显下降,且NF-κB p65核转移明显减少,表明FN可降低OGD/R后小胶质细胞的凋亡及炎症反应。同时,在OGD/R后加入PARP抑制剂PJ34、PARG抑制剂Ethacridine lactate孵育,BV2细胞中p53、AIF、TLR4表达同样下降,NF-κB p65核转移也明显减少,这表明抑制PARP1/PARG可抑制OGD/R诱导的BV2细胞凋亡及炎症反应。
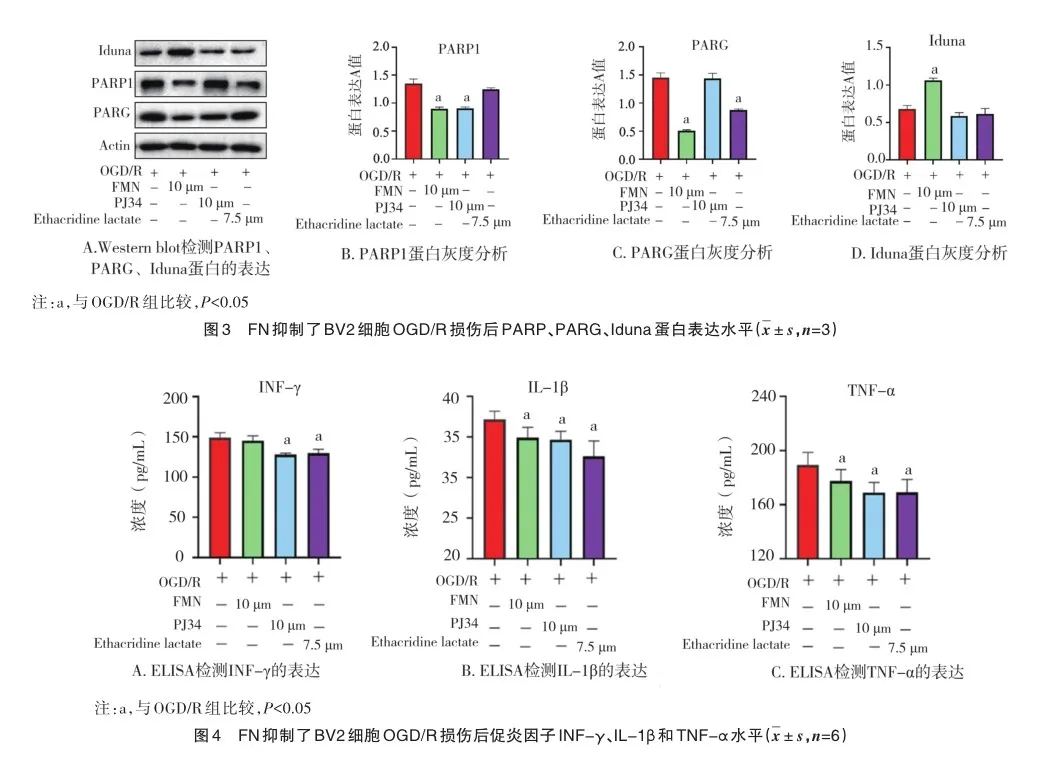
2.3 FN对PARP1、PARG、Iduna蛋白表达的影响
如图3A所示,与OGD/R组细胞相比,在OGD/R后加入FN处理,BV2细胞中PARP1(图3B,0.852±0.018)和PARG(图3C,1.430±0.027)蛋白表达量较OGD/R组(1.430±0.027)明显下降(P=0.000),Iduna(图3D,1.182±0.028)蛋白表达量较OGD/R组(0.613±0.024)明显上升(P=0.000);与OGD/R组相比,在OGD/R后加入PARP抑制剂PJ34处理后,PARP1(图3B,0.843±0.017)蛋白表达量较OGD/R组(1.430±0.027)明显下降(P=0.000),但PARG和Iduna蛋白表达量无明显变化;与OGD/R组(1.480±0.045)比较,在OGD/R后加入PARG抑制剂Ethacridine lactate处理后,BV2细胞中PARG(图3C,0.859±0.015)蛋白表达量明显下降(P=0.000),但PARP1和Iduna蛋白表达量无明显变化。这表明FN可通过同时抑制PARP1/PARG对OGD/R诱导的小胶质细胞损伤进行保护。
2.4 FN对OGD/R后BV2细胞促炎因子INFγ、IL-1β和TNFα水平的影响
如图4所示,与OGD/R组BV2细胞相比,在OGD/R后加入FN处理,BV2细胞中IL-1β(图4B,34.855±8.437,P= 0.004)和TNF-α(图4C,180.430±14.590,P=0.044)表达水平较OGD/R组(37.240±6.361,190.122±15.350)明显降低,但INF-γ水平无明显变化(148.530±10.240,146.325±12.682,P=0.056);与OGD/R组比较,在OGD/R后加入PARP抑制剂PJ34处理后,BV2细胞中促炎因子INF-γ(图4A,34.213±5.842,P=0.000)、IL-1β(图4B,34.213±5.842,P=0.021)和TNF-α(图4C,168.654±16.543,P=0.003)表达水平较OGD/ R组均明显降低;与OGD/R组比较,在OGD/R后加入PARG抑制剂Ethacridine lactate处理后,BV2细胞中促炎因子INF-γ(图4A,139.786±13.620,P=0.000)、IL-1β(图4B,32.675±9.158,P=0.000)和TNF-α(图4C,167.885±18.541,P=0.000)表达水平较OGD/R组均明显降低。这表明FN能明显抑制糖氧剥夺诱导的BV2小胶质细胞中促炎因子IL-1β和TNF-α的分泌抑制神经炎症。
3 讨 论
