投喂不同饵料对斑鳜生长及消化性能的影响





摘要:【目的】探明斑鳜(Siniperca scherzeri)摄食配合饲料后其生长与消化性能的变化规律,为开展以配合饲料替代传统饵料鱼的斑鳜规模养殖提供参考依据。【方法】选取驯化和未驯化斑鳜苗种各180尾,分别投喂配合饲料和活饵鱼,饲养20周后饥饿24 h,测定分析其生长性能、消化酶活性、肠道组织结构及肠道微生物群落结构。【结果】至饲养20周后,饲料组斑鳜的存活率、脏体比与活饵组斑鳜相比无显著差异(P>0.05,下同),但终末体质量、增重率及饲料系数显著低于活饵组斑鳜(P<0.05,下同);饲料组斑鳜肠道α-淀粉酶活性显著高于活饵组斑鳜,胰蛋白酶活性显著低于活饵组斑鳜,而胃蛋白酶和脂肪酶活性无显著差异。此外,饲料组斑鳜肠道的肌层厚度和黏膜褶皱高度均显著低于活饵组斑鳜,但二者间的肠黏膜褶皱数无显著差异。在肠道微生物群落方面,饲料组斑鳜肠道微生物群落的ACE指数和Chao1指数显著低于活饵组斑鳜,但Shannon指数和Simpson指数无显著差异;2种饵料投喂模式下斑鳜肠道微生物群落结构均以变形菌门、厚壁菌门、软壁菌门和放线菌门为绝对优势菌门,其相对丰度之和在98.00%以上;在属分类水平上,饲料组斑鳜肠道内的伯克霍尔德菌属相对丰度显著高于活饵组斑鳜,而鞘氨醇盒菌属和支原体属相对丰度显著低于活饵组斑鳜。【结论】以配合饲料替代活饵投喂斑鳜,其生长速度显著降低,肠道组织结构、消化酶活性及肠道微生物群落结构出现适应性变化,尤其是配合饲料的投喂有助于维持肠道微生物稳定性。可见,以配合饲料替代活饵养殖斑鳜具有可行性,但还需进一步优化饲料营养组分及投喂策略,提高配合饲料养殖下斑鳜的生长速率。
关键词:斑鳜;配合饲料;活饵;生长性能;消化酶活性;肠道组织;肠道微生物
中图分类号:S965.127文献标志码:A文章编号:2095-1191(2024)08-2523-12
Effects of feeding different diets on growth and digestive perfor⁃mance of spotted mandarin fish(Siniperca scherzeri)
LAI Ming-yong
(Fujian Freshwater Fisheries Research Institute,Fuzhou,Fujian 35000 China)
Abstract:【Objective】To study the changes of growth and digestive performance of spotted mandarin fish(Siniperca scherzeri)after feeding on the compound feed,which provided reference for the large-scale culture of S.Scherzeri by using the compound feed instead of the traditional forage fish.【Method】A total of 180 domesticated individuals and 180 undomesticated individuals were fed with compound feed and live forage fish respectively,and starved for 24 h after feeding 20 weeks.The growth performance,digestive enzyme activity,intestinal structure and intestinal microbiota struc-ture of S.scherzeris were determined and analyzed.【Result】After 20 weeks of feeding,the survival rate,viscero-body ratio of S.scherzeri in compound feed group had no significant differences compared with those in live bait group(P>0.05,the same below),but the final body weight,weight gain rate and feed coefficient were significantly lower than those in live bait group(P<0.05,the same below).The intestinalα-amylase activity of compound feed group was signifi-cantly higher than that of the live bait group,and the trypsinactivity was significantly lower,but there was no significant difference in pepsin and lipase activities between the two groups.In addition,the thickness of muscle layer and the height of mucosal folds of S.scherzeri in compound feed group were significantly lower than those in live bait group,but there was no significant difference in the number of intestinal folds between the two groups.In terms of intestinal microbiota,the ACE index and Chao1 index of intestinal microbiota of S.scherzeri in compound feed group were significantly lower than those in live bait group,but there was no significant difference in Shannon index and Simpson index between the twogroups.Under the two feeding