刺葡萄类钙调蛋白基因VdCML8克隆与表达分析及其启动子转录活性测定





摘要:【目的】克隆刺葡萄类钙调蛋白基因VdCML8基因及其启动子序列,并对VdCML8基因进行表达分析,对其启动子进行转录活性测定,为深入探究该基因在葡萄抗炭疽病中的生物学功能提供理论参考。【方法】以刺葡萄紫秋为材料,采用RT-PCR技术克隆VdCML8基因及其启动子序列,对VdCML8蛋白的理化性质和二级结构进行生物信息学分析,并采用实时荧光定量PCR检测刺葡萄紫秋和欧洲葡萄红地球CML8基因在接种胶孢炭疽菌及外施水杨酸(SA)和茉莉酸(JA)处理后的表达特征,通过构建β-葡萄糖苷酶(GUS)融合载体转化烟草进行转录活性检测。【结果】VdCML8基因的开放阅读框(ORF)长度为450 bp,编码149个氨基酸残基,具有EF-hand结构域,其二级结构中α-螺旋占65.10%,延伸链占4.70%,无规则卷曲占20.13%,β-转角占10.07%,该蛋白定位于细胞膜中。由系统发育进化树可知,刺葡萄VdCML8蛋白与欧洲葡萄VvCML8和河岸葡萄VrCML8的亲缘关系较近。VdCML8基因的启动子序列(pVdCML8)长度为1050 bp,除含有大量的CAAT-box和TATA-box外,还含有一些光响应元件(L-box、chs-CMALa和TCT-motif)、脱落酸(ABA)响应元件(ABRE)、厌氧诱导响应元件(ARE)、防御和应激元件(TC-rich repeats)、伤害响应元件(WUN-motif)等。构建pVdCML8的瞬时表达载体pVdCML8::GUS,瞬时转化烟草后发现pVdCML8具有转录活性,且能驱动VdCML8基因表达。在接种胶孢炭疽菌后,刺葡萄紫秋VdCML8基因和欧洲葡萄红地球VvCML8基因表达均上调,均在接种后12 h达峰值,二者的相对表达量是对照组(清水处理)的22.08和9.30倍。SA处理3 h时,VdCML8基因的相对表达量是对照组的7.68倍,是VvCML8基因的2.76倍。JA处理6 h时,VdCML8基因达峰值,是对照组的22.25倍,是VvCML8基因的9.04倍。【结论】VdCML8基因是SA和JA信号途径的下游调控基因,SA和JA可诱导其高效表达,参与葡萄炭疽病响应过程,对提高植株抗病性具有一定作用。
关键词:刺葡萄;胶孢炭疽病;VdCML8;启动子;转录活性;表达分析
中图分类号:S663.103.6文献标志码:A文章编号:2095-1191(2024)08-2225-12
Cloning and expression analysis of calmodulin-like protein geneVdCML8 in Vitis davidii and determination of its promotertranscriptional activity
DUAN Feng-feng,CHEN Man-ying,LEI Tian-ci,ZHANG Meng-qi,WEN Zhi-feng*
(College of Horticulture,Fujian Agriculture and Forestry University,Fuzhou,Fujian 35000 China)
Abstract:【Objective】Calmodulin-like protein gene(VdCML8)in Vitis davidii and its promoter sequence were cloned,and the expression of VdCML8 gene was analyzed,and the transcriptional activity of its promoter was determinedto provide theoretical reference for further exploring the anti-anthracnose biological function of this gene in grapes.【Method】V.davidiiZiqiu was as mate-rials.The VdCML8 gene and its promoter sequence were cloned by RT-PCR,andthe physicochemical properties and se-condary structure of VdCML8 protein were analyzed by bioinformatics.Real-timefluorescence quantitative PCR was used to detect the expression characteristics of CML8 gene in V.davidiiZiqiu and V.vi-niferacv.Red Globe after inoculation with Colletotrichum gloeosporioides and external salicylic acid(SA)and jasmonicacid(JA)treatments.β-glucosidase(GUS)fusion vector was constructed and transformed tobacco for transcriptional ac-tivity detection.