基于代谢组学与蛋白组学的广东烟区沙泥田烤烟上部叶挂灰成因分析









摘要:【目的】基于代谢组学和蛋白组学联合分析广东烟区沙泥田与紫色土烤烟上部叶物质代谢和蛋白表达差异,为减少沙泥田烟叶挂灰提供参考依据。【方法】以烤后不易挂灰的紫色土烟叶(对照)和烤后易挂灰的沙泥田烟叶为研究对象,选择2种土壤类型烟叶上部叶欠熟、尚熟、成熟和过熟4个时期的鲜烟叶样品,测定多酚氧化酶(PPO)活性;再以成熟时期烟叶进行代谢组学和蛋白组学联合分析,鉴定沙泥田上部成熟烟叶中的差异代谢物和差异表达蛋白,并分析筛选影响沙泥田上部烟叶褐变的关键通路。【结果】上部叶成熟过程中,紫色土烟叶PPO活性极显著低于沙泥田烟叶(Plt;0.01),且二者在成熟时期差值最大。从2种土壤类型成熟时期烟叶样品中共筛选出差异代谢物129个,差异表达蛋白1314个,差异代谢物、差异表达蛋白均以上调为主;差异代谢物的聚类和KEGG代谢通路分析主要富集到酪氨酸代谢、苯丙氨酸—酪氨酸—色氨酸生物合成、苯丙素生物合成等通路上。差异表达蛋白的GO功能注释和KEGG信号通路富集分析主要富集到苯丙素生物合成、酪氨酸代谢、异喹啉生物碱生物合成和植物激素信号传导等过程。多酚类物质生物合成过程中,沙泥田烟叶差异表达蛋白苯丙氨酸解氨酶、4-香豆酸辅酶A连接酶、羟基肉桂酰辅酶A莽草酸酯/奎宁酸羟基肉桂酰转移酶下调;差异代谢物阿魏酸、松柏醇、芥子醇下调。脱落酸生物合成和代谢过程中,沙泥田烟叶差异表达蛋白八氢番茄红素脱氢酶、玉米黄质环氧化酶、9-顺式环氧类胡萝卜素双加氧酶、黄氧素脱氢酶下调;差异代谢物红花菜豆酸、二氢红花菜豆酸下调。【结论】与紫色土烤烟上部叶相比,沙泥田烤烟上部叶生长过程中更易受环境胁迫,导致烟叶素质下降,脱落酸合成增加,激素信号激活,酚类代谢增强,PPO活性上升,在烘烤过程中易导致酶促褐变更加剧烈,从而造成烤后烟叶易挂灰。
关键词:烤烟;沙泥田;上部叶;挂灰;差异代谢物;差异表达蛋白
中图分类号:S572文献标志码:A文章编号:2095-1191(2024)10-2926-12
Analysis of the causes of ash hanging in flue-cured tobacco upperleaves in sandy mud field in Guangdong tobacco-growing areasbased on metabolomics and proteomics
YANG Qing-yue1,2,LIU Lan3,WANG Song-feng2*,WANG Xiao-bin4,YAO Yuan-hua3,WANG Ai-hua2,FAN Miao-miao3,LI Wei2,SONG Zhao-peng1,WANG Hang3*
(1College of Tobacco Science,Henan Agricultural University,Zhengzhou,Henan 450002,China;2Tobacco ResearchInstitute,Chinese Academy of Agricultural Sciences/Key Laboratory of Tobacco Biology and Processing,Ministry ofAgriculture and Rural Affairs,Qingdao,Shandong 266101,China;3Guangdong Institute of Tobacco Sciences,Shaoguan,Guangdong 512029,China;4Guangdong Tobacco Company,China National TobaccoCorporation,Guangzhou,Guangdong 510627,China)
Abstract:【Objective】Based on the combined analysis of metabolomics and proteomics,the differences in material metabolism and protein expression of flue-cured tobacco upper leaves in sandy mud field and pruple soil of Guangdongtobacco-growing areas were analyzed to provide reference for reducing ash deposition of tobacco leaves in sandy mudfield.【Method】Purple soil flue-cured tobacco leaves that were less prone to ash hanging after curing(control)and sandy mud field flue-cured tobacco leaves that were prone to ash hanging after curing were used as the research objects.Thefresh tobacco leaves of the upper leaves of the 2 soil types at 4 stages of before maturity,just mature,mature and over-mature were selected to determine the activity of polyphenol oxidase(PPO).Subsequently,a combined metabolomic and proteomic analysis was conducted on mature tobacco leaves to identify differential metabolites and differentially ex-pressed proteins present in mature upper leaves from sandy mud fields.Additionally,this study aimed to analyze andscreen for key pathways influencing browning in these upper tobacco leaves grown in sandy mud field.【Result】Duringthe maturation of the upper leaves,the PPO activity of the purple soil tobacco leaves was extremely significantly lowerthan that of the sandy mud field tobacco leaves(rlt;0.01),and the difference between the two was the largest during the maturation period.A total of 129 differential metabolites and 1314 differentially expressed proteins were screened fromthe mature tobacco samples of the 2 soil types,and the differential metabolites and differentially expressed proteins were mainly up-regulated.The clustering of differential metabolites and KEGG metabolic pathway analysis were mainly en-riched in tyrosine metabolism,phenylalanine-tyrosine-tryptophan biosynthesis,phenylpropanoid biosynthesis.GOfunc-tional annotation and KEGG signal pathway enrichment analysis of differentially expressed proteins were mainly enrichedin phenylpropanoid biosynthesis,tyrosine metabolism,isoquinoline alkaloids biosynthesis and plant hormone signaltransduction.During the biosynthesis of polyphenols,the differentially expressed proteins of phenylalanine ammonialyase,4-coumaric acid coenzyme A ligase and hydroxycinnamoyl coenzyme A shikimate/quinic acid hydroxycinnamoyltransferase were down-regulated in sandy mud field tobacco leaves.The differentially expressed proteins of phytoene de-saturase,zeaxanthin epoxidase,9-cis-epoxycarotenoid dioxygenase andxanthooxin dehydrogenase were down-regulatedin sandy mud field tobacco leaves.Differential metabolites phaseic acid and dihydrophaseic acid were down-regulated.【Conclusion】Compared with the flue-cured tobacco upper leaves in purple soil,the flue-cured tobacco upper leaves insandy mud field are more susceptible to environmental stress during the growth process,resulting in a decrease in the quality of tobacco leaves,an increase in abscisic acid synthesis,activation of hormone signals,enhancement of phenolic metabolism,and an increase in PPO activity.During the curing process,it is easy to lead to more severe enzymatic browning,resulting in easy ash hanging of cured tobacco leaves.