patterns,Proteobacteria,Firmicutes,Tenericutes and Actinobacteria were the dominant microbiota,and the sum of their relative abundance was more than 98.00%.At the genus classification level,the relative abundance of Burkholderia-Caballeronia-Paraburkholderia in the intestine of S.scherzeri in compound feed group was significantly higher than that in live bait group,while the relative abundance of Sphingopyxis and Mycoplasma ofS.scher-zeri in compound feed group was significantly lower than that in live bait group.【Conclusion】The growth rate of S.scher-zeri decreases significantly when fed with compound feed instead of live bait,and the intestinal structure,digestive en-zyme activity and intestinal microbiota structure show adaptive changes.Especially,the feeding of compound feed helps maintain the stability of intestinal microorganisms.In conclusion,it is feasible to breed S.scherzeri with compound feed instead of live bait,but it is necessary to further optimize feed nutrient composition and feeding strategies to improve the growth rate of S.Scherzeri underfeeding with compound feed.
Key words:Sinipercascherzeri;compound feed;live bait;growth performance;digestive enzyme activity;intes-tine tissue;intestinal microorganisms
Foundation items:China Agriculture Research System(CARS-46);Basic Research Project of Fujian Public Wel-fare Research Institute(Mincaizhi〔2023〕600);Fujian Seed Industry Innovation and Industrialization Project(Minnong-zong〔2021〕5]
0引言
【研究意义】斑鳜(Siniperca scherzeri)俗称黑鳜、岩鳜、老虎鳜等,隶属于鲈形目(Perciformes)鳜属(Siniperca),为东亚特有种类,广泛分布在我国内陆水域,是一种典型的肉食性鱼类(Liu et al.,2017;田田,2023)。斑鳜肉质细嫩、味道鲜美,不含肌间刺,营养与药用价值高,深受广大消费者青睐(Yang et al.,2012),是目前我国养殖的三大鳜类之一。当前,斑鳜养殖仍以传统的鲜活饵料鱼为主,生产上存在诸多不利因素(李松林等,2021):(1)饵料鱼的养殖与运输条件要求较高,直接增加了养殖成本;(2)饵料鱼可能携带病原微生物,增加了养殖过程中的疾病传播风险;(3)饵料鱼养殖还会造成渔业资源浪费,影响养殖业的绿色转型(Wang et al.,2023)。因此,探索配合饲料替代饵料鱼对斑鳜生长性能、肠道功能结构和微生物群落的影响,对开展斑鳜规模化、标准化养殖及促进斑鳜养殖业可持续发展具有重要意义。【前人研究进展】相对于翘嘴鳜(S.chuatsi)而言,斑鳜更易驯化,但生长速度及养殖规模远不及翘嘴鳜(李传阳等,2016;李松林等,2021)。至今,国内外有关斑鳜驯化与养殖的研究报道较少,研究者更多关注鳜驯食技术、营养需求及饲料开发等。王贵英等(2005)研究指出,鳜配合饲料的最适蛋白含量为44.27%~48.41%;Li等(2017)研究发现,使用人工饲料投喂杂交鳜(S.chuatsi♀×S.scherzeri♂)的生长性能及消化道蛋白酶活性均显著低于饵料鱼;班赛男等(2020)、马林等(2023)研究表明,摄食配合饲料不会影响翘嘴鳜的生长性能,且其消化酶活性与饵料鱼组无显著差异;任萍等(2020)研究证实,鳜摄入糖后可促进糖原和脂肪的合成,转化为糖原和甘油三酯,但幼9cd2ae23c87df7e5f53b9ab4cb9f4b12鳜对葡萄糖的利用效率低于糊精。肠道是鱼类重要的消化吸收器官,其形态结构与食性、食物关系紧密,且相互适应(周景祥等,2001;高红云等,2021)。消化酶活性是衡量肉食性鱼类消化能力的重要指标之一(Fernández et al.,2001),其活性与饲料吸收、生理状态、肠道环境及其他因素有关(Buddington etal.,1997)。鱼类肠道微生物与宿主的种类、营养状况及生长环境等因素密切相关(Sullam etal.,2012;Miyake et al.,2015;Huanget al.,2020),在促进营养物质消化吸收、抵御疾病、促进生长等方面发挥重要作用(Dawood et al.,2016;陈俭等,2022;何琴等,2023),其中摄食的饵料组成是影响鱼类肠道菌群结构的主要因素之一(Sullam etal.,2012)。因此,开展鱼类肠道组织结构、消化酶活性及肠道菌群结构分析不仅有助于揭示鱼类食物变化与肠道组织结构、消化酶活性及微生物群落适应性之间的关系,还能综合评价养殖鱼类的健康水平。辛晴晴等(2022)以鲫的肠道组织结构、抗氧化性能和肠道菌群结构为指标,探究了鲫对饲料添加不同剂量柠檬黄色素的生理响应;魏孟申等(2024)以肠道组织结构、消化酶活性和微生物群落结构为指标,探究了大口黑鲈对慢性氨氮胁迫的适应性,并确立了大口黑鲈高密度养殖的氨氮安全阈值。在鳜养殖方面,曾萌冬等(2021)研究发现鳜对配合饲料的摄食量和利用率均低于饵料鱼,消化道组织结构及其消化酶活性也发生适应性变化;陈剑斌等(2023)研究证实摄食配合饲料的鳜在生长、饲料效率、胃肠功能、肌肉品质、抗氧化和非特异性免疫能力等方面更具优势,但加重了肝脏和肾脏的代谢负担。【本研究切入点】至今,有关斑鳜的研究主要集中在系统分类学、遗传学、繁殖生物学及营养评价等方面(蒲德永等,2013;田田等,2023;Wang et al.,2023),针对其饲料营养、消化组织结构、消化酶活性及肠道微生物结构特征等的研究较少。【拟解决的关键问题】比较分析配合饲料与饵料鱼对斑鳜生长、肠道功能结构及其微生物群落结构的影响,探明斑鳜摄食配合饲料后其生长与消化性能的变化规律,为开展以配合饲料替代传统饵料鱼的斑鳜规模养殖提供参考依据。