【Result】The open reading frame(ORF)of VdCML8 gene was 450 bp,encoded 149 amino acids residues,and had an EF-hand domain.In the secondary structure,the proportion ofα-helix was 65.10%,that of extension chain was 4.70%,that of random coil was 20.13%,and that ofβ-turn was 10.07%.The protein was localized in the cell mem-brane.According to phylogenetic tree,VdCML8 protein in V.davidii was closely related to V.vinifera VvCML8 and V.ri-paris VrCML8.The VdCML8 gene promoter sequence(pVdCML8)was 1050 bp.It contained a large number of CAAT-box and TATA-box,and also contained some light response elements(L-box,chs-CMALa and CTT-motif),episisic acid(ABA)response element ABRE,anaerobic induction response element(ARE),defense and stress element(TC-rich re-peats),wounding response element(WUN-motif).pVdCML8::GUS,a transient expression vector of pVdCML8,was constructed.It was found that pVdCML8 had transcriptional activity and could drive the expression of VdCML8 gene aftertransient transformation of tobacco.The expression of V.davidiiZiqiu VdCML8 gene and V.vinifera VvCML8 gene were up-regulated and reached the peak at 12 h after inoculation.The relative expression levels of both were as 22.08 times and 9.30 times as that of control group(water treatment).After SA treatment for 3 h,the relative expression of VdCML8 gene was as 7.68 times as that of control and as 2.76 times as that of VvCML8 gene.After JA treatment for 6 h,VdCML8 genereached itspeak and was as 22.25 times as that of control group and as 9.04 times as that of VvCML8 gene.【Conclusion】VdCML8 gene is the downstream regulatory gene of SA and JA signaling pathways.SA and JA can induce its efficient ex-pression,participate in the response process of grape anthracnose,and have certain effects in improving plant disease re-sistance.
Key words:Vitisdavidii;Colletotrichum gloeosporioides;VdCML8;promoter;transcriptional activity;expression analysis
Foundation items:National Natural Science Foundation of China(31701907)
0引言
【研究意义】葡萄(Vitis vinifera L.)属于葡萄科葡萄属,因其独特的风味和广泛的用途,具有很高的经济价值(张洁,2022)。葡萄霜霉病、黑痘病、炭疽病等是葡萄生产中常见的病害,其中由胶孢炭疽菌引发的炭疽病危害较为严重,成为葡萄的主要病害之一,对葡萄生产造成了严重损失。目前我国葡萄种质资源圃已有3000个以上(刘伟等,2016;段长青等,2019;张新龙等,2023)。研究发现,部分葡萄种质资源携带有效的抗病基因,从中挑选培养出最具经济效益的抗炭疽病葡萄品种,是预防炭疽病发生的有效方法(李顺雨等,2010)。刺葡萄(V.davidii)是葡萄属中东亚种群的一种野生种质资源,其品质好、耐粗放管理,有良好的抗病性及耐湿热性,对炭疽病近乎免疫,故刺葡萄可作为抗真菌病害育种的优良资源(阮仕立和李记明,2002;程大伟等,2015),但其抗性基因尚未被挖掘。类钙调蛋白(CML)在植物对病原菌的防御反应中发挥调控作用,但葡萄中CML基因的相关研究较少。因此,深入研究CML基因在葡萄抗炭疽病过程中的调控机制,有助于揭示其在抗病原菌响应过程中的具体功能和调控网络。【前人研究进展】钙是植物体中调节信息转导的重要物质,是生物体中的第二信使。