Key words:flue-cured tobacco;sandy mud field;upper leaves;ash hanging;differential metabolites;differentially expressed proteins
Foundation items:Science and Technology Key Project of China National Tobacco Corporation(110202102007);Science and Technology Innovation Project of Chinese Academy of Agricultural Sciences(ASTIP-TRIC03);Technology Project of Guangdong Tobacco Company of China National Tobacco Corporation(2021440000240145)
0引言
【研究意义】广东烟区为我国浓香型烤烟产区之一,植烟土壤类型主要有沙泥田和紫色土。该烟区约有50%以上的烤烟种植在沙泥田中,但与紫色土种植的烟叶不同,沙泥田种植的烟叶烤后易挂灰,且上部叶挂灰现象表现更突出,致使烤后烟叶质量下降(王军等,2015;王行等,2023)。烤后烟叶正表面形成灰色或褐色细微斑点的现象称为挂灰。烟叶挂灰除与鲜烟素质有关外,也可能受土壤状况影响。沙泥田土壤酸化明显,部分矿物质营养元素缺乏,可能使烟株体内的生理功能紊乱,造成相关化合物大量积累;此外,其土壤粉粒含量高,质地较疏松,加之广东烟区降水时空分布不均及烟叶生长后期易出现连续高温情况,烟叶为缓解环境胁迫而增强相关生理活动,导致烟叶素质下降(王军等,2016;王行等,2023)。因此,研究影响沙泥田烟叶易挂灰的关键因素,对提高广东烟区烤烟质量具有重要意义。【前人研究进展】影响烤烟上部叶挂灰的首要因素是烟叶素质,有研究表明,烟叶生长过程中受到的非生物胁迫会导致其鲜烟素质和烤后烟叶质量下降(Li etal.,2021;Begum etal.,2021)。脱落酸(ABA)可缓解植物非生物胁迫并调节其生长发育,当植物受到非生物胁迫时,ABA含量上升(Yang et al.,2011)。ABA生物合成途径分为C15直接途径和C40间接途径。在C40间接途径中,9-顺式环氧类胡萝卜素双加氧酶(NCED)、玉米黄质环氧化酶(ZEP)是其关键酶。ABA代谢途径主要为由细胞色素P450单加氧酶(CYP707A)介导的ABA 8'位甲基羟基化和由葡萄糖基转移酶介导的螯合2条途径(Chen et al.,2020)。酚类化合物是茄科植物中广泛存在的重要次生代谢产物,参与多种非生物胁迫反应(Wang et al.,2024)。已有研究表明,植物可通过增强酚类物质代谢来缓解环境胁迫(Ampofoetal.,2020;Kohler et al.,2020;Zhang et al.,2021)。烤烟上部叶挂灰主要是由烘烤过程中酶促褐变导致(Sui et al.,2023)。前人研究发现,可通过降低或抑制多酚氧化酶(PPO)活性(Maioli et al.,2020;Ma et al.,2023;Song et al.,2023),也可通过提高抗氧化酶活性或降低酚类和醌类物质含量来减轻烟叶或食品的褐变程度(Li etal.,2023a)。随着研究深入,多组学联合分析已成为植物学研究的重要手段之一(黄亚成等,2023)。Tang等(2020)通过代谢组学和基因表达分析,发现延缓富士苹果褐变过程中,金丝桃苷可能是抑制褐变的关键多酚,较高的抗氧化酶活性也起重要作用。Tong等(2024)利用代谢组学和转录组学研究生姜的褐变机制,推测绿原酸和阿魏酸在PPO和过氧化物酶(POD)的催化作用下发生聚合反应,从而加剧生姜的木质化,使生姜发生褐变。【本研究切入点】烘烤中烟叶挂灰属于褐变反应,目前,通过调节PPO活性和酚类物质代谢来降低酶促褐变的研究主要集中在食品方面,而基于多组学联合分析烤烟褐变的研究较少,也尚无针对广东沙泥田烟叶褐变机理研究的相关报道。【拟解决的关键问题】通过代谢组学与蛋白组学手段,研究广东烟区沙泥田与紫色土成熟烤烟上部叶代谢物和蛋白表达差异与功能注释,挖掘影响烟叶褐变的关键过程,为减少广东烟区沙泥田烟叶挂灰提供参考依据。
1材料与方法
1.1试验材料
供试烤烟品种为粤烟97,由广东省烟草科学研究所提供。
1.2样品采集