1材料与方法
1.1试验鱼养殖
试验用斑鳜亲本取自福建省闽江水系的野生群体,经人工繁育,获得斑鳜苗种(体质量10.8±0.5 g)。斑鳜苗种在直径2.0 m、高0.8 m的圆形移动养殖桶内养殖,养殖用水来源于周边的溪水,每3 d更换1/3养殖水体。试验过程采用纳米微孔曝气管曝气增氧,溶解氧含量保持在6.0 mg/L以上;养殖水体pH为6.5~7.6,水温控制在20.0~28.0℃。试验前,初孵稚鱼统一以团头鲂(Megalobramaamblycephala)水花苗种为活饵,养殖4周后,活饵组斑鳜鱼苗改用鲜活的鲮(Cirrhinusmolitorella)喂养直至试验结束;同时部分斑鳜采用饥饿→少量活饵鱼→活饵鱼+冰鲜饵料鱼→冰鲜饵料鱼→冰鲜饵料鱼+饲料→饲料的方式逐步驯化摄食人工配合饲料(表1),3周内完全转换到人工配合饲料。同步养殖3个月后,分别选取摄食活饵鱼和配合饲料的斑鳜鱼苗各180尾进行试验,每组设3个重复,每个重复60尾。各处理组每天饱食投喂3次(7:00、12:00和17:00),试验周期为20周。动物试验由福建省淡水水产研究所动物伦理委员会批准,批准号FFRIFJ-DW-2024-1。
1.2生长性能指标测定
饲养20周结束后,计算斑鳜存活率(SR);每处理组每个重复随机取10尾斑鳜,分别测量其体长、体质量和内脏总质量,然后计算增重率(WGR)、脏体比(VSI)及饲料系数(FC),具体公式如下:
式中,Mi表示试验结束时的斑鳜存活数量(尾);M0表示试验开始时的斑鳜数量(尾);Wi表示斑鳜终末体质量(g);W0表示斑鳜初始体质量(g);Wv表示试验结束时的斑鳜内脏总质量(g);Feed表示饲料投喂量(g)。
1.3消化酶活性测定
每处理组取6尾斑鳜,在冰上解剖分离出肠道前中段,剔除脂肪组织,以PBS(pH 7.4)冲洗,滤纸吸干后称重,并装入1.5 mL无菌冻存管中,液氮速冻保存。样品带回实验室后,取100 mg左右的组织样品,分别加入9倍量的PBS进行匀浆,匀浆液在4℃下5000×g离心15min,吸取上清液进行消化酶活性测定。胃蛋白酶、胰蛋白酶、脂肪酶和α-淀粉酶试剂盒购自南京建成生物工程研究所,参照各试剂盒使用说明,通过iMarker酶标仪(美国Thermo Fisher Scientific公司)测定各处理组斑鳜肠道样本的光密度(OD),再通过标准曲线计算肠道消化酶活性。
1.4肠道组织结构观察
每处理组取6尾斑鳜,在冰上解剖分离出肠道,剔除脂肪组织和黏连物,以Bouin’s固定24 h后用70%乙醇反复浸洗至无色;经石蜡包埋、切片(切片厚度4~5μm)及苏木精—伊红染色后,置于Leica 3000显微镜下观察拍照。
1.5基因组DNA提取和16S rRNA测序
每处理组取6尾斑鳜,取其肠道内容物100 mg,放入1.5 mL无菌冻存管中,利用液氮速冻将其打碎至粉末,取150~200 mg样品进行基因组DNA提取,DNA提取试剂盒为E.Z.N.A.®Stool DNA Kit(美国Omega Bio-Tek公司)。提取的DNA以1.2%琼脂糖凝胶电泳检测其纯度,然后利用NanoDrop Lite微量分光光度计测定其浓度。以适当稀释的DNA为模板,使用通用引物(338F:5'-ACTCCTACGGGAGG CAGCAG-3',806R:5'-GGACTACHVGGGTWTCTA AT-3')对16S rRNA序列V3~V4变异区进行PCR扩增(Liu et al.,2016)。PCR反应体系20.0μL:DNA模板15 ng,5×TransStartFastPfu缓冲液4.0μL,上、下游引物(5μmol/L)各0.8μL,TransStartFastPfu DNA聚合酶0.4μL,ddH2O补足至20.0μL。扩增程序:95℃预变性3 min;95℃30 s,55℃30 s,72℃30 s,进行27个循环;72℃延伸10min。PCR扩增产物经2.0%琼脂糖凝胶电泳后,通过凝胶回收试剂盒进行纯化回收,使用Qubit 4.0(美国ThermoFisherScien-tific公司)进行定量分析,同时将阳性扩增产物送至上海美吉生物医药科技有限公司构建测序文库,并通过Illumina PE300/PE250平台完成高通量测序。
1.6测序数据比对分析
获得的Raw reads使用FASTP v0.19.6进行质控(Chen et al.,2018),并以FLASH v1.2.11进行拼接(Magočand Salzberg,2011):(1)设置50 bp的过滤窗口,若窗口内Reads平均质量值低于20,则从窗口开始截去后端碱基,去除质控后长度在50bp以下或含N碱基的Reads;(2)根据PE Reads间的重叠关系,对Reads进行拼接,最小重叠长度设为10bp;(3)拼接序列重叠区允许的最大错配比率为0. 去除不合格的Reads;(4)根据Reads上的条形码和引物序列区分样本,条形码允许的错配数为0,最大引物错配数为2。使用UPARSE v7.1对质控拼接获得的Clean reads进行操作分类单元(OTU)聚类分析(Edgar,2013),相似度设为97%,去除嵌合体,并剔除包含叶绿体和线粒体基因的序列。为减少测序深度对后续Alpha和Beta多样性分析数据的影响,将所有样本序列数抽平至20000,抽平后单个样本的平均序列覆盖度均在99.0%以上。通过RDP Classi-fier(v 2.11)和SILVA数据库进行OTU物种分类学注释(Wang et al.,2007),置信度阈值设为70%,在不同物种分类水平下统计单个样本的群落组成;采用Mothur(http://www.mothur.org/wiki/Calculators)计算Alpha多样性指数(ACE、Chao1、Shannon和Simpson)(Schloss et al.,2009;Douglas et al.,2020),通过Wilxocon秩和检验进行Alpha多样性的组间差异分析;以基于Bray-Curtis距离算法的主坐标分析(PCoA)检验样本间微生物群落结构的相似性,并结合PERMANOVA非参数检验分析样本组间的微生物群落结构差异。
