克氏原螯虾duox1基因抵御金黄色葡萄球菌侵染的先天免疫机制


摘要:【目的】探究双氧化酶1基因(duox1)在克氏原螯虾抵抗金黄色葡萄球菌(Staphylococcus aureus)侵染先天免疫应答中的作用机制,为确保克氏原螯虾产业的持续健康发展提供技术支撑。【方法】采用实时荧光定量PCR检测金黄色葡萄球菌刺激后,duox1基因在克氏原螯虾血细胞、肝胰腺、肠道及鳃组织中的表达情况;通过RNA干扰(RNAi)敲低duox1基因表达再进行金黄色葡萄球菌刺激,统计克氏原螯虾存活率,采用H2O2含量检测试剂盒检测肝胰腺H2O2含量,电子显微镜下观察血淋巴黑化现象,并以实时荧光定量PCR检测肝胰腺中抗菌肽基因(toll1、dorsal、crustin3和crustin4)的表达情况。【结果】经金黄色葡萄球菌刺激后,克氏原螯虾duox1基因在血细胞、肝胰腺、肠道及鳃组织中的相对表达量较PBS组整体上呈上升趋势,故推测duox1基因参与克氏原螯虾的抗菌先天免疫应答,具有潜在的抵抗细菌侵染作用。与dsGFP+金黄色葡萄球菌组相比,经RNA干扰及金黄色葡萄球菌刺激后,克氏原螯虾存活率呈明显下降趋势,肝胰腺H2O2含量呈先降低后回升的变化趋势(在刺激后24 h达最低值),且克氏原螯虾血淋巴黑化反应程度明显减弱。干扰duox1基因表达并感染金黄色葡萄球菌后,克氏原螯虾肝胰腺Toll信号通路上的抗菌肽基因(toll1、dorsal、crustin3和crustin4)表达被抑制,导致参与抗菌反应的先天免疫能力下降,最终引起克氏原螯虾存活率下降。【结论】克氏原螯虾duox1基因通过调控H2O2产生、影响血淋巴黑化现象及调控toll1、dorsal、crustin3和crustin4等抗菌肽基因的表达,参与机体的先天免疫应答,进而协助机体抵御金黄色葡萄球菌的侵染。
关键词:克氏原螯虾;双氧化酶1基因(duox1);金黄色葡萄球菌;RNA干扰;抗菌肽基因
中图分类号:S945.49文献标志码:A文章编号:2095-1191(2024)08-2485-10
Innate immune mechanism of duox1 gene in Procambarus clarkii against Staphylococcus aureus infection
LIU Shu-yao LI Qian-qian WEN Jing JIN Bo-yang ZHANG Ming-da TAN Ming-yue SHEN Xiu-li DU Zhi-qiang1*
(1School of Life Science and Technology,Inner Mongolia University of Science and Technology,Baotou,InnerMongolia 014010,China;2Library of Inner Mongolia University of Science and Technology,Baotou,Inner Mongolia 014010,China)
Abstract:【Objective】The study aimed to elucidate the role and mechanism of double oxidase 1 gene(duox1)in the innate immune response of Procambarus clarkii against Staphylococcus aureus infection,providing technical support for the sustainable and healthy development of P.clarkii industry.【Method】The real-time fluorescence quantitative PCR was used to detect the relative expression of duox 1 gene in hemocytes,hepatopancreas,intestine and gill tissues of P.clarkii after stimulation of S.aureus.By using RNA interference(RNAi)to down-regulate the expression of the duox1 gene and subsequently stimulating with S.aureus,the survival rate of the P.clarkii was determined.The contents of H2O2 in the he-patopancreas were measured with an H2O2 detection kit.Melanization in the hemolymph was observed under an electronmicroscope.The expression of antimicrobial peptide genes(toll dorsal,crustin3 and crustin4)in the hepatopancreas was assessed using real-time fluorescence quantitative PCR.【Result】After stimulation by S.aureus,the relative expres-sion of the duox1 gene in hemocytes,hepatopancreas,intestine and gill tissues of P.clarkii showed an overall upwaHpfc30FFHSigBKZjUR0tzw==rd trend compared to the PBS group.This suggested that the duox1 gene may be involved in the innate immune response of P.clarkii against bacterial infection,with a potential role in combating bacterial invasion.Compared to the dsGFP+S.au-reus group,after RNA interference and S.aureus stimulation,the survival rate of P.clarkii greatly decreased.The H2 O2 content in the hepatopancreas showed a trend of initial reduction followed by recovery(reaching its lowest point 24 h after stimulation),and the degree of hemolymph melanization was markedly weakened.After duox1 gene expression was inter-fered and S.aureus infection,the expression of antimicrobial peptide genes(toll dorsal,crustin3 and crustin4)in the Toll signaling pathway of the hepatopancreas was suppressed,leading to a reduction in innate immune capacity against bacterial infection,ultimately resulting in a decreased survival rate of P.clarkii.【Conclusion】The duox1 gene ofP.clarkii participates in the innate immune response of the body by regulating H2O2 production,affecting themelanization of hemo-lymph,and regulating the expression of antimicrobial peptide genes such as toll dorsal,crustin3 and crustin4,thereby assisting the body in resisting infection by S.aureus.