试验在广东省南雄市广东省烟草科学研究所试验基地进行。以烤后不易挂灰的紫色土烟叶(对照,Z)和烤后易挂灰的沙泥田烟叶(S)为研究对象,依据GB/T 23219—2008《烤烟烘烤技术规程》,采集2种土壤类型烟叶上部叶欠熟、尚熟、成熟、过熟4个时期的鲜烟叶样品进行PPO活性测定;再以成熟时期样品进行代谢组学和蛋白组学分析,样品采集后立即用液氮速冻,保存在-80℃冰箱中,分析前进行预混,各处理烟叶用液氮研磨成粉末,放入试管中做好标记,每项分析均设3个生物学重复。
1.3 PPO活性测定
PPO活性使用苏州格锐思生物科技有限公司生产的试剂盒(GO113F/48样),采用分光光度法测定。
1.4广泛靶向代谢组学分析
1.4.1代谢物提取将各样品置于冻干机(Scientz-100F)中真空冷冻干燥;利用研磨仪(MM400,Retsch)研磨样品至粉末状(30 Hz,1.5 min);使用电子天平(RADWAGAS 60/220.R2)称取50 mg样品粉末,溶解于1000μL的70%甲醇内标提取液中,不足50 mg的样本,按每50 mg样本加入1000μL提取液的比例加入;再加入500μL石油醚,涡旋5min,静置分层,4℃条件下以12000 r/min离心10 min。移取全部提取液过0.22μm PTFE滤膜至棕色进样瓶玻璃内衬管内,-20℃保存备用。
1.4.2检测条件使用超高效液相色谱(UHPLC)和三重四级杆质谱(QQQ)联用进行代谢组学分析。液相条件主要包括:色谱柱Agilent SB-C18(1.8µm,2.1 mm×100 mm);流动相A相为超纯水(加入0.1%的甲酸),B相为乙腈(加入0.1%的甲酸);洗脱梯度:0 min B相比例为5%,9 min内B相比例线性增加到95%,并维持在95%1 min;随后,在1.1 min(10~11.1 min)内将B相比例降为5%,并以5%平衡至14min;流速0.35 mL/min;柱温40℃;进样量2μL。质谱条件主要包括:电喷雾离子源(ESI)温度550℃;离子喷雾电压(IS)5500 V(正离子模式)/-4500 V(负离子模式);离子源气体I(GSI)、气体II(GSII)和气帘气(CUR)分别设置为50、60和25psi,碰撞诱导电离参数设置为高。QQQ扫描使用MRM模式,并将碰撞气体(氮气)设置为中等。通过进一步的去簇电压(DP)和碰撞能(CE)优化,完成各个MRM离子对的DP和CE。根据每个时期内洗脱的代谢物,在每个时期监测一组特定的MRM离子对。
1.4.3代谢组学分析通过超高效液相色谱串联质谱(UHPLC-MS/MS)对样品中代谢物进行鉴定。相关数据进行多变量统计分析,采用无监督主成分分析(PCA)对2个样本的总体方差进行剖析。代谢组学分析包括3个生物学重复,采用变量重要性投影(VIP)≥1和差异倍数(Fold Change,FC)≥2、FC≤0.5及rlt;0.05来筛选差异代谢物。
1.5 4D-DIA定量蛋白组学分析
试验样本进行总蛋白的提取、胰酶酶解、UHPLC-MS/MS上机检测,再对下机数据进行归一化处理。随后用MaxQuant搜索烟草全基因组信息数据库(Uniprot),得到蛋白定性定量结果。显著性差异表达蛋白筛选以差异倍数FCgt;1.2倍(上调gt;1.2倍或下调lt;0.8倍)且rlt;0.05为标准,得到比较组间的上调和下调蛋白数目。采用亚细胞结构预测软件CELLO对所有差异表达蛋白进行亚细胞定位分析。采用Blast2Go(https://www.blast2go.com/)对差异表达蛋白进行GO功能注释,主要分为3类:生物过程(Bio-logical process,BP),分子功能(Molecular function,MF)和细胞组分(Cellular component,CC)。通过KEGG通路数据库对蛋白进行解析注释。差异表达蛋白通过Fisher精确检验方法进行KEGG信号通路富集分析。
1.6统计分析
试验数据采用Excel 2024和SPSS 24.0进行统计分析,利用LSD法、Duncan’s法进行多重比较分析处理间差异显著性,采用Origin 2024作图。
2结果与分析
2.1 2种土壤类型烟叶PPO活性比较
如图1所示,随着烟叶成熟进程的推移,2种土壤类型烟叶PPO活性均表现出先升后降的变化趋势,在成熟时期达最大值,各时期沙泥田烟叶PPO活性均极显著高于紫色土烟叶(rlt;0.01,下同),且二者在成熟时期差值最大。
2.2 2种土壤类型烟叶差异代谢物筛选
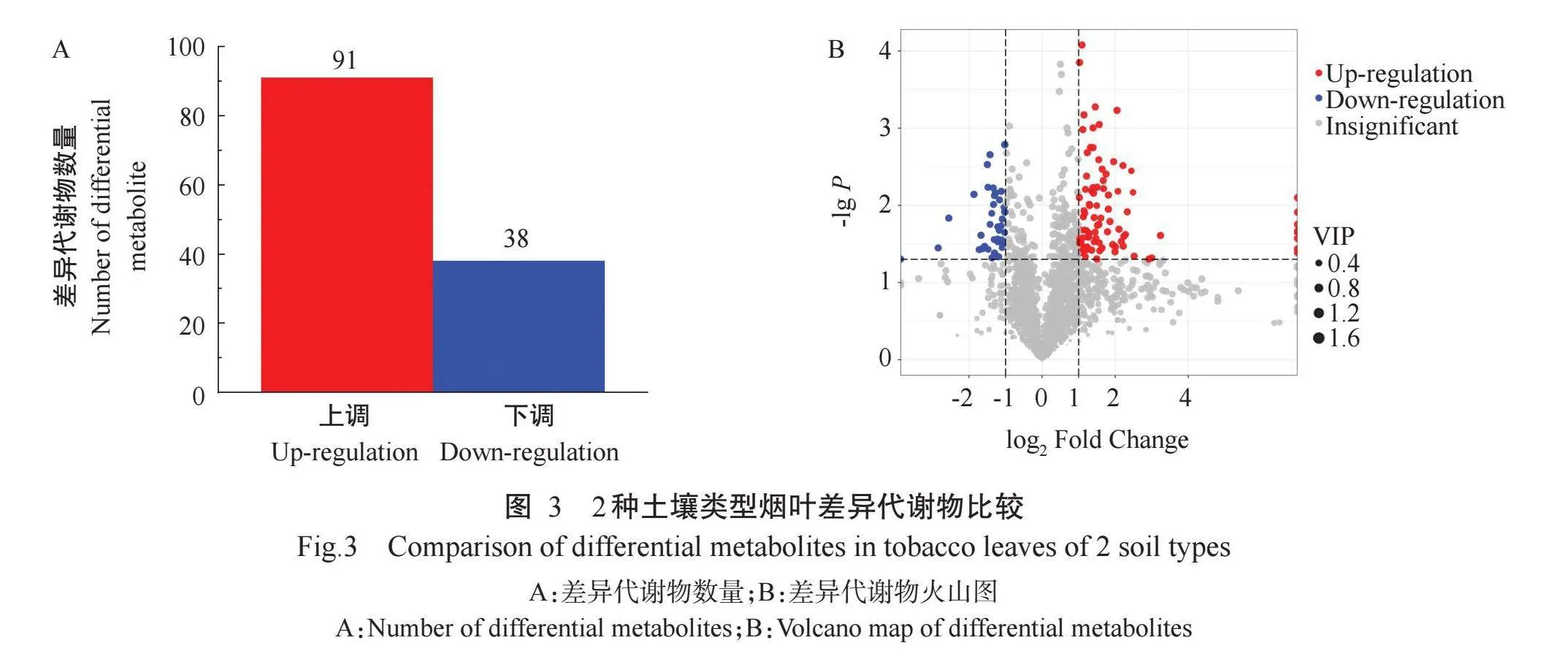
为更清楚展现2种土壤类型烟叶代谢组学差异,选取成熟时期烟叶进行对比分析。PCA分析结果(图2)显示,沙泥田烟叶与紫色土烟叶在第一主成分(PC1)上分离显著,可用于后续分析。在所检测样本中,以VIPgt;1和rlt;0.05为依据进行筛选,共筛选出129个差异代谢物,其中91个代谢物上调、38个代谢物下调(图3-A和图3-B)。