1.7统计分析
试验数据采用SPSS 22.0进行单因素方差分析(One-way ANOVA)和独立样本t检验,并以Graph-Pad Prism 8.0绘图。
2结果与分析
2.1不同饵料投喂模式对斑鳜生长性状的影响
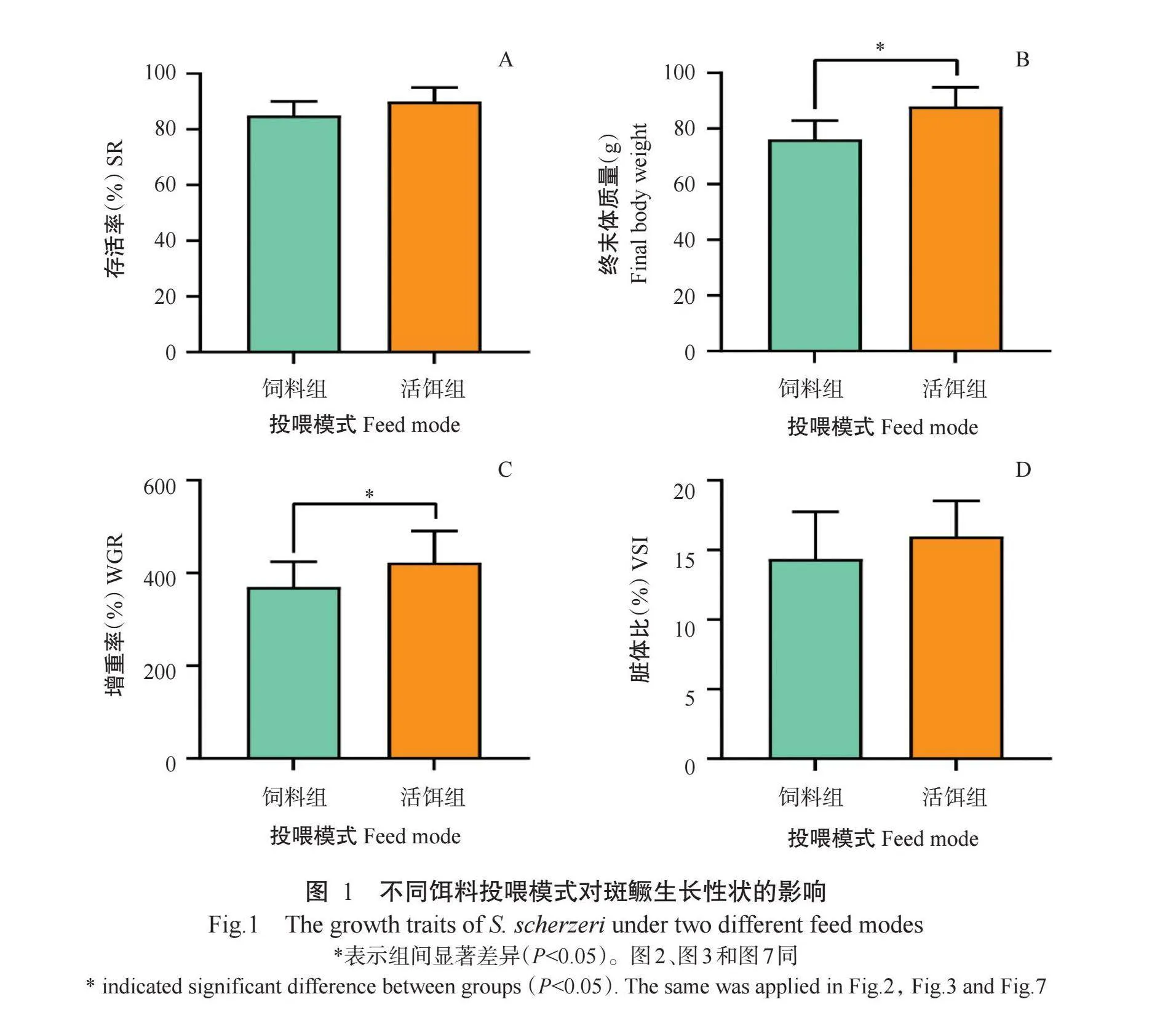
在不同饵料投喂模式下,活饵组、饲料组的斑鳜存活率分别为90%和85%(图1),组间差异不显著(P>0.05,下同);活饵组斑鳜的终末体质量、增重率较饲料组斑鳜分别显著提高15.5%和14.1%(P<0.05,下同),但在脏体比方面两处理组间无显著差异。
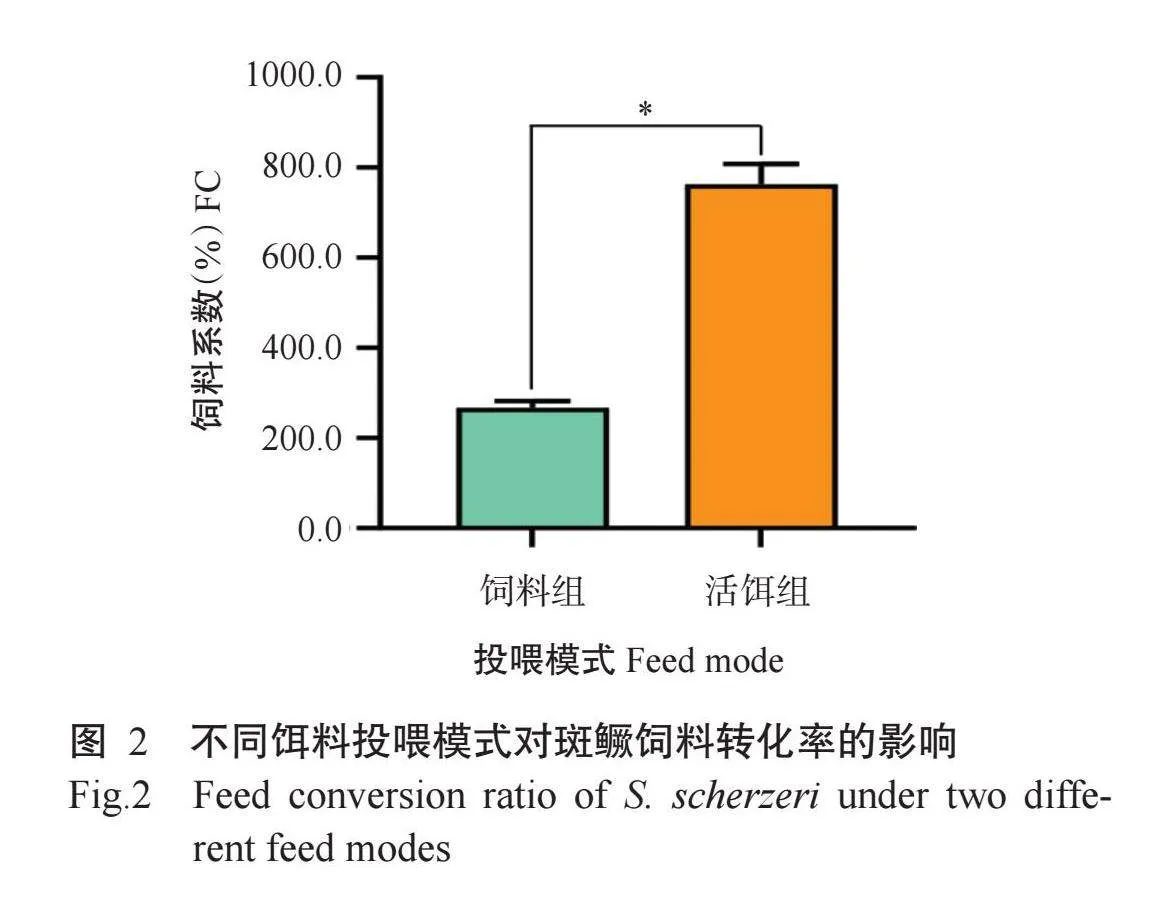
2.2不同饵料投喂模式对斑鳜饲料转化率和肠道消化酶活性的影响
由图2可看出,不同饵料投喂模式下斑鳜的饲料系数存在显著差异,活饵组斑鳜的饲料系数为763.3%,是饲料组(266.7%)的2.86倍,表明人工配合饲料投喂模式下斑鳜的饲料转化率更高。在斑鳜肠道消化酶活性方面,不同饵料投喂模式下斑鳜肠道的胰蛋白酶和α-淀粉酶活性存在显著差异(图3)。其中,活饵组斑鳜肠道胰蛋白酶活性为275.00 U/mg,是饲料组斑鳜(30.10 U/mg)的9.12倍;而活饵组斑鳜肠道α-淀粉酶活性(46.30 U/mg),仅为饲料组斑鳜(81.80 U/mg)的56.6%。此外,斑鳜肠道胃蛋白酶和脂肪酶活性受饵料投喂模式的影响均不显著。
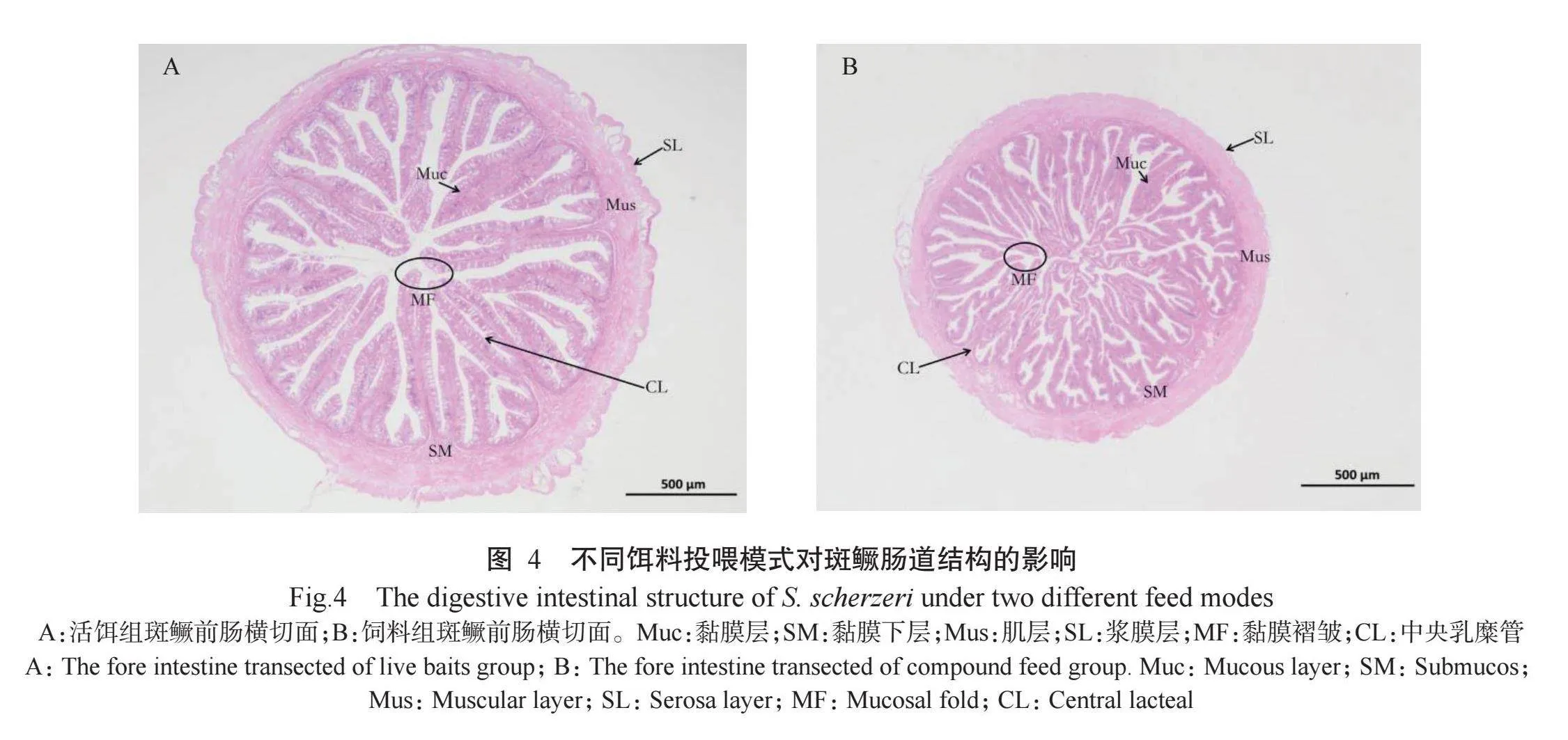
2.3不同饵料投喂模式下斑鳜肠道结构特征比较
斑鳜肠道结构由黏膜层、黏膜下层、肌层和浆膜层组成。由图4可看出,不同饵料投喂模式下,活饵组斑鳜肠道的肌层厚度(122.01±19.86μm)显著高于饲料组斑鳜(75.41±15.21μm),活饵组斑鳜的肠黏膜褶皱数(26.97±6.58个)与饲料组斑鳜(22.67±5.51个)无显著差异,但黏膜褶皱高度表现为活饵组斑鳜(969.52±32.65μm)显著高于饲料组斑鳜(598.03±127.63μm)。
2.4不同饵料模式下斑鳜肠道微生物群落多样性比较
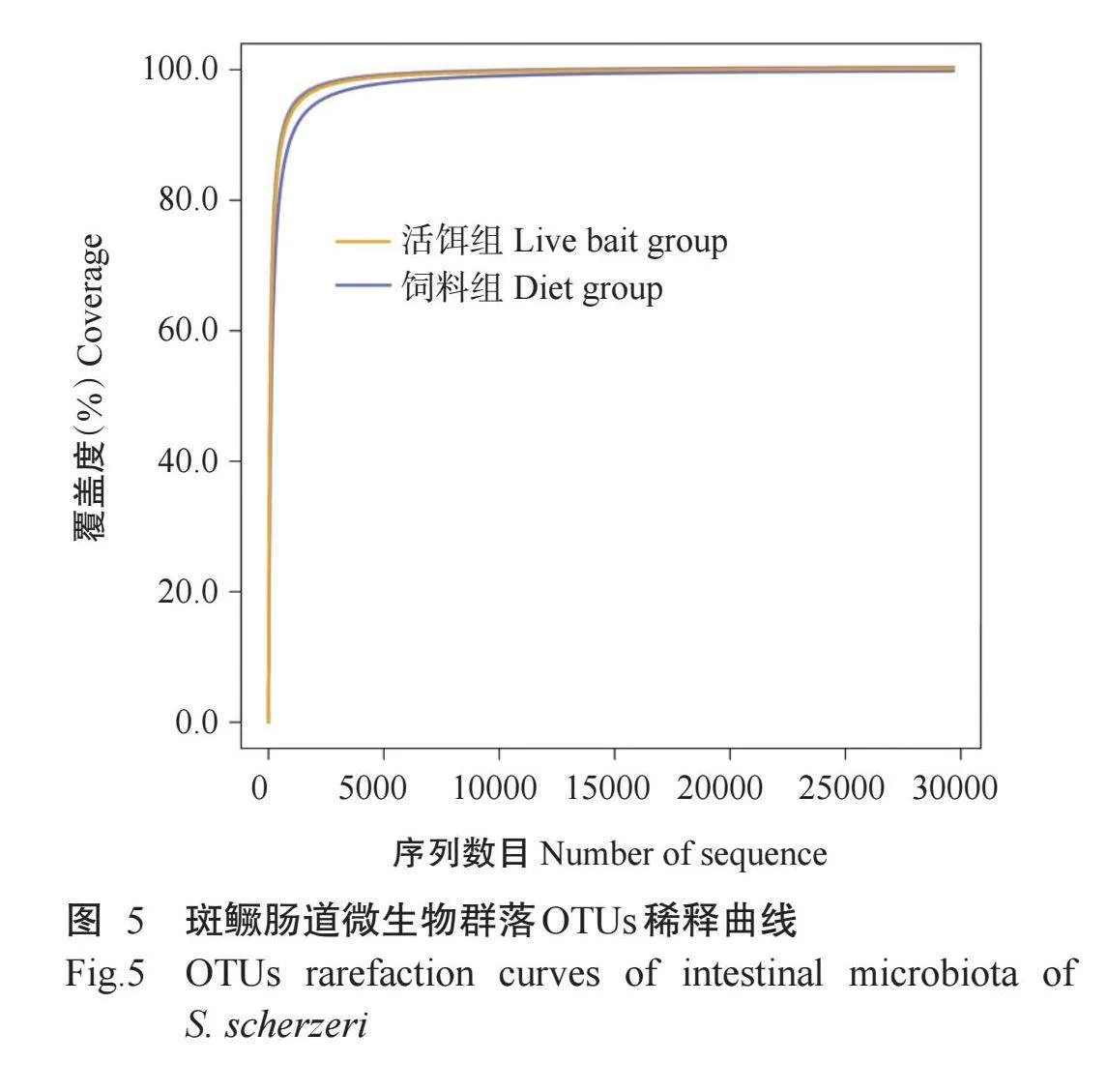
经质控处理后,活饵组和饲料组样本共获得910521条Clean reads,平均每个样本拥有75876条Clean reads,平均长度为423.51 bp。这些Clean reads注释到1629个OTUs,归属于22个门(Phyla)、46个纲(Classes)、109个目(Orders)、191个科(Families)、447个属(Genera)和642个种(Species)。稀释曲线(图5)显示,所有样本在20000条测序序列时均趋于平缓,样本覆盖度均超过99.0%,表明所有样本序列几乎全部被检出且达到饱和状态,测序结果能全面反映斑鳜肠道微生物群落结构组成及多样性的真实性,测序深度能反映每个样本中的微生物群落信息,可用于后续数据分析。
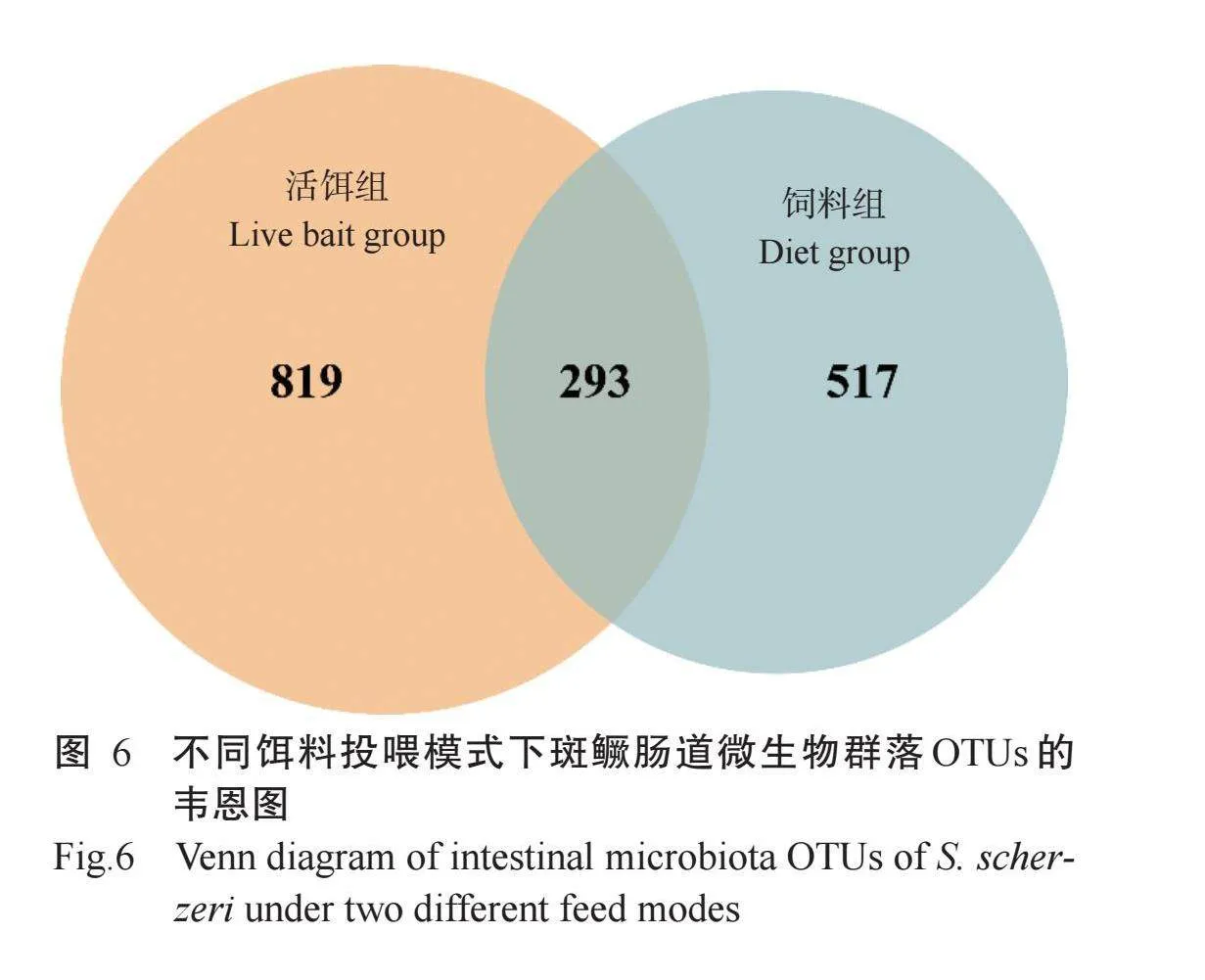
由图6可知,活饵组与饲料组斑鳜肠道微生物群落的OTUs数目分别为1112个和810个,其中共有OTUs数目为293个。为进一步了解活饵组与饲料组斑鳜肠道微生物的物种丰度及多样性,采用Mothur对OTUs进行Alpha多样性分析,结果(图7)显示,活饵组斑鳜肠道微生物群落的ACE指数和Chao1指数显著高于饲料组斑鳜肠道微生物群落,Shannon指数和Simpson指数也高于饲料组斑鳜肠道微生物群落,但差异不显著。
2.5不同饵料模式下斑鳜肠道微生物组成和相对丰度比较
Beta多样性主要反映不同样本间肠道微生物组成的相似性,PCoA分析结果(图8)显示,PCoA1贡献率为16.2%,PCoA2贡献率为11.9%。图中每个点的间距可反映个体间差异程度,两点间的距离越近表明相似性越高。在不同饵料投喂模式iV0o6YAlh06Y62bd9xYZzuV+KQVSPHM/cNnUDKOQigE=下,活饵组与饲料组斑鳜个体形成的圆圈有交集,且活饵组斑鳜个体较饲料组斑鳜个体有扩大趋势,即投喂活饵的斑鳜肠道微生物群落类型更复杂多变。
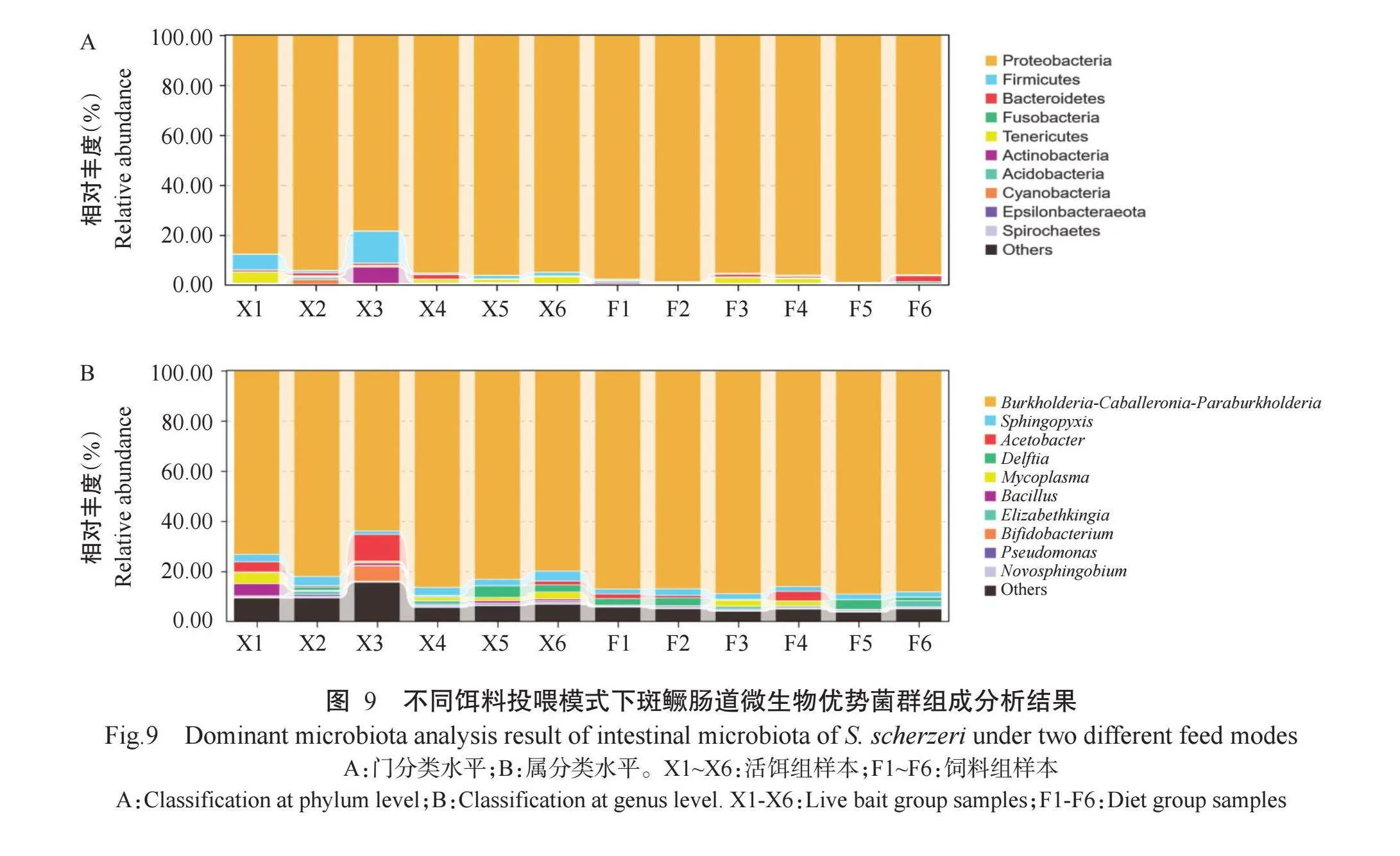
在门分类(图9-A)水平下,活饵组斑鳜肠道内相对丰度排名前10的优势菌群分别为变形菌门(Proteobacteria,91.12%)、厚壁菌门(Firmicutes,3.90%)、软壁菌门(Tenericutes,1.79%)、放线菌门(Actinobacteria,1.39%)、拟杆菌门(Bacteroidetes,0.89%)、蓝藻门(Cyanobacteria,0.47%)、酸杆菌门(Acidobacteria,0.32%)、弯曲菌门(Epsilonbacteraeota,0.03%)、梭杆菌门(Fusobacteria,0.02%)和螺旋体门(Spirochaetes,0.01%);饲料组斑鳜肠道内相对丰度排名前10的优势菌群依次为变形菌门(97.12%)、拟杆菌门(0.83%)、软壁菌门(0.72%)、放线菌门(0.37%)、厚壁菌门(0.34%)、酸杆菌门(0.18%)、梭杆菌门(0.18%)、螺旋体门(0.04%)、蓝藻门(0.02%)和弯曲菌门(0.03%)。此外,活饵组斑鳜肠道内的厚壁菌门、软壁菌门和放线菌门相对丰度显著高于饲料组斑鳜,而变形菌门相对丰度显著低于饲料组斑鳜。
在属分类(图9-B)水平下,活饵组斑鳜肠道内相对丰度排名前10的优势菌群分别为伯克霍尔德菌属(Burkholderia-Caballeronia-Paraburkholderia,78.14%)、鞘氨醇盒菌属(Sphingopyxis,2.98%)、醋酸杆菌属(Acetobacter,2.83%)、支原体属(Mycoplasma,1.79%)、戴尔福特菌(Delftia,1.60%)、芽孢杆菌属(Bacillus,1.30%)、双歧杆菌属(Bifidobacterium,1.02%)、假单胞菌属(Pseudomonas,0.52%)、鞘脂单胞菌属(Novosphingobium,0.50%)和伊丽莎白菌属(Elizabethkingia,0.47%);饲料组斑鳜肠道内相对丰度排名前10的优势菌群分别为伯克霍尔德菌属(87.76%)、鞘氨醇盒菌属(2.21%)、醋酸杆菌属(1.07%)、戴尔福特菌(1.90%)、支原体属(0.72%)、伊丽莎白菌属(0.72%)、假单胞菌属(0.46%)、鞘脂单胞菌属(0.42%)、芽孢杆菌属(0.01%)和双歧杆菌属(0.01%)。此外,活饵组斑鳜肠道内的伯克霍尔德菌属相对丰度显著低于饲料组斑鳜,而鞘氨醇盒菌属和支原体属相对丰度显著高于饲料组斑鳜。