目前已发现4类钙信号系统:钙调蛋白(CaMs)、CML、类钙调蛋白因子(CBL)和改依赖性蛋白激酶(CDPK)(曹绍玉等,2018),其中ChKi//Yvbs1N2TwLL1/ptng==ML蛋白是一类广泛存在于植物界中含有钙离子结合EF-hand结构域的蛋白,对于生物的多种生物学过程具有调控功能(Day et al.,2002)。例如,已从大豆(陈超等,2015)、番茄(Munir et al.,2016)、葡萄(Vandelleet al.,2018)、陆地棉(杨秀等,2019)和银杏(Zhang et al.,2022b)中分别鉴定到68、52、62、154和26个CML基因,这些基因在植物响应多种环境胁迫过程中发挥重要作用。拟南芥中的AtCML24基因编码1种潜在的钙离子传感器,该传感器可能有助于对脱落酸(ABA)、日长和各种盐的存在做出反应。从巴西橡胶树、美国山核桃等作物中克隆获得CML基因,其参与调控抗高盐和高温等非生物胁迫响应(刘辉等,2015)。山葡萄(V.amu-rensisRupr)中32个VaCMLs基因积极响应非生物胁迫(Dubrovina et al.,2019),其中VaCML21基因的所有4个剪接变异体受冷应激的高度诱导(Aleynova et al.,2020)。植物在长期进化过程中逐渐形成一系列有效机制来感知和抑制病原菌的入侵(Zipfel and Felix,2005),在病原体侵染植物的过程中,部分CMLs家族成员可能通过与其他蛋白质的相互作用和信号传递,参与调控植物的抗病响应(曹绍玉等,2018)。拟南芥受丁香假单胞菌侵染时,AtCML41基因参与防御响应(Xu etal.,2017);AtCML9基因可通过鞭毛蛋白依赖途径响应病原菌,从而提高自身免疫力(Leba et al.,2012)。对于生物胁迫,山葡萄VaCML65基因作为正调控因子参与二苯乙烯的生物合成信号通路,而二苯乙烯可在葡萄中迅速积累来应对伤害或病原体,从而提高葡萄的抗性(Aley-nova etal.,2022)。使用病毒诱导基因沉默(VGIS)技术对AtCML24基因进行沉默,会导致植株中一氧化氮和超敏物质含量下降(Ma etal.,2008)。在一些病原物的侵染下,拟南芥AtCML37和AtCML42基因发挥防御调控作用(Heyer et al.,2022)。棉花GhCML11和GhMYB108基因对大丽轮枝菌具有正向的协同调控作用,导致植株对大丽轮枝菌抗性增强,但将GhCML11和GhMYB108基因沉默,协同调控作用消失,一些与钙信号相关基因表达量下调(Cheng et al.,2016)。【本研究切入点】目前,CML基因在非生物胁迫下的表达变化及功能响应是目前主要的研究方向,对于其响应生物胁迫的研究较少,尤其是鲜见有关CML基因参与葡萄抗炭疽病方面的研究报道。【拟解决的关键问题】以刺葡萄紫秋为试验材料,采用RT-PCR技术克隆VdCML8基因及其启动子序列,对VdCML8蛋白的理化性质和二级结构进行生物信息学分析,并采用实时荧光定量PCR检测刺葡萄紫秋和欧洲葡萄红地球CML8基因在接种胶孢炭疽菌及外施水杨酸(SA)和茉莉酸(JA)处理后的表达特征,通过构建β-葡萄糖苷酶(GUS)融合载体转入烟草进行转录活性检测,为深入探究CML基因抗葡萄炭疽病的生物学功能提供理论参考。
1材料与方法
1.1试验材料
供试材料为树龄5年且生长状态良好的刺葡萄紫秋和欧洲葡萄红地球(V.viniferacv.Red Globe),栽培于福州市农业科学研究所(25°58′N,119°22′E),采集其叶片液氮速冻后,-80℃保存备用。葡萄胶孢炭疽菌菌株(FZ-1)由福建农林大学园艺学院果树抗病与遗传育种实验室提供。
主要试剂:RNAprep Pure Plant Kit多糖多酚RNA提取试剂盒购自南京诺唯赞生物科技股份公司;DNA纯化回收检测试剂盒、质粒小提检测试剂盒和植物基因组DNA提取试剂盒等购自天根生化科技(北京)有限公司;SYBR Green PCR Mastermix试剂盒购自宝生物(大连)有限公司。卡那霉素(Kana)和氨苄西林(Amp)购自生工生物工程(上海)股份有限公司,用于筛选阳性菌落。主要仪器设备:实时荧光定量PCR仪(德国Analytik Jena公司)、凝胶成像系统(美国BIO-RAD公司)、离心机(美国ThermoFisher Scientific公司)、高压灭菌锅(日本HIRAYAMA公司)、恒温水浴锅(上海精宏实验设备有限公司)、紫外分光光度计(德国Analytik Jena公司)。
1.2试验方法
1.2.1胶孢炭疽菌接种及SA和JA处理胶孢炭疽菌接种处理:喷施前,将浓度为1×106个/mL的胶孢炭疽菌分生孢子加入0.2%吐温20,选择生长健康的刺葡萄紫秋和欧洲葡萄红地球植株进行喷施,以叶片不滴水为宜,同时设无菌水处理作为对照组(CK)。将植株保持在25℃、80%湿度的条件下培养。接种后0、6、12、24、48和72 h,收集被处理的葡萄叶片样品,使用液氮速冻后于-80℃保存备用。每个时间点设3次重复试验。
SA和JA处理:使用SA和JA(浓度均为100μmol/L)均匀喷施于刺葡萄紫秋和欧洲葡萄红地球植株上,以无菌水处理为对照组(CK)。取样、培养方法同上述一致。分别在0、3、6、12和24 h时收集葡萄叶片,使用液氮速冻后于-80℃保存备用。每个时间点设3次重复。
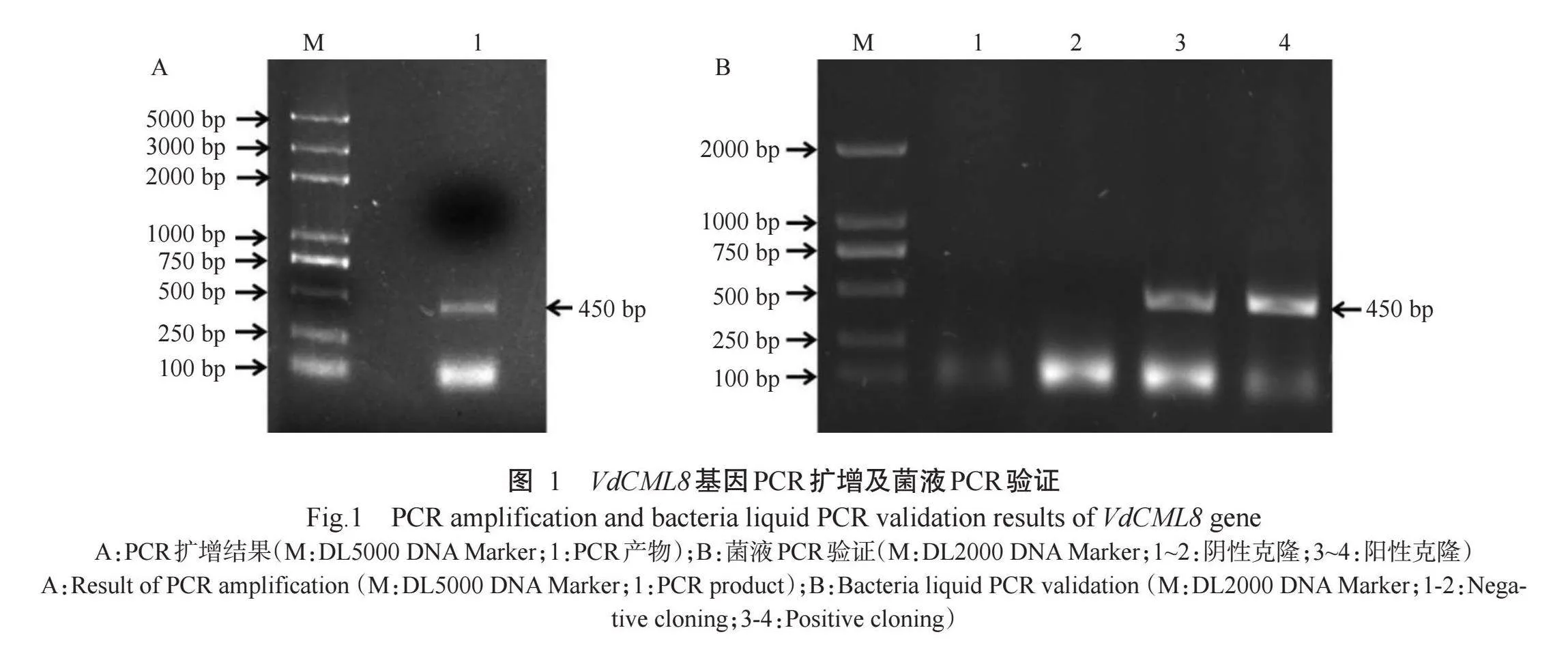