2.3 2种土壤类型烟叶差异代谢物聚类和KEGG代谢通路富集分析
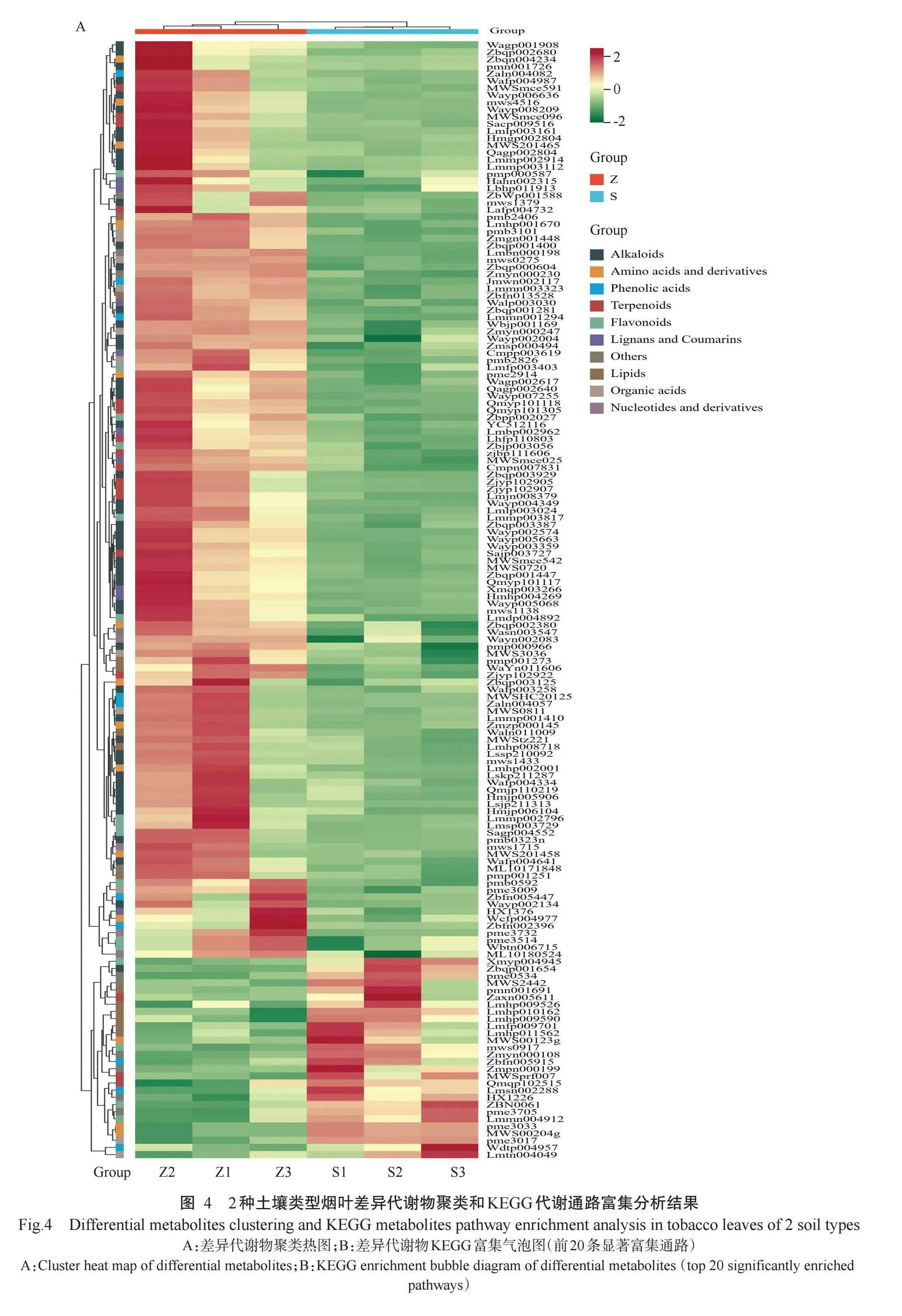
对2种土壤类型烟叶进行聚类分析,结果(图4-A)显示,下调差异代谢物主要为D-果糖-6-磷酸(MWS 2442)、D-葡萄糖醛酸(pme3705)、D-蔗糖酸(Zmyn 000108)等糖类物质和3-O-甲基槲皮素(Lmmn00 4912)、山茶苷A(Xmyp004945)等黄酮类物质以及脱落酸(Lmtn004049)、2-氨基异丁酸(pme3017)等有机酸类物质;上调差异代谢物主要是咖啡酰腐胺(pmb0323n)、N-阿魏酰腐胺(Lmlp003161)、去甲基烟碱(mws1379)等生物碱酚胺类物质和L-哌啶酸(MWS0811)、L-苹果酸(mws0275)、反式乌头酸(pme3009)等有机酸类物质以及棉黄素-3-O-芸香糖苷(Lmmp002796)、杨梅素-3-O-芸香糖苷(Lmsp 003729)等黄酮类物质。KEGG代谢通路富集分析结果(图4-B)显示,差异代谢物共富集到47条代谢通路上,其中显著富集的有5条,分别为酪氨酸代谢(Tyrosine metabolism,ko00350)、苯丙氨酸—酪氨酸—色氨酸生物合成(Phenylalanine,tyrosine andtryptophan biosynthesis,ko00400)、苯丙素生物合成(Phenylpropanoid biosynthesis,ko00940)、异喹啉生物碱生物合成(Isoquinoline alkaloids biosynthesis,ko00950)和植物次生代谢产物的生物合成(Bio-synthesis of various plant secondary metabolites,ko00990)。可见,不同土壤类型烟叶差异代谢物主要富集在多酚类物质的合成通路中,其中,酪氨酸代谢、苯丙氨酸—酪氨酸—色氨酸生物合成、苯丙素生物合成等通路在烟草多酚类物质的合成中起重要调控作用,对烟叶酶促褐变反应具有重要影响。
2.4 2种土壤类型烟叶差异表达蛋白鉴定
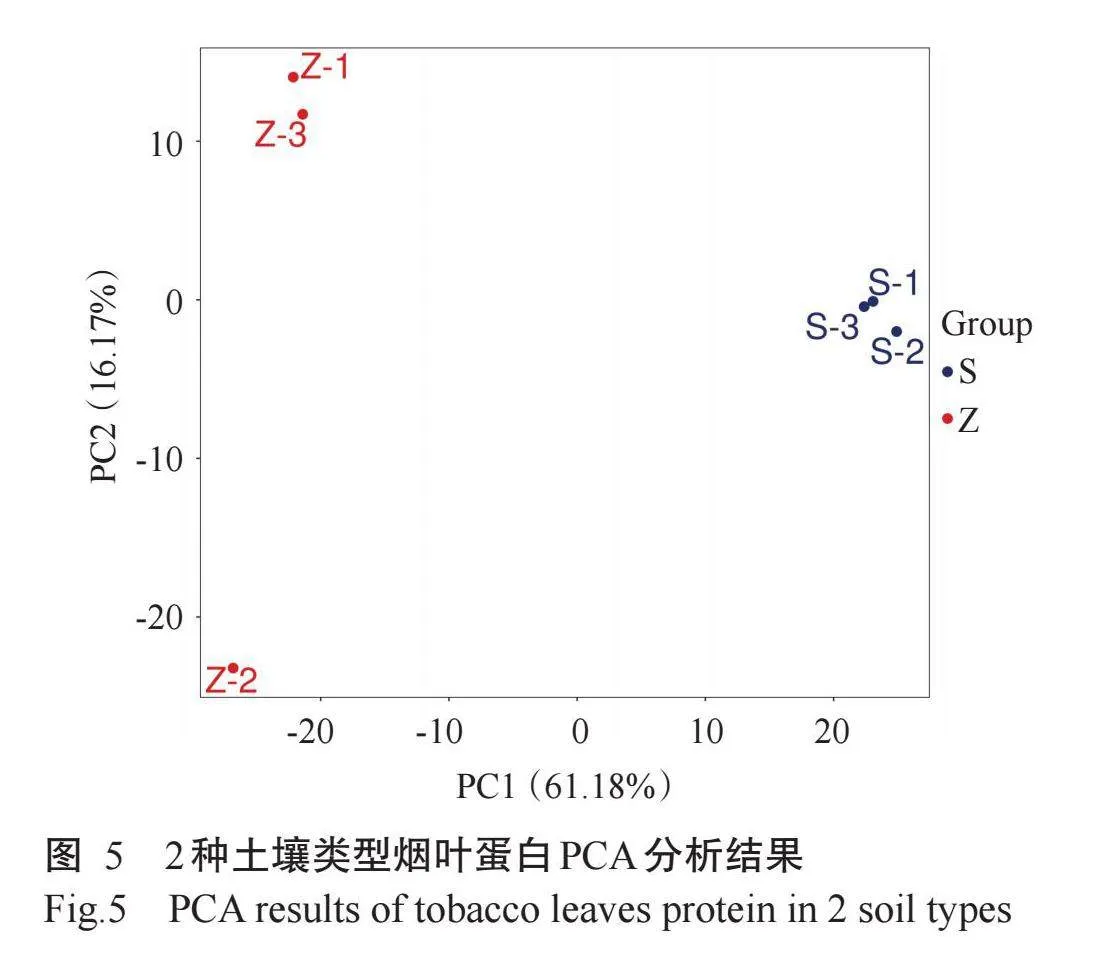
采用基于4D-DIA定量蛋白组学方法,选取成熟时期烟叶进行蛋白组学比较分析。PCA分析结果(图5)显示,2组样品在PC1上分离显著,可用于后续分析。鉴定共获得7428个蛋白,以FCgt;1.2倍或lt;0.8倍且rlt;0.05为依据共筛选出1314个差异表达蛋白,其中767个上调、547个下调(图6-A和图6-B)。
2.5 2种土壤类型烟叶差异蛋白功能注释
为了解差异表达蛋白在细胞内的分布,对其进行亚细胞定位分析,结果(图7)表明,306个差异表达蛋白能进行亚细胞定位分析,其中,189个上调、117个下调。189个上调差异表达蛋白主要位于细胞膜(Cell membrane,39.7%,75个)、参与分泌的细胞器(Secreted,22.8%,43个)和内质网(Endoplasmicreticulum,11.1%,21个)。117个下调差异表达蛋白主要定位于细胞核(Nucleus,40.2%,47个)、细胞膜(Cell membrane,39.3%,46个)和细胞质(Cytoplasm,17.9%,21个)。
为评估差异表达蛋白对生理过程的影响,采用Blast2Go对其进行GO功能注释和解析。其中,差异表达蛋白数占总差异表达蛋白数较多的条目为生物过程中的细胞过程(Cellular process,9.6%)、代谢过程(Metabolic process,9.5%);细胞组分中的细胞部分(Cell part,10.5%)、细胞器(Organelle,6.9%);分子功能中的催化活性(Catalytic activity,10.7%)、结合(Binding,8.8%)等(图8-A)。分别在3类GO功能条目中筛选出r值最小的前20个GO条目,可看出其生物过程类别主要富集在响应胁迫(Response to stress,GO:0006950)等条目(图8-B);细胞组分类别主要富集在胞外区(Extracellular region,GO:0005576)、外部封装结构(External encapsulating structure,GO:0030312)、细胞壁(Cell wall,GO:0005618)等条目(图8-C);分子功能类别主要富集在水解酶活性(Hydrolase activity,hydrolyzing O-glycosyl com-pounds/hydrolase activity,acting on glycosyl bonds;GO:0004553/GO:0016798)、氧化还原酶活性(Oxi-doreductase activity,acting on peroxide as acceptor,GO:0016684)、过氧化物酶活性(Peroxidase activity,GO:0004601)、抗氧化活性(Antioxidant activity,GO:0016209)等条目(图8-D)。