3讨论
目前,我国超过90%的鳜养殖仍以活饵或冰冻杂鱼为食(Nirmal etal.,2022),与水产养殖的绿色可持续发展理念不符,亟待研发人工配合饲料替代活饵或冰冻杂鱼。为此,本研究设计并开发出专门针对斑鳜营养的配合饲料,其替代活饵的养殖结果表明,以配合饲料与活饵喂养斑鳜的存活率无显著差异,有效解决了以往饲料替代活饵养殖过程中斑鳜返口或闭口死亡的问题,对今后开展斑鳜的规模化和标准化养殖具有重要指导意义。饲料的适口性和营养水平对鱼类的生长发育也有重要影响,决定了水产养殖的成功与否(Li etal.,2013)。配合饲料的营养组分为粗蛋白占46.00%、粗脂肪占12.40%、粗灰分占15.90%、水分占8.00%,活饵的营养组分为粗蛋白占15.61%、粗脂肪占1.52%、粗灰分占5.45%、水分占76.20%。综合2种饵料投喂模式养殖的斑鳜存活率,可初步确定配合饲料具有替代活饵养殖斑鳜的可行性。
近年来,使用人工配合饲料替代活饵投喂鳜的生长跟踪研究已有相关报道。李燕等(2016)研究发现,投喂以石斑鱼粉料调配人工饲料的翘嘴鳜生长速率低于活饵组;Ding等(2022)研究表明,使用现有鳜商业饲料养殖的翘嘴鳜苗种生长速率低于活饵组。本研究中,饲料组斑鳜的增重率也显著低于活饵组,与李燕等(2016)、Ding等(2022)的研究结果基本一致。活饵养殖鳜的生长速度优于使用配制饲料,可能是由于鳜的生性以活饵为食,即使经人工驯化后可摄食人工配合饲料,但天生的偏好活饵特性使其面对活饵的摄食行为更积极(曾萌冬等,2024)。尽管活饵在促进鳜的生长方面表现良好,但也存在一些不利因素,如活饵组斑鳜个体的脏体比偏高,可能与活饵养殖斑鳜的内脏处于亚健康状态有关(陈剑斌等,2023)。
鱼类消化酶的活性与饲料吸收、生理状态、肠道环境及其他因素有关(Buddington etal.,1997;余友斌等,2023)。Zhu等(2014)研究报道,添加外源酶制剂可增强黄颡鱼等淡水鱼类的消化酶活性,改善饲料中干物质和粗蛋白的表观消化率,减少粪便中的蛋白含量,进而有效改善其生长性能。本研究结果表明,经过20周的饲喂试验后,饲料组斑鳜肠道胰蛋白酶活性显著低于活饵组斑鳜,可能与其特定生长率有关(李燕等,2016;曾萌冬等,2024)。“-淀粉酶活性与碳水化合物的利用有关(高梅等,2006)。饲料组斑鳜肠道“-淀粉酶活性显著高于活饵组斑鳜,表明配方饲料中的淀粉含量高于活饵,因此需要更多“-淀粉酶进行消化和吸收(曾萌冬等,2024)。一般而言,肉食性鱼类的肠道较短,杂食性鱼类的肠道较长,草食性鱼类则介于二者之间(曾端和叶元土,1998)。若摄食的饵料发生改变,鱼类的消化道结构也会随之发生适应性变化(欧红霞等,2020;陈剑斌等,2023;荣华等,2023;曾萌冬等,2024),因此肠道结构完整性是消化能力的重要保证。本研究通过对比活饵组与饲料组斑鳜肠道组织切片,发现2种饵料投喂模式下的斑鳜肠道结构均由黏膜层、黏膜下层、肌层和浆膜层组成,结构完整。活饵组斑鳜肠道的肌层厚度显著高于饲料组斑鳜,说明试验周期内斑鳜食性改变对其肠道节律性收缩运动已产生一定影响。此外,活鱼组斑鳜的肠黏膜褶皱相对排列整齐,且黏膜褶皱高度显著高于饲料组斑鳜,轴心中央乳糜管明显,表现出更强的消化吸收79D44uk9Zk10xKdIqvPhwQ==能力。
肠道微生物群落组成对鱼类营养物质的消化和吸收过程起重要作用,可生产短链脂肪酸和维生素等必需营养素,促进鱼类宿主的生长发育(陈秀梅等,2022;Cao et al.,2024);同时肠道微生物群落结构受饲料营养水平、养殖环境及宿主状态等因素的影响(Pelusio etal.,2020;Kim etal.,2021;Liu et al.,2023)。Alpha多样性是反映肠道微生物群落丰富度和均匀度的综合指标(Sullam et al.,2012;Huang et al.,2020),其中,ACE指数和Chao1指数主要表征鱼类肠道菌群物种的丰富度,Shannon指数和Simpson指数主要指示鱼类肠道微生物群落的丰度和均匀度。本研究中,虽然活饵组斑鳜肠道微生物群落的ACE指数和Chao1指数显著高于饲料组斑鳜肠道微生物群落,但Shannon指数和Simpson指数无显著差异,说明以活饵投喂斑鳜在肠道微生物群落均匀度上并无明显优势。Beta多样性又称为群落间多样性,主要用于比较群落间的物种差异性(Willis and Whittaker,2002)。本研究结果表明,在2种饵料投喂模式下的斑鳜肠道微生物群落类型存在一定差异,其中饲料组斑鳜个体聚集得更加紧密,说明饲料组斑鳜个体间的肠道微生物含量差异程度较小,肠道微生物群落结构更加趋于一致。
鱼类肠道微生物群落结构受食物种类、宿主及水环境等多个因素的影响(Sullam etal.,2012;Wong and Rawls et al.,2012;Bolnick et al.,2014;Miyake et al.,2015)。在淡水养殖鱼类肠道中,变形菌门、厚壁菌门和拟杆菌门等是肠道微生物群落结构中的常见优势菌群(马阿敏等,2021)。本研究结果表明,2种饵料投喂模式下斑鳜肠道微生物群落结构中变形菌门、厚壁菌门、软壁菌门和放线菌门的相对丰度之和均在98.00%以上。虽然不同的饵料投喂模式并未改变斑鳜肠道中优势菌门的类别,但在相对丰度方面存在明显差异,活饵组斑鳜肠道内的厚壁菌门、软壁菌门和放线菌门相对丰度显著高于饲料组斑鳜,而变形菌门相对丰度显著低于饲料组斑鳜,究其原因可能是活饵鱼体内的微生物群落对斑鳜产生了影响。在属分类水平上,饲料组斑鳜肠道内的伯克霍尔德菌属相对丰度显著高于活饵组斑鳜,而鞘氨醇盒菌属和支原体属相对丰度显著低于活饵组斑鳜。伯克霍尔德菌属作为变形菌门中参与糖类分解代谢的一类微生物,对宿主的健康和代谢具有促进作用(Zhang et al.,2024);支原体属则是软壁菌门中的条件致病菌,其较高的相对丰度对宿主免疫和疾病防控具有负面效应(McAuliffe et al.,2005)。综上所述,饲料组斑鳜肠道内的条件致病菌相对丰度较低,而有益微生物相对丰度较高,即饲料组斑鳜具有更好的自我调节功能,能有效缓解外界环境导致的应激反应,而有助于降低养殖过程中的致病风险。