近年来,越来越多的研究证实中药异黄酮类成分在各种神经系统疾病中的神经保护作用。FN在抗氧化、神经保护、抗癌、抗炎、促进血管生成等方面均有研究[12]。已有研究证明,FN能够减少H2O2诱导的活性氧(reactive oxygen species,ROS)产生和细胞凋亡[14],并通过调节磷脂酰肌醇3-激酶(phospha? tidylinositol 3-kinase,PI3K)/蛋白激酶B(protein ki? nase B,Akt)信号通路抑制大鼠神经元缺血/再灌注损伤。此外,它还可能通过减少神经元的炎症反应,在脑外伤动物中产生神经保护作用[15]。本课题组前期研究发现,FN对急性脑梗死具有明显疗效,可明显减少脑缺血后梗塞体积和促炎因子表达。提示FN可能通过降低PAR水平发挥神经保护作用。PAR降解是由聚合酶介导的,其中涉及水解酶PARG,但其机制尚不清楚。大量研究表明,聚ADP-核糖参与神经元损伤和脑缺血炎症反应的关键调节机制[16]。然而,FN对PARP1酶活性和多聚(ADP-核糖基)化在小胶质细胞转录激活的作用,以及PARP-1活性依赖性调节NF-κB对脑缺血的保护作用尚未见报道。因此,本研究旨在探讨FN对OGD/R刺激的BV2小胶质细胞PARP1/PARG信号通路及神经炎症的影响。结果观察到FN及PARP/PARG抑制剂处理抑制了小胶质细胞在OGD/ R的凋亡因子表达及促炎症反应,同时抑制了PARP1/PARG信号通路的活性。
本研究中,OGD/R后的BV2细胞中PARP1/ PARG表达呈动态变化。在复氧初期(OGD/R 1 h),PARP1和PARG蛋白表达明显上调达到峰值,但在OGD/R 2 h后开始下降。过度活化的PARP1可能促进受体蛋白的形成,导致细胞毒性作用和促进细胞凋亡[17]。相反,Iduna作为一种神经保护因子,是一种PAR聚合物依赖的E3泛素连接酶,可抑制线粒体中AIF的核转移,减少PARP1激活引起的细胞死亡[18]。本研究结果显示,FN可降低OGD/R后PARP1和PARG的活性,促进Iduna的表达,同时抑制了细胞中凋亡因子P53和AIF的表达。Stoica BA等[19]研究发现PARP1抑制剂PJ34可抑制神经细胞中凋亡因子AIF的释放,这与本研究结果一致。PARP抑制剂PJ34和PARG抑制剂Ethacridine lac?tate分别抑制了OGD/R后PARP1和PARG的活性,同时也抑制了细胞中凋亡因子p53和AIF的表达。这表明,FN通过抑制PARP1和PARG的活性,从而抑制BV2小胶质细胞的凋亡。
PARP1已被证实参与炎症过程,包括调节转录因子以及促进细胞因子、黏附因子和炎症介质的表达[20]。NF-κB是一种经典的促炎信号通路,在IL-1β和TNF-α等促炎细胞因子的作用下被激活,进而参与促炎因子的表达[21]。小胶质细胞作为中枢神经系统的巨噬细胞,在OGD/R后被激活,分泌促炎因子,进而引发脑内炎症反应[22]。本研究结果显示,FN可以逆转OGD/R对BV2小胶质细胞炎症的影响。FN能够降低BV2细胞中TLR4和NF-κB p65的表达,并抑制NF-κB p65的核转移,从而抑制TLR4及其下游蛋白NF-κB的活化,同时抑制促炎细胞因子INF-γ、IL-1β和TNF-α的分泌。此外,有研究表明,BV2小胶质细胞暴露在脂多糖中可诱导NF-κB的形成和TNF-α的释放,而这些效应可以被PARP抑制剂PJ34减弱[19],本研究同样也证实了这一结论。PARP抑制剂PJ34和PARG抑制剂Ethacridine lactate同样抑制了OGD/R后BV2小胶质细胞中TLR4和NF-κB的炎症激活,以及促炎因子INF-γ、IL-1β和TNF-α的分泌。针对PARP1的促炎作用,PARP1抑制剂已被证明是治疗许多炎症相关疾病的方法。
综上所述,本研究发现FN能够抑制糖氧剥夺小鼠小胶质细胞中PARP1/PARG活性,促进神经保护因子Iduna的表达,抑制NF-κB转录,从而降低炎症反应。这些结果为FN治疗脑梗死急性期小胶质细胞介导的神经炎症提供了新的见解。
参 考 文 献
[1] Herpich F,Rincon F. Management of acute ischemic stroke[J]. Crit Care Med,2020,48(11):1654-1663.