说明沙泥田上部成熟烟叶对周围环境存在胁迫响应,使其部分蛋白显著富集在抗逆性方面。对2种土壤类型烟叶的差异表达蛋白进行KEGG富集分析,筛选出r值最小的前20个途径,主要富集在苯丙素生物合成、酪氨酸代谢、异喹啉生物碱生物合成和植物激素信号传导(Plant hormone signal transduction)等通路(图8-E)。其中,酪氨酸代谢、异喹啉生物碱生物合成途径中多酚氧化酶(PPO,EC:1.10.3.1)、天冬氨酸转氨酶(GOT2,EC:2.6.1.1)均下调,与烟叶PPO活性表现一致。
2.6 2种土壤类型烟叶多酚类物质生物合成分析
将2种土壤类型烟叶差异代谢物和差异表达蛋白注释到KEGG通路中进行联合分析,差异物质富集程度前3的通路分别为氨基糖和核苷酸糖代谢(Amino sugar and nucleotide sugar metabolism,ko00520)、苯丙素生物合成(ko00940)和辅因子的生物合成(Biosynthesis of cofactors,ko01240)(图9)。
结合PPO活性表现和代谢组学、蛋白组学分析结果,选择苯丙素生物合成途径进行联合分析。其中,苯丙氨酸解氨酶(PAL,EC:4.3.1.24)、4-香豆酸辅酶A连接酶(4CL,EC:6.2.1.12)、羟基肉桂酰辅酶A莽草酸酯/奎宁酸羟基肉桂酰转移酶(HCT,EC:2.3.1.133)、咖啡酰辅酶A 3-O-甲基转移酶(CCoAOMT,EC:2.1.1.104)、松柏醛脱氢酶(REF1,EC:1.2.1.68)下调(图10)。筛选出来的差异表达蛋白主要集中在通路的上游且均表现为下调,上游差异代谢物阿魏酸(Ferulic aeid)和下游差异代谢物松柏醇(Coniferyl alcohol)、芥子醇(Sinapyl alcohol)均表现出下调,说明相较于紫色土烟叶,沙泥田烟叶中多酚类物质合成速率加快,使酚类物质的积累量提高。
2.7 2种土壤类型烟叶ABA生物合成分析
除多酚类物质合成途径外,ABA生物合成和代谢途径也有差异代谢物和差异表达蛋白富集。ABA生物合成分类萜和类胡萝卜素2条途径。联合分析结果(图11)显示,类胡萝卜素生物合成(Carotenoids biosynthesis,ko00906)途径上富集到较多差异表达蛋白和差异代谢物。其中,八氢番茄红素脱氢酶(PDS,EC:2.5.1.32)、玉米黄质环氧化酶(ZEP,EC:1.14.15.21)、9-顺式环氧类胡萝卜素双加氧酶(NCED,EC:1.14.15.21)、黄氧素脱氢酶(ABA2,EC:1.1.1.288)下调。ABA代谢途径主要分由P450型单加氧酶(CYP707A)介导的羟基化和由葡萄糖基转移酶介导的螯合2条途径。在前者中,差异代谢物红花菜豆酸(Phaseic acid)、二氢红花菜豆酸(Dihydrophaseic acid)下调;后者中,葡萄糖基转移酶(AOG,EC:2.4.1.263)下调(图11)。整体上,在ABA生物合成和代谢途径中,筛选出来的差异代谢物和差异表达蛋白均表现为下调,说明与紫色土烟叶相比,沙泥田上部烟叶生长过程中受到胁迫,导致ABA的物质合成、代谢均较旺盛。
3讨论
烤后烟叶挂灰现象受生态条件、栽培措施、烘烤调制技术等多种因素影响。广东烟区沙泥田土壤质地较疏松,保肥性能较差,土壤pH偏酸性,有效铁和有效锰含量较高,交换性钙和交换性镁含量较低,水溶性硼普遍缺乏(王军等,2015;王行等,2023),可能会引起烟草出现生理性中毒和生理功能紊乱,使烟叶生长后期易受土壤因子胁迫,最终经调制后出现挂灰烟叶。目前,普遍认为挂灰烟叶形成与鲜烟素质及酶促褐变密切相关(孙皓月等,2022)。同时,PPO在植物抗逆性活动中也起着重要作用(赵伶俐等,2005)。本研究中,以紫色土成熟烟叶(不易挂灰)为对照,发现沙泥田烟叶PPO活性在各时期均显著升高,与烟叶挂灰程度一致,说明鲜烟叶时期的沙泥田上部叶易受外界胁迫,PPO活性已显著高于紫色土烟叶,采收时应注意烟叶素质,同时在后期烟叶烘烤中更需注意烟叶所处环境的温湿度条件,以降低烟叶酶促褐变的剧烈程度。