4结论
以配合饲料替代活饵投喂斑鳜,其生长速度显著降低,肠道组织结构、消化酶活性及肠道微生物群落结构出现适应性变化,尤其是配合饲料的投喂有助于维持肠道微生物稳定性。可见,以配合饲料替代活饵养殖斑鳜具有可行性,但还需进一步优化饲料营养组分及投喂策略,提高配合饲料养殖下斑鳜的生长速率。
参考文献(References):
班赛男,朱传忠,杨新冬,陈伟军,李栋,夏冬梅,杨生灿,陈晶,孙云章,易敢峰.2020.摄食不同饵料对翘嘴鳜生长、体成分和消化酶活性的影响[J].淡水渔业,50(1):93-100.[Ban S N,Zhu C Z,Yang X D,Chen W J,Li D,Xia D M,Yang S C,Chen J,Sun Y Z,Yi G F.2020.Effect of different diet on the growth performance,body composi-tion and digestive enzymes active of mandarin fish(Siniperca chuatsi)[J].Freshwater Fisheries,50(1):93-100.]doi:10.3969/j.issn.1000-6907.2020.01.014.
陈俭,代冰涛,王红明,宋守钢,谭北平,章双.2022.饲料中添加β-葡聚糖对珍珠龙胆石斑鱼生长性能、免疫指标、转录组及肠道菌群的影响[J].南方农业学报,53(5):1434-1447.[Chen J,Dai B T,Wang H M,Song S G,Tan B P,Zhang S.2022.Effects of addingβ-glucan to feed on the growth performance,immune indexes,transcriptome andintestinal flora of Epinephelusfuscoguttatus♀×Epinephe-lus lanceolatus♂[J].Journal of Southern Agriculture,53(5):1434-1447.]doi:10.3969/j.issn.2095-1191.2022.05.026.
陈剑斌,于俊琦,徐杭忠,马俊康,刘天骥,李洪琴,刘匆,罗浩,李虹,翟旭亮,薛洋,罗莉.2023.配合饲料和饵料鱼对鳜生长、胃肠结构功能及肉质的影响[J].水产学报,47(10):82-96.[Chen J B,Yu J Q,Xu H Z,Ma J K,Liu T J,Li H Q,Liu C,Luo H,Li H,Zhai X L,Xue Y,Luo L.2023.Effects of compound feed and bait fish on growth,gastrointestinal structure and function and meat quality of Siniperca chuatsi[J].Journal of Fisheries of China,47(10):82-96.]doi:10.11964/jfc.20230113886.
陈秀梅,王桂芹,单晓枫,钱爱东.2022.鱼类肠道屏障损伤与肠道炎症发生发展关系的研究进展[J].河南农业科学,51(5):1-9.[Chen X M,Wang G Q,Shan X F,Qian A D.2022.Research progress on the relationship between intes-tinal barrier damage and intestinal inflammation develop-ment in fish[J].Journal of Henan Agricultural Sciences,51(5):1-9.]doi:10.15933/j.cnki.1004-3268.2022.05.001.
高梅,罗毅平,曹振东.2006.饲料碳水化合物对南方鲇(Silu-rus meridionalis Chen)幼鱼消化酶活性的影响[J].西南师范大学学报(自然科学版),31(2):119-123.[Gao M,Luo Y P,Cao Z D.2006.Effect of dietary carbohydrate on digestive enzyme activities in southern catfish(Silurus meridionalis Chen)juveniles[J].Journal of Southwest China Normal University(Natural Science),31(2):119-123.]doi:10.3969/j.issn.1000-5471.2006.02.028.
高云红,景琦琦,黄滨,关长涛,张佳伟,李文升,翟介明,贾玉东.2021.云龙石斑鱼胃排空特征和摄食消化特性研究[J].渔业科学进展,42(1):92-99.[Gao Y H,Jing Q Q,Huang B,Guan C T,Zhang J W,Li W S,Zhai J M,Jia Y D.2021.Characteristics of gastric evacuation and feeding digestion in“Yunlong”groupers(Epinephelus moara♀×E.lanceolatus♂)[J].Progress in Fishery Sciences,42(1):92-99.]doi:10.19663/j.issn2095-9869.20191216001.
何琴,王利,段荟芹,苟小兰.2023.枯草芽孢杆菌和粪肠球菌对鲫鱼生长性能、血清学指标和肠道微生物多样性的影响[J].江苏农业学报,39(1):142-147.[He Q,Wang L,Duan H Q,Gou X L.2023.Effects of Bacillus subtilis and Enterococcus faecalis on growth performance,serum bio-chemical indices and intestinal microflora of Carassius auratus[J].Jiangsu Journal of Agricultural Sciences,39(1):142-147.]doi:10.3969/j.issn.1000-4440.2023.01.017.