1.2.2 VdCML8基因克隆提取条带完整、OD260/OD280约为2.0的RNA作为模板。使用Primer 5.0设计PCR扩增刺葡萄紫秋VdCML8基因完整开放阅读框(ORF)的上、下游引物(表1)。反应体系25.0µL:2×Taq PCR Master Mix 12.5µL,上、下游引物各1.0µL,cDNA 1.0µL,ddH2O补足至25.0µL。扩增程序:94℃预变性90 s;94℃30 s,58℃60 s,72℃90 s,进行34个循环;72℃延伸10min。将PCR产物用1%琼脂糖凝胶电泳检测。将目的片段与pMD20-T克隆载体连接,再利用冻融法将连接产物转化大肠杆菌,将转化菌液涂布在含100 mg/LAmp的LB固体培养基上。挑选阳性单菌落,并将其送至尚亚生物技术(福州)有限公司进行测序。
1.2.3生物信息学分析使用ORFfinder对VdCML8基因进行ORF预测;利用NCBI数据库NCBI Con-served Domain Database(CDD)工具预测VdCML8蛋白的保守结构域;使用DNAMAN 8.0对不同物种的CML基因序列进行多重比对及系统发育分析;利用SOPMA工具预测VdCML8蛋白的二级结构。利用ProtParam预测VdCML8蛋白的理化性质。使用PlantCARE预测VdCML8基因启动子的顺式作用元件。
1.2.4 VdCML8蛋白亚细胞定位以VdCML8-GFP-F和VdCML8-GFP-R(携带Hind III和Xba I酶切位点)为引物(表1),对VdCML8基因的ORF进行PCR扩增。随后将PCR产物与pBI221-35S-GFP载体连接获得表达载体pBI221-35S-VdCML8-GFP,将其送至尚亚生物科技(福州)有限公司进行测序,并通过农杆菌介导法将表达载体转化洋葱内表皮细胞,在培养48h后,使用激光扫描共聚焦显微镜观察洋葱内表皮中GFP的荧光反应。
1.2.5 VdCML8基因启动子克隆及GUS融合表达载体构建从NCBI数据库的葡萄基因组中搜索到CML8基因启动子序列,并利用Primer 5.0设计其特异性引物(表1),以刺葡萄DNA为模板,PCR扩增VdCML8基因启动子序列(pVdCML8),反应体系与1.2.2相同。将PCR产物送至尚亚生物技术(福州)有限公司进行测序,以验证PCR产物的正确性。使用BamH I和Pst I对中间载体pMD20-T-pVdCML8和pC0380::GUS进行双酶切。使用T4连接酶将两者连接成重组载体(pVdCML8::GUS),并转化大肠杆菌感受态细胞,菌液PCR检测阳性克隆。通过BamH I和Pst I双酶切验证,挑选阳性克隆送至尚亚生物技术(福州)有限公司进行测序,并采用冻融法转化农杆菌GV3101。
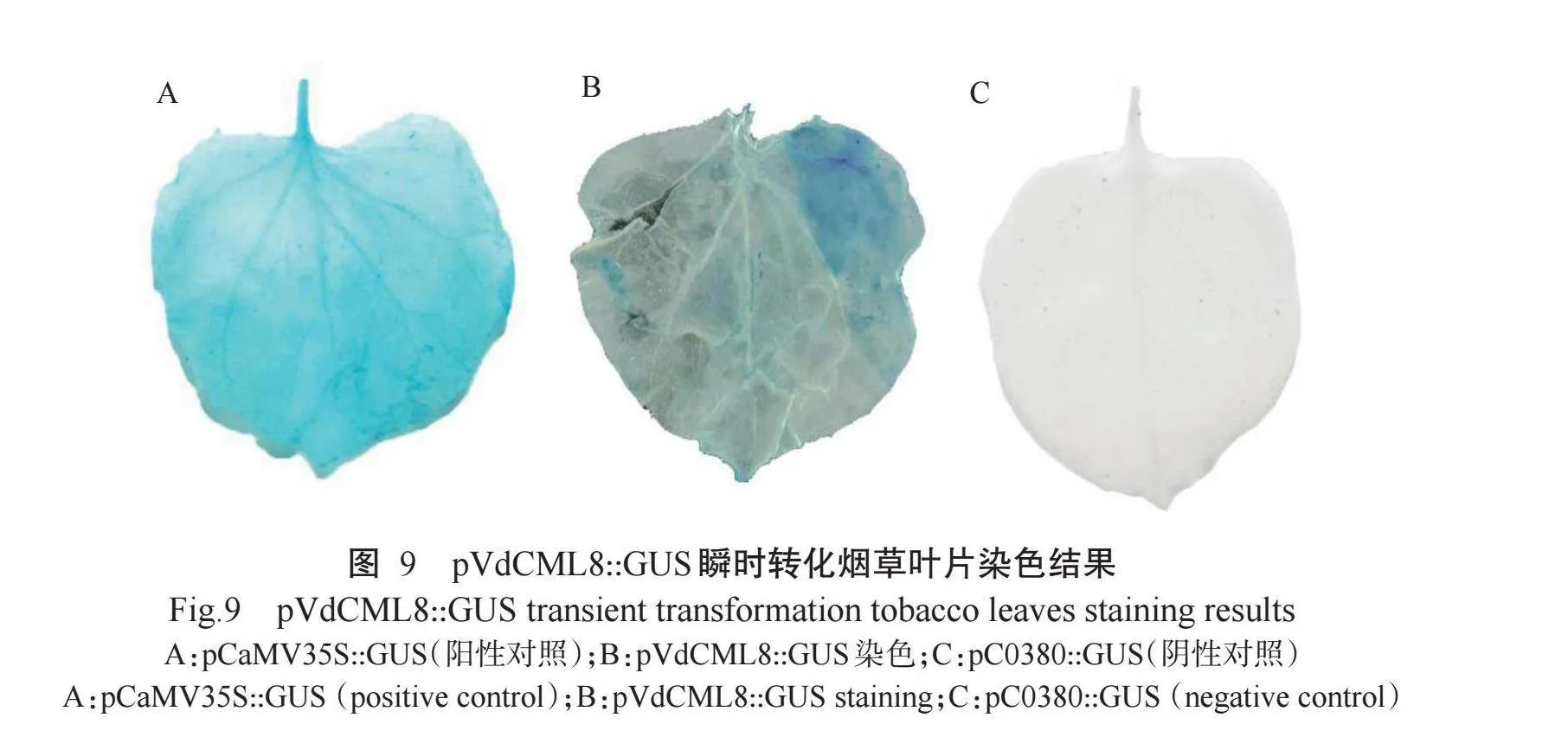
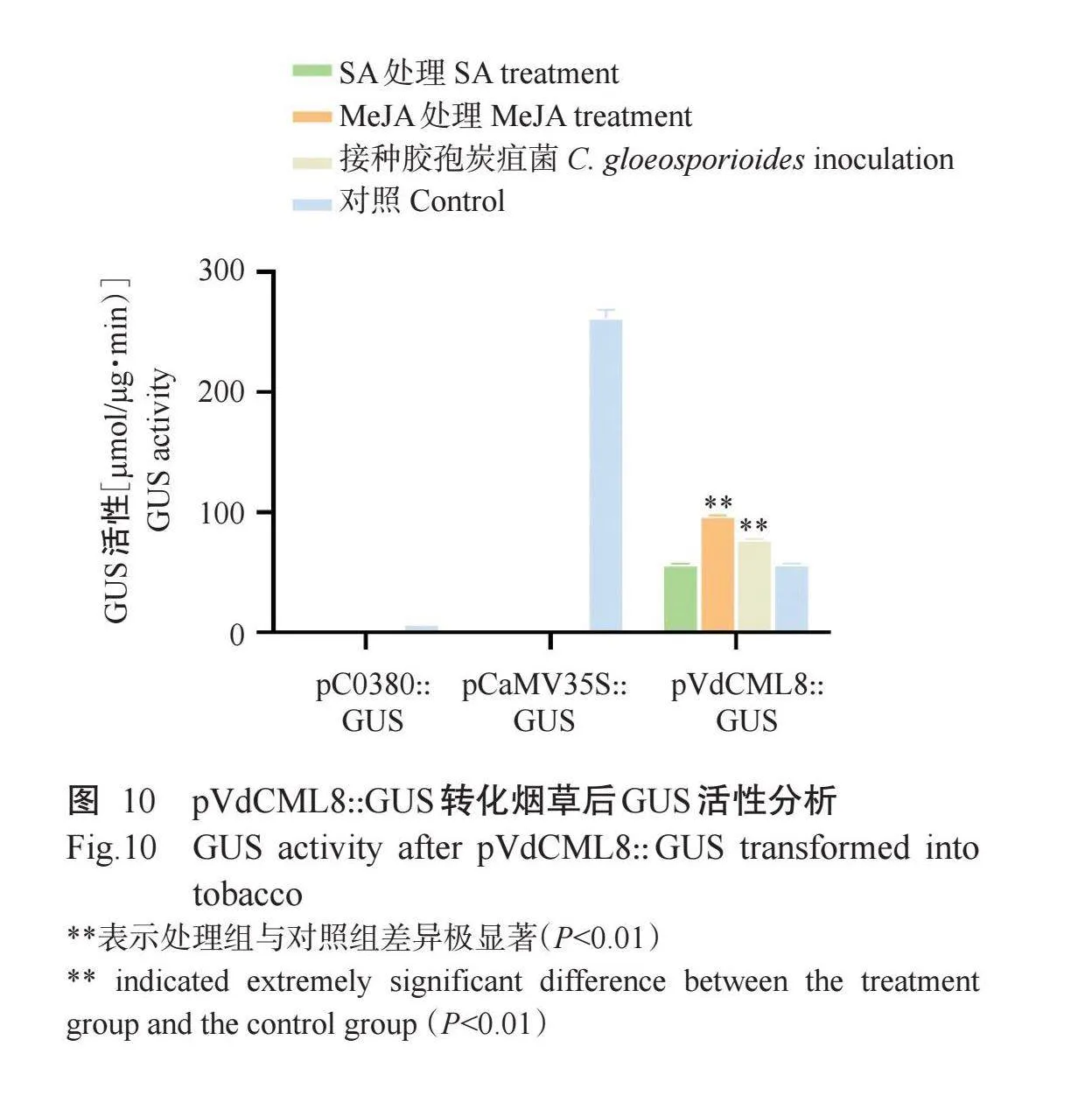
1.2.6 GUS化学染色分析与GUS活性测定使用农杆菌注射渗透法瞬时转化本式烟草,将叶片放入GUS染色液中37℃避光染色1 d,用75%乙醇进行多次脱色处理,直至完全脱色,然后使用体视显微镜拍照记录。将含pVdCML8::GUS、pC0380::GUS(阴性对照)和pCaMV35S::GUS(阳性对照)的农杆菌重悬液通过叶片背面注射瞬时转化烟草叶片中,暗培养2 d后采集叶片液氮速冻进行GUS活性测定对叶片进行胶孢炭疽菌、SA和MeJA处理2 d后收集叶片,液氮速冻后进行GUS活性测定。
1.2.7实时荧光定量PCR检测以葡萄Actin为内参基因,实时荧光定量PCR检测刺葡萄紫秋和欧洲葡萄红地球CML8基因在胶孢炭疽菌、SA、JA处理后不同时间点的相对表达量。反应体系10.0µL:5.0µL:SYBR Green I qPCR Mix,10µmol/L上、下游引物0.8µL,cDNA模板2.0µL,ROX 0.4µL,ddH2O补足至10.0µL。扩增程序:95℃预变性180 s;94℃30 s,60℃30 s,进行42个循环。采用2-ΔΔCt方法计算目的基因的相对表达量(Schmittgen and Livak,2008),并用SPSS 26.0进行差异显著性分析。
2结果与分析
2.1 VdCML8基因cDNA克隆及序列分析结果
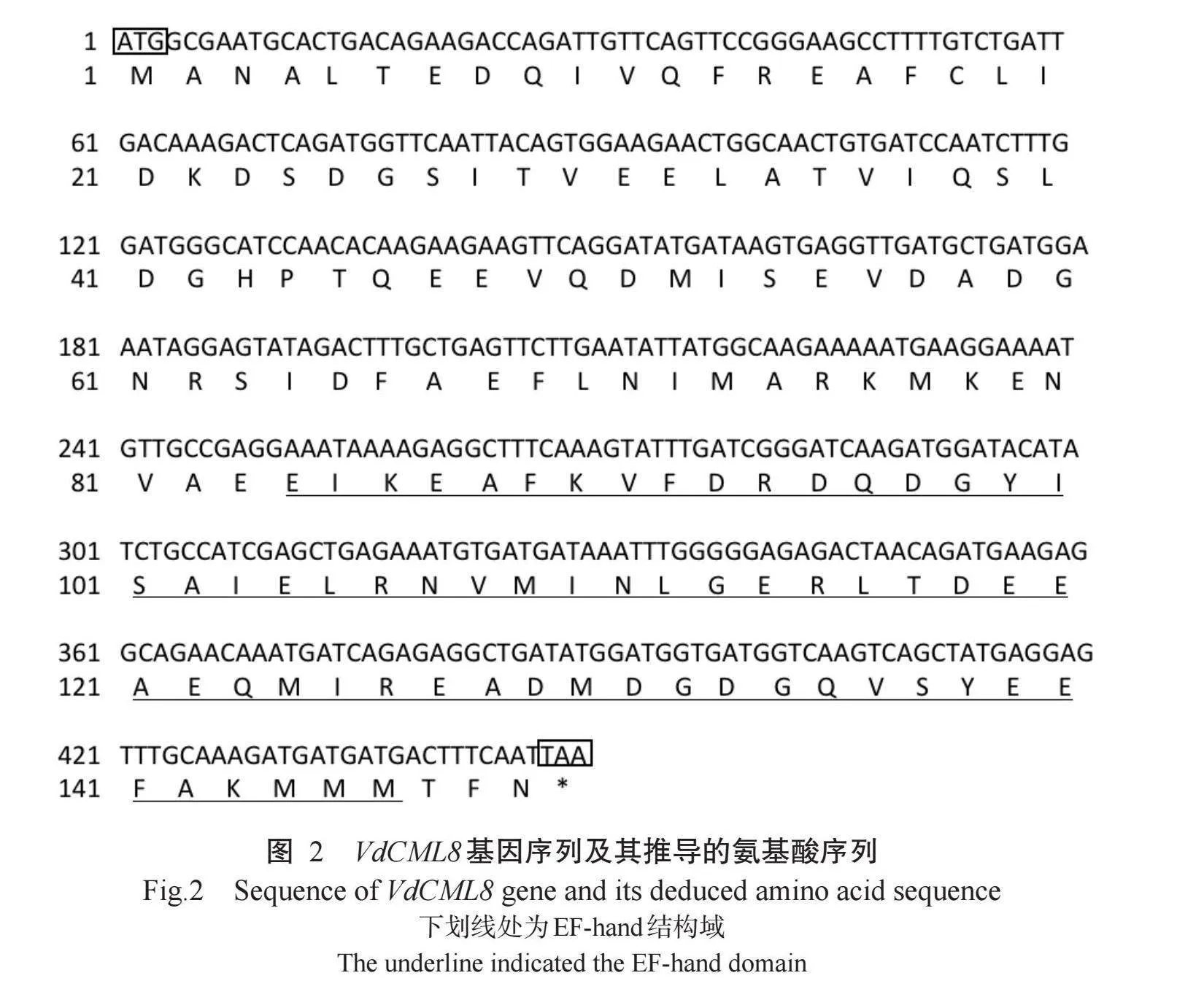
PCR扩增获得VdCML8基因的ORF序列(Gen-Bank登录号为MN913567.1),长度为450 bp(图1),编码149个氨基酸残基,包含EF-hand结构域(84~146个氨基酸)(图2)。VdCML8蛋白分子式为C727H1147N193O252S1 相对分子量为16.97 kD,理论等电点4.08,不稳定系数44.5 半衰期30 h,脂肪系数78.59,亲水指数-0.429,说明VdCML8为带正电荷且不稳定的亲水蛋白。VdCML8蛋白的二级结构中“-螺旋占比最高,达65.10%,无规则卷曲、β-转角和延伸链占比分别为20.13%、10.07%和4.70%(图3-A)。VdCML8蛋白无信号肽(图3-B)。
2.2 VdCML8蛋白系统发育分析及同源对比结果
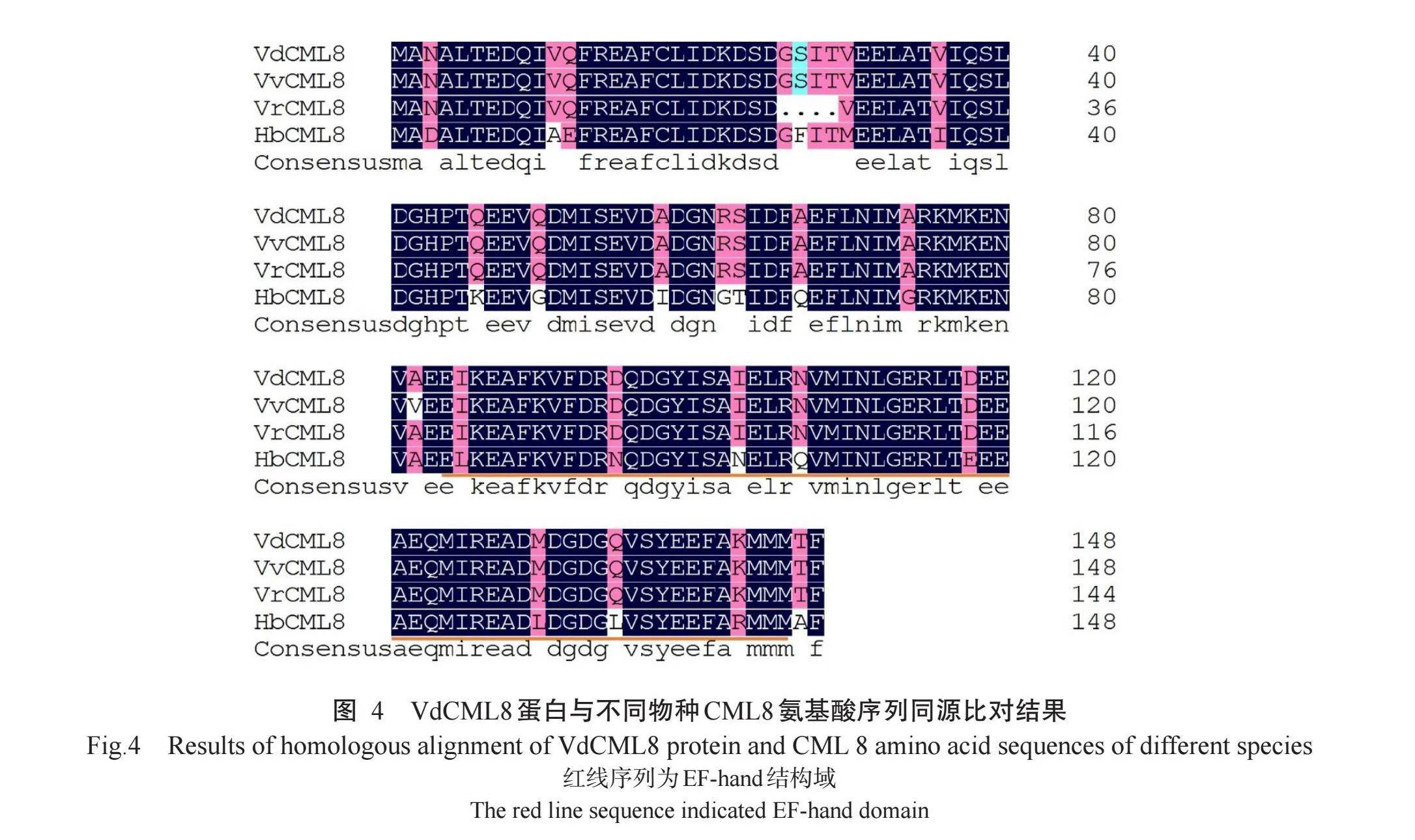
对从NCBI数据库中下载的不同物种CML8氨基酸序列进行同源比对,结果显示,刺葡萄VdCML8(GenBank登录号MN913567.1)与GenBank上已经公布的欧洲葡萄(V.vinifera,RVW54726.1)VvCML8、河岸葡萄(V.riparis,XP_034690901.1)VrCML8、橡胶树(Hevea brasiliensis,XP_021635369.1)HbCML8、苹果(Malus domestica,XP_008368689.2)、石榴(Punica granatum,XP_031401729.1)PgCML8的氨基酸序列相似性分别为99.33%、97.32%、85.14%、83.56%和84.93%,推测VdCML8与VvCML8、VrCML8、HbCML8为同源蛋白,氨基酸序列比对结果如图4所示。