本研究从代谢组学和蛋白组学层面解析2种土壤类型成熟烟叶差异表达并进行富集分析,发现差异代谢物和差异表达蛋白主要富集在酪氨酸代谢、苯丙素生物合成、类胡萝卜素生物合成等途径;在其联合分析中,氨基糖和核苷酸糖代谢、苯丙素生物合成和辅因子生物合成3条通路显著富集,与前人发现褐变涉及通路相似(Li et al.,2023b;Guan et al.,2024)。PAL、C4H和4CL活性与酚类物质的合成密切相关(Zhou et al.,2018;Shahidi et al.,2024)。HCT在木质素单体的合成中起重要作用(Zhou et al.,2018)。本研究结果表明,相较于紫色土烟叶,沙泥田烟叶的PAL、4CL和HCT下调,说明沙泥田上部烟叶烤后易挂灰,可能与多酚类物质合成相关酶活性上升有关。而阿魏酸、芥子酸等酚类和类黄酮物质作为酶促褐变的关键底物,与组织褐变、抗逆性等密切相关(Sukhonthara et al.,2016;Liao et al.,2020)。本研究中,差异代谢物阿魏酸、松柏醇、芥子醇等差异代谢物下调,也表明沙泥田上部烟叶更易受外界胁迫,导致PPO活性和多酚类物质含量增加。
已有研究表明,ABA是植物感知逆境信号并响应胁迫的关键因子(Habibpourmehrabanetal.,2023)。非生物胁迫诱导ABA含量增加,使其可通过调节植物中的各种生理和生化信号转导级联反应来应对胁迫(Yang et al.,2011)。Castro-Cegri等(2023)对冷藏西葫芦果实施用外源ABA,通过UPLC/MS-MS对特定酚类进行定量检测,观察到外源ABA主要激活类黄酮的产生,使抗坏血酸、类胡萝卜素和多酚类化合物积累,从而提高西葫芦果实在贮藏过程中的抗氧化能力。本研究发现,相较于紫色土烟叶,沙泥田烟叶差异代谢物ABA下调,同时,ABA生物合成途径中NCED、PDS、ZEP等差异表达蛋白下调。其中,NCED、ZEP是催化形成植物激素ABA生物合成的关键酶。说明沙泥田上部烟叶生长过程中更易受到环境胁迫,导致烟叶素质下降,ABA含量上升,进而激活与植物激素信号传导相关的重要蛋白来增强其适应能力。
4结论
与紫色土烤烟上部叶相比,沙泥田烤烟上部叶生长过程中更易受环境胁迫,导致烟叶素质下降,ABA合成增加,激素信号激活,酚类代谢增强,PPO活性上升,在烘烤过程中易导致酶促褐变更加剧烈,从而造成烤后烟叶易挂灰。
参考文献(References):
黄亚成,任东立,何斌,赵艳妹,龚小见,陈锦秀,刘林娅.2023.转录组学和代谢组学在植物非生物胁迫中的研究进展[J].江苏农业科学,51(22):1-7.[Huang Y C,Ren D L,He B,Zhao Y M,Gong X J,Chen J X,Liu LY.2023.Research progress in transcriptomics and metabolomics in plant abiotic stress[J].Jiangsu Agricultural Sciences,51(22):1-7.]doi:10.15889/j.issn.1002-1302.2023.22.001.
孙皓月,贾宏昉,谢良文,冯长春,伍德洋,秦艳青,陈汉发,郭仕平.2022.不同采烤方式对烤烟上部叶脂质褐变的影响[J].南方农业学报,53(6):1616-1624.[Sun H Y,Jia H F,Xie L W,Feng C C,Wu D Y,Qin Y Q,Chen H F,Guo S P.2022.Effects of different harvesting methods on lipid browning of upper leaves of flue-cured tobacco[J].Southern Agricultural Journal,53(6):1616-1624.]doi:10.3969/j.issn.2095-1191.2022.06.015.
王军,丁效东,何振峰,田俊岭,刘兰,陈泽鹏.2015.广东南雄烟区植烟土壤肥力特征及综合评价[J].中国烟草科学,36(6):30-36.[Wang J,Ding X D,He Z F,Tian J L,Liu L,Chen Z P.2015.The soil fertility characteristics and comprehensive evaluation of Nanxiong tobacco-growing area in Guangdong Province[J].China Tobacco Science,36(6):30-36.]doi:10.13496/j.issn.1007-5119.2015.06.006.