李传阳,许淼洋,THAMMRATSUNTORN Jeerawat,赵金良,钱叶洲,吴超,钱德.2016.3种鳜鱼生长与摄食量、胃蛋白酶活性和胃蛋白酶原基因表达相关分析[J].上海海洋大学学报,25(1):1-7.[Li C Y,Xu M Y,Thammratsun-torn J,Zhao J L,Qian Y Z,Wu C,Qian D.2016.Compari-son of growth,food intake,pepsin activity and pepsinogen genes expression among Siniperca species[J].Journal of Shanghai Ocean University,25(1):1-7.]
李松林,韩志豪,王小源,陈乃松.2021.鳜养殖概况及摄食调控机制研究进展[J].水产学报,45(10):1787-1795.[Li S L,Han Z H,Wang X Y,Chen N S.2021.Research prog-ress on aquaculture and feeding regulation mechanism of Mandarin fish[J].Journal of Fisheries of China,45(10):1787-1795.]doi:10.11964/jfc.20200812371.
李燕,李永强,李建忠,骆志强,施顺昌,陆锦天.2016.配合饲料完全替代鲜活饵料对翘嘴鳜生长、体成分及消化能力的影响[J].水产科技情报,43(3):164-168.[Li Y,Li YQ,Li J Z,Luo Z Q,Shi S C,Lu J T.2016.Effects of com-plete replacement of live feed with compound feed on growth,body composition and digestive ability of Siniperca chuatsi[J].Fisheries Science&Technology Information,43(3):164-168.]doi:10.16446/j.cnki.1001-1994.2016.03.012.
马阿敏,李娜,覃虹焱,王子悦,姚曲.2021.淡水鱼类肠道微生物菌群研究进展[J].甘肃畜牧兽医,51(5):9-13.[Ma A M,Li N,Qin H Y,Wang Z Y,Yao Q.2021.Research progress of intestinal microbiota of freshwater fish[J].Gansu Animal and Veterinary Sciences,51(5):9-13.]doi:10.3969/j.issn.1006-799X.2021.05.003.
马林,李明泽,毕相东,逯云召,薄其康,刘克明,王胜利,尤宏争.2023.摄食不同饵料对翘嘴鳜生长性能、肌肉营养成分及消化酶活性的影响[J].饲料研究,46(6):44-49.[Ma L,Li M Z,Bi X D,Lu Y Z,Bo Q K,Liu K M,Wang S L,You H Z.2023.Effect of different diets on growth per-formance,muscle nutrient composition and digestive enzyme activity of Sinipercachuatsi[J].Feed Resrarch,46(6):44-49.]doi:10.13557/j.cnki.issn 1002-2813.2023.06.010.
欧红霞,王广军,李志斐,余德光,龚望宝.2020.不同饲料对大口黑鲈肠道组织结构的影响[J].水产科学,39(6):902-907.[Ou H X,Wang G J,Li Z F,Yu D G,Gong W B.2020.Influence of different diets on intestinal histological morphologic structure of largemouth bass Micropterus salmoides[J].Fisheries Science,39(6):902-907.]doi:10.16378/j.cnki.1003-1111.2020.06.015.
蒲德永,黄小琪,魏刚.2013.大眼鳜和斑鳜消化道组织结构的比较研究[J].淡水渔业,43(2):26-31.[Pu D Y,Huang X Q,Wei G.2013.Histological studies and comparison on the digestive tract in Siniperca kneri and Siniperca scher-zeri[J].Freshwater Fisheries,43(2):26-31.]doi:10.3969/j.issn.1000-6907.2013.02.005.
任萍,梁旭方,方刘,何珊,肖倩倩,史登勇.2020.鳜对葡萄糖和糊精利用差异比较研究[J].水生生物学报,44(2):364-371.[Ren P,Liang X F,Fang L,He S,Xiao Q Q,Shi D Y.2020.Comparative study of the difference in glucose and dextrin utilization in the Chinese perch(Siniperca chuatsi)[J].Acta Hydrobiologica Sinica,44(2):364-371.]doi:10.7541/2020.044.
荣华,张雷,王晓雯,武祥伟,王金浩,胡青,毕保良,孔令富,豆腾飞.2023.四种不同食性鱼类的消化酶活性及肠道组织形态学比较研究[J].淡水渔业,53(2):29-35.[Rong H,Zhang L,Wang X W,Wu X W,Wang J H,Hu Q,Bi B L,Kong L F,Dou T F.2023.Comparative study on diges-tive enzyme activity and intestinal tissue morphology of four fishes with different feeding habits[J].Freshwater Fisheries,53(2):29-35.]doi:10.3969/j.issn.1000-6907.2023.02.004.
田田,张风光,王茂元,黄洪贵,秦志清,赖铭勇,刘银华,黄柳婷,吴妹英.2023.2月龄斑鳜形态性状与体质量的相关性研究[J].河南农业科学,52(8):126-134.[Tian T,Zhang F G,Wang M Y,Huang H G,Qin Z Q,Lai M Y,Liu Y H,Huang L T,Wu M Y.2023.Correlation between morpho-logical traits and body weight of 2-month-old Sinipercascherzeri[J].Journal of Henan Agricultural Sciences,52(8):126-134.]doi:10.15933/j.cnki.1004-3268.2023.08.014.
田田.2023.人工养殖斑鳜(Siniperca scherzeri)形态性状与体质量的相关性研究[J].水产学杂志,36(3):68-74.[Tian T.2023.Correlation analysis of morphological traits and body weight of spotted mandarin fish(Siniperca scherzeri)under artificial cultivation[J].Chinese Journal of Fisheries,36(3):68-74.]doi:10.3969/j.issn.1005-3832.2023.03.010.
王贵英,曾可为,高银爱,李清,夏儒龙.2005.鳜配合饲料的最适蛋白质含量[J].水生生物学报,29(2):189-192.[Wang G Y,Zeng K W,Gao YA,Li Q,Xia R L.2005.The opti‐mum dietary protein level for Siniperca chuatsi[J].Acta Hydrobiologica Sinica,29(2):189-192.]doi:10.3321/j.issn:1000-3207.2005.02.015.
魏孟申,郑涛,路思琪,强俊,陶易凡,李岩,徐跑.2024.氨氮胁迫对大口黑鲈幼鱼组织结构、酶活及肠道微生物的影响[J].水生生物学报,48(1):10-22.[Wei M S,Zheng T,Lu S Q,Qiang J,TaoY F,Li Y,Xu P.2024.Ammonia-N stress on tissue structure,enzyme activity and intestinal microbiota of Macropterussalmoides[J].Acta Hydrobio‐logica Sinica,48(1):10-22.]doi:10.7541/2023.2023.0054.
辛晴晴,吕茜茜,吴利敏,田雪,马文阁,李学军.2022.饲料中添加柠檬黄对鲫肝、肠组织结构、抗氧化指标及肠道菌群的影响[J].水产学报,46(10):1902-1911.[Xin Q Q,LüX X,Wu L M,Tian X,Ma W G,Li X J.2022.Effects of tartrazine consumption on liver and intestine structure,antioxidant indices and intestinal microbiota in crucian carp(Carassius auratus)[J].Journal of Fisheries of China,46(10):1902-1911.f118380060750d93dab76311e2b533a57fec905cc06e951600e232235c55fced]doi:10.11964/jfc.20220313401.
余友斌,黄温赟,崔铭超.2023.养殖密度对大黄鱼生长、血清生化、营养成分、消化酶和代谢酶活力的影响[J].渔业现代化,50(3):64-71.[Yu Y B,Huang W Y,Cui M C.2023.Effects of stocking densities on growth performance,nutrient composition,serum biochemical,digestive and metabolic enzymes activities of large yellow croaker(Lar-imichthyscrocea)[J].Fishery Modernization,50(3):64-71.]doi:10.3969/j.issn.1007-9580.2023.03.008.
曾端,叶元土.1998.鱼类食性与消化系统结构的研究[J].西南农业大学学报,20(4):81-84.[Zeng D,Ye Y T.1998.Studies on digestive system and different feeding habits of some fishes in freshwater[J].Journal of Southwest Agri‐cultural University,20(4):81-84.]doi:10.13718/j.cnki.xdzk.1998.04.017.
曾萌冬,马晨夕,赵亮亮,赵金良.2024.活饵与饲料投喂对幼鳜肠肽酶活力及小肽转运、吸收的影响[J].水生生物学报,48(1):53-62.[Zeng M D,Ma C X,Zhao L L,Zhao J L.2024.Feeding live bait and feed on the peptidase acti-vity,transport and absorption of small peptides in juvenile mandarin fish[J].Acta Hydrobiologica Sinica,48(1):53-62.]doi:10.7541/2023.2021.0108.
曾萌冬,徐俊,宋银都,赵金良.2021.配合饲料替代活饵对鳜生长性能、消化功能及小肽转运载体基因表达的影响[J].南方农业学报,52(1):228-237.[Zeng M D,Xu J,Song Y D,Zhao J L.2021.Effects of replacing live bait with compound feed on growth,digestion and expression of small peptide transporter(PepT1)gene of Siniperca chuatsi[J].Journal of Southern Agriculture,52(1):228-237.]doi:10.3969/j.issn.2095-1191.2021.01.028.