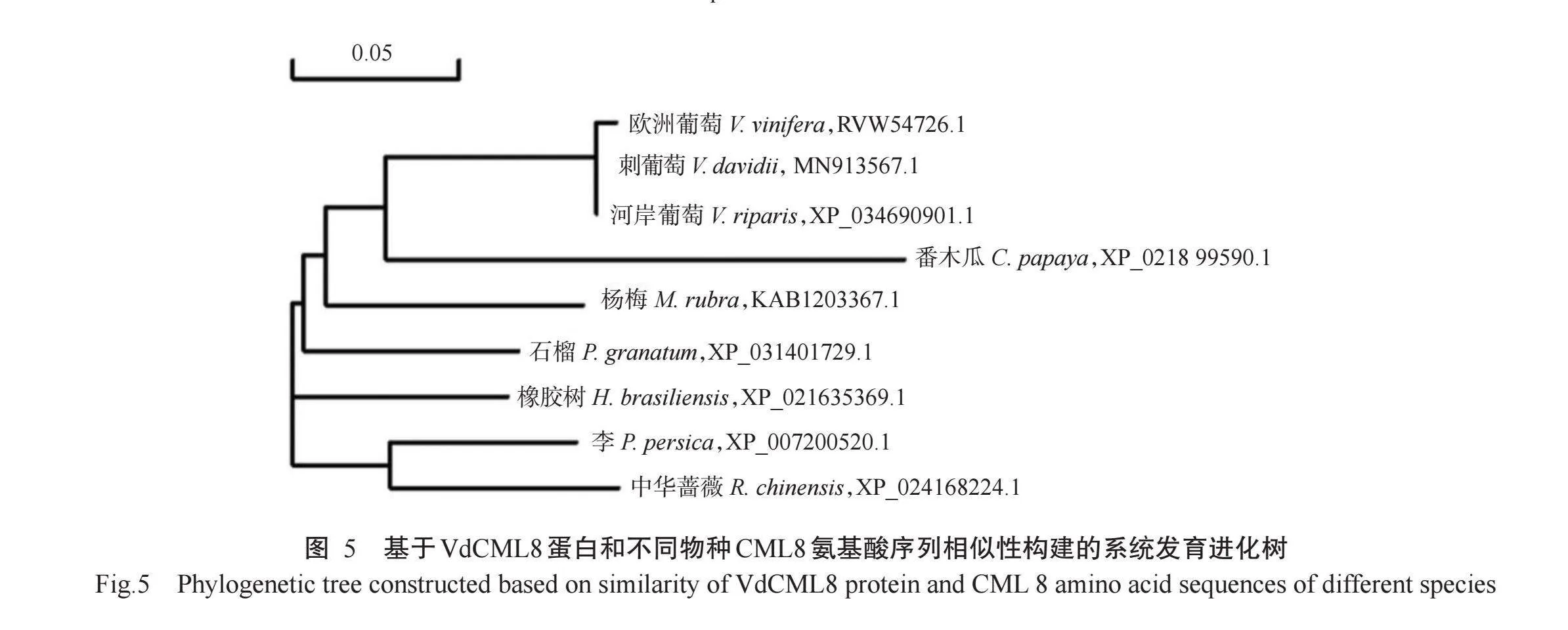
由系统发育进化树(图5)可知,刺葡萄VdCML8与欧洲葡萄(V.vinifera,RVW54726.1)VvCML8和河岸葡萄(V.riparis,XP_034690901.1)VrCML8聚在一起,形成同一个小分支,说明三者间亲缘关系较近,其次是VdCML8与番木瓜(Carica papaya,XP_0218 99590.1)CpCML8亲缘关系较近,与杨梅(Morella rubra,KAB1203367.1)MrCML8、石榴(Punica gra-natum,XP_031401729.1)PgCML8、橡胶树(H.brasi-liensis,XP_021635369.1)HbCML8、李(Prunus per-sica,XP_007200520.1)PpCML8和中华蔷薇(Rosa chinensis,XP_024168224.1)RcCML8的亲缘关系较远。欧洲葡萄、河岸葡萄和刺葡萄紫秋均属于葡萄科葡萄属,表明刺葡萄VdCML8在进化上较为保守。
2.3 VdCML8蛋白亚细胞定位结果
通过农杆菌介导的洋葱表皮细胞瞬时表达分析VdCML8蛋白亚细胞定位,结果如图6所示。含有空载体pBI221-35S-GFP洋葱细胞的细胞核和细胞膜中有绿色荧光;将含有VdCML8的融合蛋白表达载体(pBI221-35S-VdCML8-GFP)注射到洋葱表皮细胞中后,在细胞膜中观察到绿色荧光,结果表明VdCML8蛋白定位在细胞膜上。
2.4 VdCML8基因启动子克隆及顺式作用元件分析结果
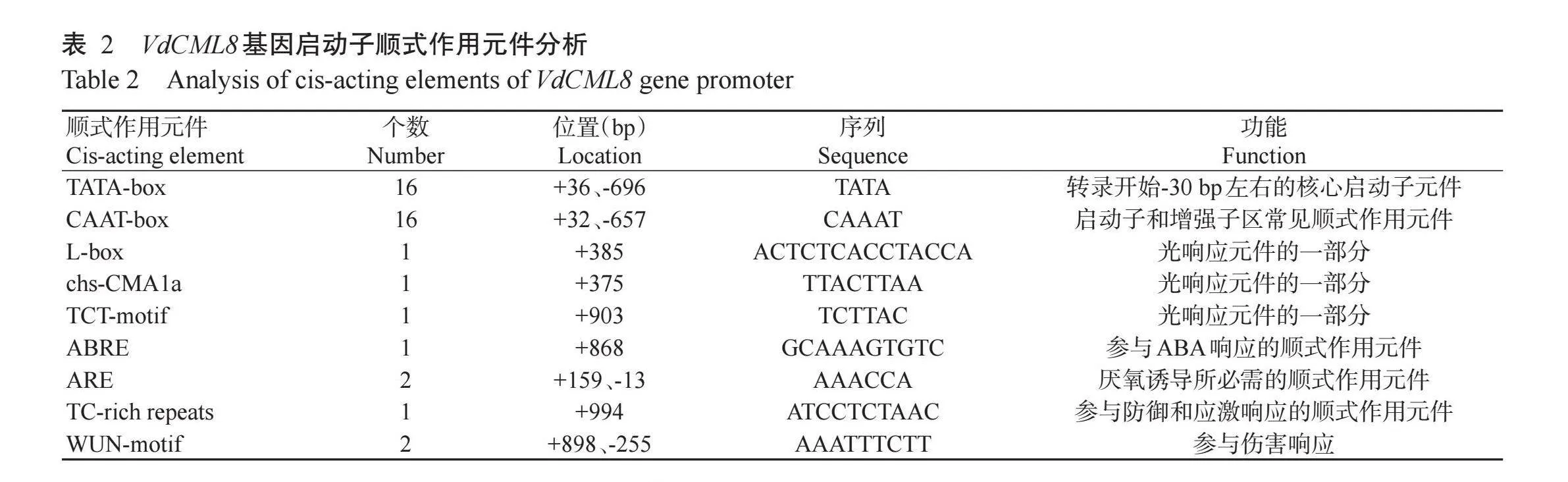
以刺葡vOCG98+i3wQM1SmVnnRhkJsElhtTKa0LUHfIVMXyPCQ=萄紫秋DNA为模板进行PCR扩增,获得长度约为1050 bp的启动子序列pVdCML8(Gen-Bank登录号:PP852686)(图7)。通过PlantCARE预测pVdCML8序列的顺式作用元件,结果(表2)显示,pVdCML8除含有大量的CAAT-box和TATA-box外,还含有一些光响应元件(L-Box、chs-CMALa和TCT-motif)、ABA响应元件(ABRE)、厌氧诱导响应元件(ARE)、防御和应激元件(TC-rich repeats)、伤害响应元件有(WUN-motif)等。由此可见,pVdCML8启动子能响应多种信号诱导,说明VdCML8基因在激素诱导和QHpPPrBED2sHHxoqmMQY7rwLsk+ycPuss9mGSu7GLww=逆境响应中扮演重要的调控角色,为进一步研究刺葡萄的应激响应机制提供了重要线索。
2.5 VdCML8基因启动子融合表达载体构建
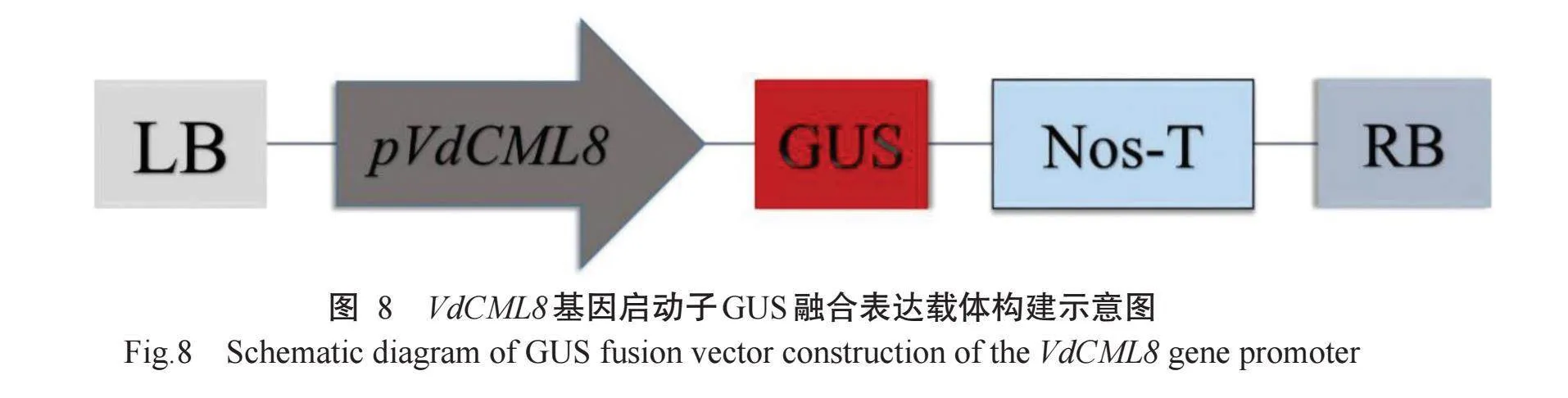
对pVdCML8进行PCR扩增后,在pVdCML8两端加入酶切位点BamH I和Pst I。将pVdCML8连接至pC0380::GUS上,获得GUS融合表达载体pVd-CML8::GUS(图8)。
2.6 VdCML8基因启动子活性测定结果
GUS化学染色结果显示,阳性对照颜色较深(图9-A),试验组pVdCML8::GUS染色的叶片较阳性对照颜色浅(图9-B),阴性对照无色(图9-C),说明VdCML8基因启动子能正常表达。通过将pVdCML8::GUS融合表达载体序列转化烟草叶片,并进行接种胶孢炭疽菌及喷施SA和MeJA处理,结果发现,与对照相比,处理样品的GUS活性极显著升高(图10)(P<0.01),推测胶孢炭疽菌和MeJA可诱导VdCML8基因表达。
2.7不同葡萄品种CML8基因在胶孢炭疽菌、JA和SA诱导下的表达模式
利用实时荧光定量PCR检测接种胶孢炭疽菌后刺葡萄紫秋VdCML8基因和欧洲葡萄红地球VvCML8基因在叶片的相对表达量,结果如图11-A所示。接种后0~72 h,VdCML8基因相对表达量呈先上升后下降的变化趋势,在接种6、12、24、48和72 h的相对表达量分别为对照组的7.89、22.08、11.72、5.36和1.19倍,尤其在接种后0~12 h相对表达量迅速增长。欧洲葡萄红地球属于易感病品种,在接种胶孢炭疽菌后,VvCML8基因的相对表达量较对照组也有提高,同样在12 h达最高,为对照组的9.30倍,但其相对表达量低于刺葡萄紫秋,表明VdCML8能响应胶孢炭疽菌的胁迫,推测VdCML8基因在炭疽病防御反应中扮演重要角色,并对其提高抗病性具有一定的调控作用。
利用实时荧光定量PCR检测SA和JA处理下刺葡萄紫秋VdCML8基因和欧洲葡萄红地球VvCML8基因在叶片的相对表达量,结果如图11-B和11-C所示。SA处理后0~24 h,VdCML8基因在3 h出现峰值,为对照组的7.68倍,是VvCML8基因的2.76倍(图11-B)。JA处理后0~24 h,VdCML8基因的表达量总体呈现先上升后下降的变化趋势,在6h的相对表达量出现峰值,为对照组的22.25倍,为VvCML8基因的9.04倍(图11-C)。综上所述,VdCML8基因可能是SA和JA信号途径的下游调控因子,参与刺葡萄紫秋对炭疽病的抗性反应。
3讨论