王军,丁效东,罗静,王晓宾,王政仁,陈泽鹏.2016.南雄烟区气候条件与烟叶产量构成及主要化学成分的关系[J].华南农业大学学报,37(3):54-61.[Wang J,Ding X D,LuoJ,Wang X B,Wang Z R,Chen Z P.2016.Effects of cli-matic conditions on yield components and main chemical constituents of tobacco in Nanxiong,Guangdong Province[J].Journal of South China Agricultural University,37(3):54-61.]doi:10.7671/j.issn.1001-411X.2016.03.008.
王行,姚远华,张丹丹,刘兰,朱文格,邱妙文.2023.提高粤北烤烟上部烟叶可用性关键技术研究进展[J].中国农学通报,39(34):16-21.[Wang H,Yao Y H,Zhang D D,Liu L,Zhu W G,Qiu M W.2023.Research progress on key tech-nologies for improving the availability of upper flue-cured tobacco leaves in Northern Guangdong[J].Chinese Agri-cultural Science Bulletin,39(34):16-21.]
赵伶俐,范崇辉,葛红,刘洪涛.2005.植物多酚氧化酶及其活性特征的研究进展[J].西北林学院学报,20(3):156-159.[Zhao L L,Fan C H,Ge H,Liu H T.2005.Progress on polyphenol oxidase and its activity characteristics in plants[J].Journal of Northwest Forestry University,20(3):156-159.]doi:10.3969/j.issn.1001-7461.2005.03.042.
Ampofo J,Ngadi M,Ramaswamy H S.2020.The impact oftemperature treatments on elicitation of the phenylpro-panoid pathway,phenolic accumulations and antioxidative capacities of common bean(Phaseolus vulgaris)sprouts[J].Food and Bioprocess Technology,13(9):1544-1555.
Begum N,Akhtar K,Ahanger M A,Iqbal M,Wang P P,Mus-tafa N S,Zhang L X.2021.Arbuscular mycorrhizal fungi improve growth,essential oil,secondary metabolism,and yield of tobacco(Nicotiana tabacum L.)under drought stress conditions[J].Environmental Science and Pollution Research International,28(33):45276-45295.doi:10.1007/s11356-021-13755-3.
Castro-Cegri A,Sierra S,Hidalgo-Santiago L,Esteban-Munoz A,Jamilena M,Garrido D,Palma F.2023.Postharvest treatment with abscisic acid alleviates chilling injury in zucchini fruit by regulating phenolic metabolism and non-enzymatic antioxidant system[J].Antioxidants,12(1):211.doi:10.3390/antiox 12010211.
Chen K,Li G J,Bressan R A,Song C P,Zhu J K,Zhao Y.2020.Abscisic acid dynamics,signaling,and functions in plants[J].Journal of Integrative Plant Biology,62(1):25-54.doi:10.1111/jipb.12899.