周景祥,余涛,黄权,李月红.2001.鲤鱼、黄颡鱼和大眼鰤鲈消化酶活性的比较研究[J].吉林农业大学学报,23(1):94-96.[Zhou J X,Yu T,Huang Q,Li Y H.2001.Compari‐son studies on the activities of the digestive enzymes of common carp,Huangsang cat-fish and walleye[J].Journal of Jilin Agricultural University,23(1):94-96.]doi:10.13327/j.jjlau.2001.01.026.
Bolnick D I,Snowberg L K,Hirsch P E,Lauber C L,Knight R,Gregory Caporaso J,Svanbäck.2014.Individualsʼdiet diversity influences gut microbial diversity in two fresh-water fish(threespine stickleback and Eurasian perch)[J].Ecology Letter,17(8):979-987.doi:10.1111/ele.12301.
Buddington R K,Krogdahl A,Bakke-McKellep A M.1997.The intestines of carnivorous fish:Structure and functions and the relations with diet[J].Acta Physiologica Scandi‐navica,638:67-80.
Cao S W,Dicksved J,Lundh T,Vidakovic A,Norouzitallab P,Huyben D.2024.A meta-analysis revealing the technical,environmental,and host-associated factors that shape the gut microbiota of Atlantic salmon and rainbow trout[J].Reviews in Aquaculture,16(4):1603-1620.doi:10.1111/raq.12913.
Chen S F,Zhou Y Q,Chen Y R,Jia G.2018.Fastp:An ultra-fast all-in-one FASTQ preprocessor[J].Bioinformatics,34(17):i884-i890.doi:10.1093/bioinformatics/bty560.
Dawood M A O,Koshio S,Ishikawa M,Yokoyama S E,El Basuini M F,Hossain M S,Nhu T H,Dossou S,Moss A S.2016.Effects of dietary supplementation of Lactobacillus rhamnosus or/and Lactococcus lactis on the growth,gut microbiota and immune responses of red sea bream,Pagrus major[J].Fish&Shellfish Immunology,49:275-285.doi:10.1016/j.fsi.2015.12.047.
Ding LY,Zhang Y P,Chen J C,Chen W J,Xie S Q,Chen Q T.2022.Growth,muscle nutrition composition,and digestive enzyme activities of the juvenile and adult Siniperca chuatsi fed on live baits and a formulated diet[J].Fishes,7(6):379.doi:10.3390/fishes7060379.
Douglas G M,Maffei V J,Zaneveld J R,Yurgel S N,Brown J R,Taylor C M,Huttenhower C,Langille M G I.2020.PIC‐RUSt2 for prediction of metagenome functions[J].Nature Biotechnology,38(6):685-688.doi:10.1038/s41587-020-0548-6.
Edgar R C.2013.UPARSE:Highly accurate OTU sequences from microbial amplicon reads[J].Nature Methods,10:996-998.doi:10.1038/nmeth.2604.
Fernández I,Moyano F J,Díaz M,Martinez T.2001.Characteri-zation ofα-amylase activity in five species of Mediterra‐nean sparid fishes(Sparidae,Teleostei)[J].Journal of Ex-perimental Marine Biology and Ecology,262(1):1-12.doi:10.1016/S0022-0981(01)00228-3.
Huang Q,Sham R C,Deng Y,Mao Y P,Wang C X,Zhang T,Leung K M Y.2020.Diversity of gut microbiomes in marine fishes is shaped by host-related factors[J].Molecu‐lar Ecology,29(24):5019-5034.doi:10.1111/mec.15699.
Kim P S,Shin N R,Lee J B,Kim M S,Whon T W,Hyun D W,Yun J H,Jung M J,Kim J Y,Bae J W.2021.Host habitat is the major determinant of the gut microbiome of fish[J].Microbiome,9(1):166.doi:10.21203/rs.3.rs-332643/v 1.
Li W,Zhang T,Ye S W,Liu J S,Li Z J.2013.Feeding habits and predator-prey size relationships of mandarin fish Siniperca chuatsi(Basilewsky)in a shaqTCeAzrtYAuQLu0F33alTM12OQINfjL4GQzqSzO55lM=llow lake,central China[J].Journal of Applied Ichthyology,29(1):56-63.doi:10.1111/j.1439-0426.2012.02044.x.
Li Y,Li J Z,Lu J T,Li Z,Shi S C,Liu Z J.2017.Effects of live and artificial feeds on the growth,digestion,immunity and intestinal microflora of mandarin fish hybrid(Sini-percachuatsi♀×Siniperca scherzeri♂)[J].Aquaculture Research,48(8):4479-4485.doi:10.1111/are.13273.
Liu C S,Zhao D F,Ma W J,Guo Y D,Wang A J,Wang Q L,Lee D J.2016.Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp.[J].Applied Microbiology and Biotechnology,100(3):1421-1426.doi:10.1007/s00253-015-7039-6.
Liu LW,Liang X F,Fang J G.2017.The optimal stocking den‐sity for hybrid of Sinipercachuatsi(♀)×Sinipercascher-zeri(♂)mandarin fish fed minced prey fish[J].Aquacul‐ture Research,48(3):1342-1345.doi:10.1111/are.12892.
Liu M K,Li Q Y,Tan L T,Wang L P,Wu F C,Li L,Zhang G F.2023.Host-microbiota interactions play a crucial role in oyster adaptation to rising seawater temperature in summer[J].Environmental Research,216(2):114585.doi:10.1016/j.envres.2022.114585.
MagočT,Salzberg S L.2011.FLASH:Fast length adjustment of short reads to improve genome assemblies[J].Bioinfor‐matics,27(21):2957-2963.doi:10.1093/bioinformatics/btr507.
McAuliffe L,Ellis R J,Lawes J R,Ayling R D,Nicholas RA J.2005.16S rDNA PCR and denaturing gradient gel electro‐phoresis:A single generic test for detecting and differentia-ting Mycoplasma species[J].Journal of Medical Microbio-logy,54(8):731.doi:10.1099/jmm.0.46058-0.
Miyake S,Ngugi D K,Stingl U.2015.Diet strongly influences the gut microbiota of surgeon fishes[J].Molecular Eco-logy,24(3):656-672.doi:10.1111/mec.13050.
Nirmal N P,Santivarangkna C,Benjakul S,Maqsood S.2022.Fish protein hydrolysates as a health-promoting ingre-dient—Recent update[J].Nutrition Reviews,80(5):1013-1026.doi:10.1093/nutrit/nuab065.
Pelusio N F,Rossi B,Parma L,Volpe E,Ciulli S,Piva A,D'Amico F,Scicchitano D,Candela M,Gatta P P,Bonaldo A,Grilli E.2020.Effects of increasing dietary level of organic acids and nature-identical compounds on growth,intestinal cytokine gene expression and gut microbiota of rainbow trout(Oncorhynchus mykiss)reared at normal and high temperature[J].Fish&Shellfish Immunology,107:324-335.doi:10.1016/j.fsi.2020.10.021.
Schloss P D,Westcott S L,Ryabin T,Hall J R,Hartmann M,Hollister E M,Lesniewsk R A,Oakley B B,Parks A H,Robinson C J,Sahl J W,Stres B,Thallinger G G,van Horn D J,Weber C F.2009.Introducing mothur:Open-source,platform-independent,community-supported soft‐ware for describing and comparing microbial communities[J].Applied and Environmental Microbiology,75(23):7537-7541.doi:10.1128/AEM.01541-09.
Sullam K E,Essinger S D,Lozupone C A,O'Connor M P,Rosen G L,Knight R,Kilham S S,Russell JA.2012.Envi‐ronmental and ecological factors that shape the gut bacte‐rial communities of fish:A meta-analysis[J].Molecular Ecology,21(13):3363-3378.doi:10.1111/j.1365-294X.2012.05552.x.
Wang M Y,Lai1 M Y,Tian T,Wu M Y,Liu Y H,Liang P,Huang L T,Qin Z Q,Ye X J,Xiao W,Huang H G.2023.Comparison of growth performance and muscle nutrition levels of juvenile Siniperca scherzeri fed on an iced trash fish diet and a formulated diet[J].Fishes,8(8):393.doi:10.3390/fishes8080393.
Wang Q,Garrity G M,Tiedje J M,Cole J R.2007.Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy[J].Applied and Environ‐mental Microbiology,73(16):5261-5267.doi:10.1128/AEM.00062-07.
Willis K J,Whittaker R J.2002.Ecology.Species diversity—Scale matters[J].Science,295(5558):1245-1248.doi:10.1126/science.1067335.
Wong S,Rawls J F.2012.Intestinal microbiota composition in fishes is influenced by host ecology and environment[J].Molecular Ecology,21(3):3100-3102.doi:10.1111/j.1365-294X.2012.05646.x.
Yang M,Liang X F,Tian C X,Gul Y,Dou Y Q,Cao L,Yu R.2012.Isolation and characterization of fifteen novel micro‐satellite loci in golden mandarin fish(Siniperca scherzeri)steindachne[J].Conservation Genetic Resources,4(3):599-601.doi:10.1007/s 12686-012-9601-1.
Zhang Q Y,Cai Y Z,Zhang L P,Lu M,Yang LY,Wang D K,Jia Q J.2024.The accumulation of active ingredients of Polygonatumcyrtonema Hua is associated with soil charac‐teristics and bacterial community[J].Frontiers in Microbio-logy,15:1347204.doi:10.3389/fmicb.2024.1347204.
Zhu Y,Qing X,Ding Q L,Duan M M,Wang C F.2014.Com‐bined effects of dietary phytase and organic acid on growth and phosphorus utilization of juvenile yellow catfish Pel-teobagrusfulvidraco[J].Aquaculture,430:1-8.doi:10.1016/j.aquaculture.2014.03.023.
(责任编辑兰宗宝)