CML作为最典型的钙离子结合蛋白,在植物生长发育和胁迫应答中发挥重要作用(Boonburapong and Buaboocha,2007)。研究表明,CML蛋白广泛参与植物抗病调控机制,其与钙离子结合后会发生空间结构改变,并在下游引发特异反应(Cheung,1980;McAinsh and Pittman,2009),下游的感受器接收钙信号,从而调节植物的生命活动(Scrase-Field and Knight,1979)。小麦TaCML25和TaCML26基因能增强对叶锈病的抗性,对有效防治叶锈病具有重要意义(刘鹏等,2020)。拟南芥受丁香假单胞菌侵染后,AtCML8和AtCML9基因表达上调(Zhu et al,2017)。从拟南芥中克隆获得1个与CaM蛋白相关的基因AtCML43,当拟南芥受丁香假单胞菌侵染时,AtCML43基因在拟南芥叶片中快速响应,通过过表达加速超敏反应,能在植物对病原菌的免疫反应中起作用(Chiasson et al,2005)。此外,CML在植物抗病毒过程中也发挥重要作用。当烟草叶片受烟草蚀纹病毒侵染时,rgs-CaM过量表达可抑制HC-Pro的活性,从而增强植物对病毒的抵抗力(Nakahara,2012)。甜瓜接种尖孢镰刀菌后,CmCMLs基因表达显著上调,推测CmCMLs对甜瓜枯萎病有抑制作用(罗澜等,2021)。小麦基因系TcLr19接种小麦叶锈菌后,TaCML25和TaCML26基因表达上调,其表达量是感病突变体mu19中表达量的4倍(刘鹏等,2020)。大丽轮枝菌Vd080侵染棉花后,GhCML41基因的表达量显著升高,说明GhCML41参与了棉花抗黄萎病响应过程(赵沛,2019)。本研究对刺葡萄紫秋(抗病品种)和欧洲葡萄红地球(感病品种)在接种胶孢炭疽菌后不同时间点检测CML8基因的表达模式,结果发现,VdCML8基因的相对表达量整体上高于VvCML8基因,推测该基因参与葡萄炭疽病的抗病反应。
植物中植物激素的调节是非常重要的抗病机制,其中SA和JA能激活不同防御信号途径,最终使植物产生防御蛋白或次生代谢物(Creelman and Mullet,1997)。棉花GhCML41基因经VIGS技术沉默后,叶片的木质素含量减少,胼胝质沉淀能力下降,SA和JA含量升高(赵沛,2019)。沉默辣椒SlCML55基因可增加抗病基因PR1的表达,激活植物中SA的免疫反应,从而增强辣椒对疫霉菌的抵抗能力(Zhang et al.,2022a)。将花生AhCML69基因过表达并瞬时转化烟草,在接种青枯菌后,其中超敏反应(HR)及JA和SA信号通路相关基因的表达均被诱导,并显著上调(Yang et al.,2024)。本研究通过SA和JA处理刺葡萄紫秋,结果显示VdCML8基因的相对表达量明显高于对照,推测外源激素SA和JA能诱导VdCML8基因表达,VdCML8能通过介导SA和JA信号参与葡萄炭疽病的抗病反应。
启动子是一段控制结构基因转录、活化,且参与转录RNA聚合酶的DNA序列(昝新丽等,2013;崔小月等,2024)。中国华东野生葡萄中的诱导型启动子pVpSTS受白粉病和赤星病诱导表达,该启动子含有TC-rich repeats、ABRE等顺式作用元件(Xu et al.,2010)。中国野生葡萄VpTNL1基因启动子序列(pVpTNL1)中的TC-rich repeats元件可能在葡萄对叶斑病胁迫的响应中起重要作用(Wen et al.,2017)。研究发现,烟草NtVQ35基因能同时被SA和青枯病菌高效诱导表达,对该基因的启动子区域进行分析,结果发现其启动子区域含有3个胁迫和防御响应相关的顺式作用元件(刘翠花,2020)。本研究克隆刺葡萄紫秋VdCML8基因的启动子序列,其含有响应逆境、激素及光等多种的顺式作用元件,因此推测该顺式作用元件在VdCML8基因对逆境胁迫的响应和防御中发挥重要作用。玉米ZmRXO1基因的启动子序列包含P-box、GARE-motif和MBS等激素响应元件。这些元件使得ZmRXO1基因能对外源激素如JA、赤霉素和ABA等作出响应,导致ZmRXO1基因的表达水平升高(Tao et al.,2015)。玉米Zmap基因启动子序列中含有茉莉酸甲酯(MeJA)响应元件,对瞬时表达的烟草喷施MeJA后GUS活性显著增加,说明该基因启动子被激活并促进了基因表达(Jin et al.,2019)。对木薯MeCML42转基因体胚用100µmol/L的MeJA和SA溶液处理后进行GUS活性测定,结果发现2种激素均能调控MeCML42基因启动子的活性,且不同激素的调控方式存在差异(侯静怡等,2024)。本研究构建GUS融合表达载体进行GUS活性测定,在胶孢炭疽菌和MeJA诱导下,GUS活性增强,推测VdCML8基因能够响应胶孢炭疽菌和调节MeJA来抵抗病原菌的侵染。
4结论
VdCML8基因是SA和JA信号途径的下游调控基因,SA和JA可诱导其高效表达,参与葡萄炭疽病响应过程,对提高植株抗病性具有一定作用。
参考文献(References):
曹绍玉,王艳芳,苏婉玉,张琳,张应华,许俊强.2018.类钙调蛋白在植物生长发育及逆境胁迫中的功能研究进展[J].植物生理学报,54(10):1517-1526.[Cao S Y,Wang Y F,Su W Y,Zhang L,Zhang Y H,Xu J Q.2018.Research progress on functions of calmodulin-like proteins in pro-cesses of plant growth and developments and stresses[J].Plant Physiology Journal,54(10):1517-1526.]doi:10.13592/j.cnki.ppj.2018.0053.