Guan Y G,Lu S N,Sun Y,Zheng X R,Wang R,Lu X H,Pang L J,Cheng J Y,Wang L.2024.Tea polyphenols inhibit the occurrence of enzymatic browning in fresh-cut potatoes by regulating phenylpropanoid and ROS metabolism[J].Plants,13(1):125.doi:10.3390/plants 13010125.
Habibpourmehraban F,Wu Y Q,Masoomi-Aladizgeh F,Amirkhani A,Atwell B J,Haynes PA.2023.Pre-treatment of rice plants with ABA makes them more tolerant to mul-tiple abiotic stress[J].International Journal of Molecular Sciences,24(11):9628.doi:10.3390/ijms24119628.
Köhler A,Forster N,Zander M,Ulrichs C.2020.Compound-specific responses of phenolic metabolites in the bark of drought-stressed Salix daphnoides and Salix purpurea[J].Plant Physiology and Biochemistry,155:311-320.doi:10.1016/j.plaphy.2020.07.004.
Li L Q,Mu Y L,Chen J,Wang Q,Lu Y F,Xin S,Yang S M,Huang X L,Wang X Y,Lu L M.2023a.Molecular mecha-nism by which StSN2 overexpression inhibits the enzy-matic browning of potato[J].Postharvest Biology and Te-chnology,203:112416.doi:10.1016/j.postharvbio.2023.112416.
Li X T,Zhang S,Wang Q G,Dong T T.2023b.Diacetyl inhi-bits the browning of fresh-cut stem lettuce by regulating the metabolism of phenylpropane and antioxidant ability[J].Foods,12(4):740.doi:10.3390/foods 12040740.
Li Y,Ren K,Hu M Y,He X,Gu K Y,Hu B B,Su J E,Jin Y,Gao W Y,Yang D S,Li F L,Zou C M.2021.Cold stress in the harvest period:Effects on tobacco leaf quality and curing characteristics[J].BMC Plant Biology,21(1):131.doi:10.1186/s 12870-021-02895-w.
Liao T,Liu J P,Sun Yu F,Zou L Q,Zhou L,Liu C M,Terefe N S,Liu W.2020.Differential inhibitory effects of organic acids on pear polyphenol oxidase in model systems and pear puree[J].LWT-Food Science and Technology,118:108704.doi:10.1016/j.lwt.2019.108704.
Ma Y,Hong T T,Xu D,Wu F F,Xu X M.2023.Inhibition of PPO-related browning in fresh noodles:A combination of chemical and heat treatment[J].Food Chemistry,404(Part B):134549.doi:10.1016/j.foodchem.2022.134549.
Maioli A,Gianoglio S,Moglia A,Acquadro A,Valentino D,Milani A M,Prohens J,Orzaez D,Granell A,Lanteri S,Comino C.2020.Simultaneous CRISPR/Cas9 editing of three PPO genes reduces fruit flesh Browning in Solanum melongena L[J].Frontiers in Plant Science,11:607161.doi:10.3389/fpls.2020.607161.
Shahidi P,Bahramnejad B,Vafaee Y,Dastan D,Heidari P.2024.Isolation and characterization of Phenylalanine Ammonia Lyase(PAL)genes in Ferulapseudalliacea:Insights into the phenylpropanoid pathway[J].Genes,15(6):771.doi:10.3390/genes 15060771.
Song Z Y,Qiao J,Tian D D,Dai M,Guan Q H,He Y,Liu P,Shi J Y.2023.Glutamic acid can prevent the browning of fresh-cut potatoes by inhibiting PPO activity and regula-ting amino acid metabolism[J].LWT-Food Science and Technology,180:114735.doi:10.1016/j.lwt.2023.114735.
Sui X,Meng Z,Dong T T,Xue T T,Wang Q G.2023.Enzy-matic browning and polyphenol oxidase control strategies[J].Current Opinion in Biotechnology,81:102921.doi:10.1016/j.copbio.2023.102921.
Sukhonthara S,Kaewka K,Theerakulkait C.2016.Inhibitory effect of rice bran extracts and its phenolic compounds on
polyphenol oxidase activity and browning in potato and apple puree[J].Food Chemistry,190:922-927.doi:10.1016/j.foodchem.2015.06.016.
Tang T T,Xie X F,Ren X,Wang W J,Tang X M,Zhang J,Wang Z D.2020.A difference of enzymatic browning unre-lated to PPO from physiology,targeted metabolomics and gene expression analysis in Fuji apples[J].Postharvest Bio-logy and Technology,170:111323.doi:10.1016/j.posthar-vbio.2020.111323.
Tong M R,Ding Ya F,Yu H,Zhang W,Wu D L.2024.Inte-grated non-targeted metabolomics and transcriptomicsreveals the browning mechanism of scraped ginger(Zingiber officinale Rosc.)[J].Journal of Food Science,89(6):3139-3275.doi:10.1111/1750-3841.17084.
Wang J,Wang J,Yue Z B,Luo S L,Zhang B,Yu J H,Liu Z C.2024.Disease and pest resistance through phenolic sub-stances in the solanaceae[J].Journal of Plant Growth Re-gulation,43(7):2121-2136.
Yang W,Liu X D,Chi X J,Wu C A,Li Y Z,Song L L,Liu X M,Wang Y F,Wang F W,Zhang C,Liu Y,Zong J M,Li H Y.2011.Dwarf apple MbDREB1 enhances plant toleranceto low temperature,drought,and salt stress via both ABA-dependent and ABA-independent pathways[J].Planta,233(2):219-229.doi:10.1007/s00425-010-1279-6.
Zhang L L,Martinelli E,Senizza B,Miras-Moreno B,Yildiztu-gay E,Arikan B,Elbasan F,Ak G,Balci M,Zengin G,Rouphael Y,Lucini L.2021.The combination of mild salinity conditions and exogenously applied phenolics modulates functional traits in lettuce[J].Plants,10(7):1457.doi:10.3390/plants 10071457.
Zhou P L,Li Q Y,Liu G L,Xu N,Yang Y J,Zeng W L,Chen A G,Wang S S.2018.Integrated analysis of transcriptomic and metabolomic data reveals critical metabolic pathways involved in polyphenol biosynthesis in Nicotiana tabacum under chilling stress[J].Functional Plant Biology,46(1):30-43.doi:10.1071/fp 18099.
(责任编辑王晖)