[2] Zhang P,Cui J. Neuroprotective effect of fisetin against the cere? bral ischemia-reperfusion damage via suppression of oxidative stress and inflammatory parameters[J]. Inflammation,2021,44(4):1490-1506.
[3] Xu Q,Guohui M,Li DD,et al. lncRNA C2dat2 facilitates au? tophagy and apoptosis via the miR-30d-5p/DDIT4/mTOR axis in cere? bral ischemia-reperfusion injury[J]. Aging,2021,13(8):11315-11335.
[4] Slade D. PARP and PARG inhibitors in cancer treatment[J]. Genes Dev,2020,34(5/6):360-394.
[5] McGurk L,Rifai OM,Bonini NM. Poly(ADP-ribosylation) in agerelated neurological disease[J]. Trends Genet,2019,35(8):601-613.
[6] Zhu HF,Tang YD,Zhan GQ,et al. The critical role of PARPs in regulating innate immune responses[J]. Front Immunol,2021,12:712556.
[7] Liu LB,Li JX,Ke YS,et al. The key players of parthanatos:oppor? tunities for targeting multiple levels in the therapy of parthanatos-based pathogenesis[J]. Cell Mol Life Sci,2022,79(1):60.
[8] Karpova Y,Johnson SJ,Bordet G,et al. Upregulation of PARG in prostate cancer cells suppresses their malignant behavior and downregu? lates tumor-promoting genes[J]. Biomedecine Pharmacother,2022,153:113504.
[9] Peng JL,Wang HX,Gong Z,et al. Idebenone attenuates cerebral inflammatory injury in ischemia and reperfusion via dampening NLRP3 inflammasome activity[J]. Mol Immunol,2020,123:74-87.
[10] Kanazawa M,Ninomiya I,Hatakeyama M,et al. Microglia and monocytes/macrophages polarization reveal novel therapeutic mecha? nism against stroke[J]. Int J Mol Sci,2017,18(10):2135.
[11] Var SR,Shetty AV,Grande AW,et al. Microglia and macro? phages in neuroprotection,neurogenesis,and emerging therapies for stroke[J]. Cells,2021,10(12):3555.
[12] Ma XY,Wang JJ. Formononetin:a pathway to protect neurons[J]. Front Integr Neurosci,2022,16:908378.
[13] Luo J,Cai YD,Wei DL,et al. Formononetin alleviates cerebral ischemia-reperfusion injury in rats by targeting the PARP-1/PARG/ Iduna signaling pathway[J]. Brain Res,2024,1829:148845.
[14] Jia WC,Liu G,Zhang CD,et al. Formononetin attenuates hydro? gen peroxide (H2O2)-induced apoptosis and NF-κB activation in RGC-5 cells[J]. Eur Rev Med Pharmacol Sci,2014,18(15):2191-2197.
[15] Liang K,Ye Y,Wang Y,et al. Formononetin mediates neuropro? tection against cerebral ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2 ratio and upregulation PI3K/Akt signaling pathway[J]. J Neurol Sci,2014,344(1/2):100-104.
[16] Morihara R,Yamashita T,Kono S,et al. Reduction of intracere? bral hemorrhage by rivaroxaban after tPA thrombolysis is associated with downregulation of PAR-1 and PAR-2[J]. J Neurosci Res,2017,95(9):1818-1828.
[17] Mamontova EM,Clément MJ,Sukhanova MV,et al. FUS RRM regulates poly(ADP-ribose) levels after transcriptional arrest and PARP-1 activation on DNA damage[J]. Cell Rep,2023,42(10):113199.
[18] Yang XX,Cheng JH,Gao YB,et al. Downregulation of Iduna is associated with AIF nuclear translocation in neonatal brain after hypoxia-ischemia[J]. Neuroscience,2017,346:74-80.
[19] Stoica BA,Loane DJ,Zhao ZR,et al. PARP-1 inhibition attenu? ates neuronal loss,microglia activation and neurological deficits after traumatic brain injury[J]. J Neurotrauma,2014,31(8):758-772.
[20] Li WH,Wang F,Song GY,et al. PARP-1:a critical regulator in radioprotection and radiotherapy-mechanisms,challenges,and thera? peutic opportunities[J]. Front Pharmacol,2023,14:1198948.
[21] Sun SC. The non-canonical NF-κB pathway in immunity and in? flammation[J]. Nat Rev Immunol,2017,17(9):545-558.
[22] Ganbold T,Bao QM,Zandan J,et al. Modulation of microglia po? larization through silencing of NF-κB p65 by functionalized curdlan nanoparticle-mediated RNAi[J]. ACS Appl Mater Interfaces,2020,12(10):11363-11374.
(责任编辑:周一青)
本文引用格式:
韦丁玲,王 湄,王文秀,等. 芒柄花素保护BV2小胶质细胞糖氧剥夺再灌注损伤及其机制研究[J]. 重庆医科大学学报,2025,50(2):244-249.