陈超,端木慧子,朱丹,刘艾林,肖佳雷,朱延明.2015.大豆CML家族基因的生物信息学分析[J].大豆科学,34(6):957-963.[Chen C,Duanmu H Z,Zhu D,Liu A L,Xiao J L,Zhu Y M.2015.Bioinformatics analysis of GmCML genes in soybean genome[J].Soybean Science,34(6):957-963.]doi:10.11861/j.issn.1000-9841.2015.06.0957.
程大伟,张国海,姜建福,樊秀彩,张颖,刘崇怀.2015.刺葡萄种内遗传多样性研究进展[J].植物遗传资源学报,16(6):1141-1151.[Cheng D W,Zhang G H,Jiang J F,Fan X C,Zhang Y,Liu C H.2015.Intraspecies genetic diver-sityof Vitis davidii[J].Journal of Plant Genetic Resources,16(6):1141-1151.]doi:10.13430/j.cnki.jpgr.2015.06.002.
崔小月,尚泓泉,吕中伟,娄玉穗,张柯,樊红杰,吴文莹,张晓锋.2024.欧洲葡萄NF-YB3基因克隆与表达分析[J].河南农业科学,53(4):111-118.[Cui X Y,Shang H Q,LüZ W,Lou Y S,Zhang K,Fan H J,Wu W Y,Zhang X F.2024.Cloning and expression analysis of NF-YB3 gene from Vitis vinifera[J].Journal of Henan Agricultural Scien-ces,53(4):111-118.]doi:10.15933/j.cnki.1004-3268.2024.04.012.
段长青,刘崇怀,刘凤之,王忠跃,刘延琳,徐丽明.2019.新中国果树科学研究70年—葡萄[J].果树学报,36(10):1292-1301.[Liu C Q,Duam C H,Liu F Z,Wang Z Y,Liu Y L,Xu L M.2019.Fruit scientific research in new Chinain the past 70 years:Grape[J].Journal of Fruit Science,36(10):1292-1301.]doi:10.13925/j.cnki.gsxb.Z05.
侯静怡,甄兴厚,王亚杰,张慧敏,李瑞梅,刘姣,郭建春,耿梦婷,姚远.2023.木薯MeCML42基因启动子克隆及激素响应分析[J/OL].分子植物育种.https://kns.cnki.net/kcms2/detail/46.1068.S.20230526.0955.002.html.[Hou J Y,Zhen X H,Wang Y J,Zhang H M,Li R M,Liu J,Guo J C,Geng M T,Yao Y.2023.Cloning and hormone response analysis of cassava MeCML42 gene promoter[J/OL].Molecular Plant Breeding.http://kns.cnki.net/kcms/detail/46.1068.S.20230526.0955.002.html.]
李顺雨,潘学军,张文娥,张素杰,刘崇怀.2010.葡萄属种质资源多样性及利用[J].种子,29(1):61-64.[Li S Y,Pan X J,Zhang W E,Zhang S J,Liu C H.2010.The diversity and utilization of Vitis L.germplasm resource[J].Seed,29(1):61-64.]doi:10.16590/j.cnki.1001-4705.2010.01.080.
刘翠花.2020.烟草NtVQ35基因的鉴定及其抗青枯病基本功能研究[D].重庆:西南大学.[Liu C H.2020.Identifica-tion of NtVQ35 gene in Nicotiana tabacum and prelimi-nary study on its function against tobacco bacterial wilt disease[D].Chongqing:Southwest University.]
刘辉,邓治,陈江淑,李德军.2015.巴西橡胶树类钙调素蛋白基因HbCML27克隆与表达分析[J].分子植物育种,13(12):2721-2727.[Liu H,Deng Z,Chen J S,Li D J.2015.Cloning and expression analysis of calmodulin-like protein gene HbCML27 from Hevea brasiliensis[J].Mo-lecular Plant Breeding,13(12):2721-2727.]doi:10.13271/j.mpb.013.002721.
刘梅,张玮,周莹,严红,乔广行,黄金宝.2011.葡萄炭疽病研究进展[J].中国植保导刊,34(1):29-33.[Liu M,Zhang W,Zhou Y,Yan H,Qiao G X,Huang J B.2011.Research progress on grape anthracnose[J].China Plant Protection,34(1):29-33.]doi:10.3969/j.issn.1672-6820.2014.01.006.
刘鹏,韦杰,杨毅清,张娜,温晓蕾,范学锋,杨文香,刘大群.2020.小麦类钙调素新亚型基因TaCML25/26调控抗叶锈性[J].中国农业科技导报,22(4):120-128.[Liu P,Wei J,Yang Y Q,Zhang N,Wen X L,Fan X F,Yang W X,Liu D Q.2020.A new subtype of calmodulin-like TaCML25/26 in wheat regulate resistance to leaf rust[J].Journal of Agricultural Science and Technology,22(4):120-128.]doi:10.13304/j.nykjdb.2019.0596.
刘伟,王芸芸,赵武娟,赵雪辉,董志刚.2016.21种不同类型葡萄种质资源遗传多样性的SSR分析[J].中国农学通报,32(34):143-148.[Liu W,Wang YY,Zhao W J,Zhao X H,Dong Z G.2016.Genetic diversity of 21 kinds of grape germplasm resources by SSR markers[J].Chinese Agricultural Science Bulletin,32(34):143-148.]
罗澜,司修洋,孙蕾,高鹏,李勇,王学征.2021.甜瓜CML基因家族的鉴定与表达特性分析[J].分子植物育种,19(24):8081-8094.[Luo L,Si X Y,Sun L,Gao P,Li Y,Wang X Z.2021.Identification and expression characteristic analy-sis of CML gene family of melon[J].Molecular Plant Breeding,19(24):8081-8094.]doi:10.13271/j.mpb.019.008081.
阮仕立,李记明.2002.野生葡萄种质资源的抗性及其利用研究进展[J].中外葡萄与葡萄酒,27(4):30-33.[Ruan S L,Li J M.2002.Progress in the study of resistance and its uti-lization of wild grapevine germplasm resources[J].Sino-Overseas Grapevine&Wine,27(4):30-33.]doi:10.13414/j.cnki.zwpp.2002.04.009.
杨秀,许艳超,杨芳芳,蔡小彦,侯宇清,王玉红,王星星,王坤波,刘方,周忠丽.2019.棉花CML基因家族成员鉴定与功能分析[J].棉花学报,31(4):307-318.[Yang X,Xu Y C,Yang F F,Cai X Y,Hou Y Q,Wang Y H,Wang X X,Wang K B,Liu F,Zhou Z L.2019.IdenA+EWlaeBABbXeJhqTCOE6O+FrJhk2em72gedDQyJyj4=tification and func-tional analysis of CML gene family in cotton[J].Cotton Science,31(4):307-318.]
昝新丽,高英,陈玉玲,赵开军.2013.病原菌诱导型启动子顺式作用元件及其互作的转录因子[J].植物学报,48(2):219-229.[Zan X L,Gao Y,Chen Y L,Zhao K J.2013.Pathogen-responsive cis-acting elements and their interac-tive transcription factors[J].Chinese Bulletin of Botany,48(2):219-229.]doi:10.3724/SP.J.1259.2013.00219.
张洁.2022.中国野生葡萄抗病相关转录因子互作筛选与功能研究[D].杨凌:西北农林科技大学.[Zhang J.2022.Interaction and functional study of transcription factorsrelated to disease resistance in Chinese wild grapevine[D].Yangling:Northwest A&F University.]
张新龙,张国福,杨素梅,金岩,张耀中,范昆,付丽.2023.葡萄炭疽病病原菌的分离鉴定及防控药剂筛选[J].中外葡萄与葡萄酒,(6):46-51.[Zhang X L,Zhang G F,Yang S M,Jin Y,Zhnag Y Z,Fan K,Fu L.2023.Isolation and identification of grape anthracnose pathogens of Colle-totrichumviniferum and fungicide screening[J].Sino-Overseas Grapevine&Wine,(6):46-51.]doi:10.13414/j.cnki.zwpp.2023.06.007.
赵沛.2019.GhCML41蛋白调控棉花抗黄萎病的分子机制[D].杨凌:西北农林科技大学.[Zhao P.2019.Molecular mechanism of cotton resistance to verticillium wilt regu-lated by GhCML41 protein[D]Yangling:Northwest A&F University.]
Aleynova O A,Kiselev K V,Ogneva Z V,Dubrovina A S.2020.The grapevine calmodulin-like protein gene CML21 is regulated by alternative splicing and involved in abiotic stress response[J].International Journal of Molecular Scien-ces,21(21):7939.doi:10.3390/ijms21217939.
Aleynova O A,Suprun A R,Ananev A A,Nityagovsky N N,Ogneva Z V,Dubrovina A S,Kiselev K V.2022.Effect of cmodulin-like gene(CML)overexpression on stilbene bio-synthesis in cell cultures of Vitis amurensis Rupr[J].Plants,11(2):171.doi:10.3390/plants 11020171.
Boonburapong B,Buaboocha T.2007.Genome-wide identifica-tion and analyses of the rice calmodulin and related poten-tial calcium sensor proteins[J].BMC Plant Biology,7:4.doi:10.1186/1471-2229-7-4.
Cheng H Q,Han L B,Yang C L,Wu X M,Zhong N Q,Wu J H,Wang F X,Wang H Y,Xia G X.2016.The cotton MYB108 forms a positive feedback regulation loop with CML11 and participates in the defense response against Verticillium dahliae infection[J].Journal of Experimental Botany,67(6):1935-1950.doi:10.1093/jxb/erw016.
Cheung W Y.1979.Calmodulin plays a pivotal role in cellular regulation[J].Science,207(4426):19-27.doi:10.1126/science.6243188.
Chiasson D,Ekengren S K,Martin G B,Dobney S L,Snedden W A.2005.Calmodulin-like proteins from Arabidopsis and tomato are involved in host defense against Pseudomo-nassyringaepv.tomato[J].Plant Molecular Biology,58(6):887-897.doi:10.1007/s 11103-005-8395-x.
Creelman RA,Mullet J E.1997.Biosynthesis and action of jas-monates in plants[J].Annual Review of Plant Biology and Plant Molecular Biology,48:355-381.doi:10.1146/an-nurev.arplant.48.1.355.
Day I S,Reddy V S,Shad Ali G,Reddy A S.2002.Analysis of EF-hand-containing proteins in Arabidopsis[J].Genome Biology,3(10):RESEARCH0056.doi:10.1186/gb-2002-3-10-research0056.
Delk N A,Johnson K A,Chowdhury N I,Braam J.2005.CML24,regulated in expression by diverse stimuli,encodes a potential Ca2+sensor that functions in responses to abscisic acid,daylength,and ion stress[J].Plant Physio-logy,139(1):240-253.doi:10.1104/pp.105.062612.
Dubrovina A S,Aleynova O A,Ogneva Z V,Suprun A R,Ananev A A,Kiselev K V.2019.The effect of abiotic stress conditions on expression of calmodulin(CaM)and calmodulin-like(CML)genes in wild-growing grapevine Vitis amurensis[J].Plants(Basel),8(12):602.doi:10.3390/plants8120602.
Jin B,Sheng Z,Muhammad I,Chen J Q,Yang H L.2019.Cloning and functional analysis of the promoter of a stress-inducible gene(Zmap)in maize[J].PLoS One,14(2):e211941.doi:10.1371/journal.pone.0211941.
Heyer M,Scholz S S,Reichelt M,Kunert G,Oelmüller R,Mithöfer A.2022.The Ca2+sensor proteins CML37 andCML42 antagonistically regulate plant stress responses byaltering phytohormone signals[J].Plant Molecular Bio-logy,109(4-5):611-625.doi:10.1007/s 11103-021-01184-2.Leba
L J,Cheval C,Ortiz-Martín I,Ranty B,Beuzón C R,Galaud J P,Aldon D.2012.CML9,an Arabidopsiscalmodulin-like protein,contributes to plant innate immu-nity through a flagellin-dependent signalling pathway[J].Plant Journal,71(6):976-989.doi:10.1111/j.1365-313X.2012.05045.x.
Ma W,Smigel A,Tsai Y C,Braam J,Berkowitz G A.2008.Innate immunity signaling:Cytosolic Ca2+elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein[J].Plant Physiology,148(2):818-828.doi:10.1104/pp.108.125104.
McAinsh M R,Pittman J K.2009.Shaping the calcium signa-ture[J].The New Phytologist,181(2):275-294.doi:10.1111/j.1469-8137.2008.02682.x.
Munir S,Khan M R,Song J W,Munir S,Zhang Y Y,Ye Z B,Wang T T.2016.Genome-wide identification,characteriza-tion and expression analysis of calmodulin-like(CML)pro-teins in tomato(Solanum lycopersicum)[J].Plant Physio-logy and Biochemistry,102:167-179.doi:10.1016/j.pla-phy.2016.02.020.
Nakahara K S,Masuta C,Yamada S,Shimura H,Kashihara Y,Wada T S,Meguro A,Goto K,Tadamura K,Sueda K,Seki-guchi T,Shao J,Itchoda N,Matsumura T,Igarashi M,Ito K,Carthew R W,Uyeda I.2012.Tobacco calmodulin-like protein provides secondary defense by binding to and directing degradation of virus RNA silencing suppressors[J].Proceedings of the National Academy of Sciences ofthe United States of America,109(25):10113-10118.doi:10.1073/pnas.1201628109.
Schmittgen T D,Livak K J.2008.Analyzing real-time PCR data by the comparative C(T)method[J].Nature Proto-cols,3(6):1101-1108.doi:10.1038/nprot.2008.73.
Scrase-Field S A M G,Knight M R.2003.Calcium:Just a chemical switch?[J].Current Opinion in Plant Biology,6(5):500-506.doi:10.1016/s 1369-5266(03)00091-8.
Tao Y,Wang F T,Jia D M,Li J T,Zhang Y M,Jia C G,Wang D P,Pan H Y.2015.Cloning and functional analysis of thepromoter of a stress-inducible gene(ZmRXO1)in maize[J].Plant Molecular Biology Reporter,33(2):200-208.doi:10.1007/s 11105-014-0741-1.
Vandelle E,Vannozzi A,Wong D,Danzi D,Digby A M,Dal Santo S,Astegno A.2018.Identification,characterization,and expression analysis of calmodulin and calmodulin-like genes in grapevine(Vitis vinifera)reveal likely roles in stress responses[J].Plant Physiology and Biochemistry,129:221-237.doi:10.1016/j.plaphy.2018.06.003.
Wen Z F,Yao L P,Singer S D,Muhammad H,Li Z,Wang X P.2017.Constitutive heterologous overexpression of a TIR-NB-ARC-LRR gene encoding a putative disease resistance protein from wild Chinese Vitis pseudoreticulata in Arabi-dopsis and tobacco enhances resistance tophytopathogenic fungi and bacteria[J].Plant Physiology and Biochemistry,112:346-361.doi:10.1016/j.plaphy.2017.01.017.
Xu B,Cheval C,Laohavisit A,Hocking B,Chiasson D,Olsson T S G,Shirasu K,Faulkner C,Gilliham M.2017.Aal-modulin-like protein regulates plasmodesmal closure du-ring acterial immune responses[J].New Phytologist,215(1):77-84.doi:10.1111/nph.14599.
Xu W R,Yu Y H,Ding J H,Hua Z Y,Wang Y J.2010.Charac-terization of a novel stilbene synthase promoter involved in pathogen-and stress-inducible expression from Chinese wild Vitis pseudoreticulata[J]Planta,231(2):475-487.doi:10.1007/s00425-009-1062-8.
Yang D,Chen T,Wu Y S,Tang H Q,Yu J Y,Dai X Q,Zheng Y X,Wan X R,Yang Y,Tan X D.2024.Genome-wide analy-sis of the peanut CaM/CML gene family reveals that the AhCML69 gene is associated with resistance to Ralstonia solanacearum[J].BMC Genomics,25(1):200.doi:10.1186/s 12864-024-10108-5.
Zhang J,Zou AH,Wen YX,Wei X F,Liu CY,Lv X,Ma X Z,Fan G J,Sun X C.2022a.SlCML55,a novel Solanum lyco-persicum calmodulin-like gene,negatively regulates plant immunity to Phytophthora pathogens[J].Scientia Horti-culturae,299:111049.doi:10.1016/j.scienta.2022.111049.
Zhang X X,Tian J,Li S,Liu Y Y,Feng T,Wang Y Y,Li Y J,Huang X X,Li D H.2022b.Characterization of the calmodulin/calmodulin-like protein(CAM/CML)family in Ginkgo biloba,and the influence of an ectopically expressed GbCML gene(Gb_30819)on seedling and fruit development of transgenic Arabidopsis[J].Plants(Basel),11(11):1506.doi:10.3390/plants 11111506.
Zhu X Y,Perez M,Aldon D,Galaud J P.2017.Respective con-tribution of CML8 and CML9,two arabidopsis calmodulin-like proteins,to plant stress responses[J].Plant Signaling Behavior,12(5):e1322246.doi:10.1080/15592324.2017.1322246.
Zipfel C,Felix G.2005.Plants and animals:A different taste for microbes?[J].Current Opinion in Plant Biology,8(4):353-360.doi:10.1016/j.pbi.2005.05.004.
(责任编辑陈燕)