Key words:Procambarus clarkii;double oxidase 1 gene(duox1);Staphylococcus aureus;RNA interference;anti-bacterial peptide genes
Foundation items:National Natural Science Foundation of China(32060834);Inner Mongolia Natural Science Foundation(2024MS03051);Basic Research Fund of Inner Mongolia University of Science and Technology(〔2022〕028)
0引言
【研究意义】克氏原螯虾(Procambarus clarkii)又称红沼泽小龙虾,隶属于节肢动物门(Arthropoda)十足目(Decapoda)拟螯虾科(Parastacidae),原产于墨西哥北部和美国南部,现已发展成为我国淡水养殖的主要经济动物(陈卫军,2019)。近年来受病害频发等因素的影响,克氏原螯虾死亡率呈逐年上升趋势,已影响到我国淡水养殖市场的健康发展(孟思妤等,2017)。金黄色葡萄球菌(Staphylococ-cus aureus)作为常见的致病菌,可诱发虾蟹等甲壳类生物发生多种细菌性疾病(Vasta and Wang,2020;Cheung et al.,2021),但目前尚缺乏有效的防治手段,给水产养殖市场带来巨大经济损失。克氏原螯虾感染细菌后不仅导致其养殖减产,在食品安全方面还会威胁人类健康(兰培利等,2024)。因此,亟待明确克氏原螯虾的先天免疫应答机制,为制定其病害防控策略提供参考依据。【前人研究进展】克氏原螯虾缺乏特异性免疫,只能通过先天免疫应答而发挥免疫抵抗作用,主要包括体液免疫和细胞免疫2种形式(Li etal.,2020)。血淋巴作为克氏原螯虾的主要免疫组织之一,同时在体液免疫和细胞免疫中发挥作用(Qin et al.,2019)。其中,血淋巴氧化性杀灭机制会产生大量活性氧(Reactive oxygen spe-cies,ROS)(张利庆,2007),而ROS作为一种重要免疫效应物,在无脊椎动物抵抗病原菌侵染时发挥免疫作用或作为第二信使激活下游相关信号通路(Zhang et al.,2022)。烟酰胺腺嘌呤二核苷酸磷酸氧化酶(Nicotinamide adenine dinucleotide phosphate oxidase,NOX)家族包括7种异构体(NOX1、NOX2、NOX3、NOX4、NOX5、DUOX1和DUOX2)(Nocella et al.,2023),其主要生理功能包括宿主防御、蛋白翻译后处理、细胞信号传导、基因表达调控及细胞分化等(Bedard and Krause,2007)。双氧化酶(Dual oxi-dase,DUOX)是NOX家族的重要成员之一(García et al.,2023),主要介导H2O2产生,在机体抗菌的先天免疫反应中发挥重要作用(Giusti etal.,2020;Gu et al.,2021)。DUOX首次在人类甲状腺细胞中鉴定获得(Caillou et al.,2001),随后陆续在果蝇(Dro-sophila)(Kim and Lee,2014)、斑马鱼(Danio rerio)(Chopra et al.,2019)、凡纳滨对虾(Litopenaeus van-namei)(Zhang et al.,2019)等生物体中发现DUOX。Yang等(2020)研究证实,经副溶血性弧菌(Vibrio parahaemolyticus)或白斑综合征病毒(White spotsyndrome virus,WSSV)感染后,拟穴青蟹(Scylla paramamosain)的duox1基因表达被激活,ROS积累增加,从而抑制拟穴青蟹血淋巴中的病原微生物增殖;Guan等(2021)以斑马鱼为研究对象,发现RIC体液因子可能是通过调控duox基因表达,而在氧化应激与组织损伤修复过程中发挥重要作用;Yu等(2023)在蛤蜊中也发现DUOX介导的ROS产生可激活Toll信号通路中抗菌肽表达,而参与无脊椎动物的抗菌先天免疫应答;Ji等(2024)研究发现,DUOX在甜菜夜蛾(Spodoptera exigua)的先天免疫中发挥抗菌作用。在无脊椎动物中,血淋巴黑化现象作为体液免疫的关键免疫反应,由无活性的酶原(prophenoloxidase,proPO)激活级联调节,在抵御病原微生物入侵的过程发挥重要作用(Tassanakajonet al.,2018)。Wilson等(2001)在非洲黏虫(Spodop-tera exempta)中发现,黑化现象与血淋巴中的酚氧化酶(PO)活性呈正相关;Huang等(2020)研究表明,黑水虻(Hermetiaillucens)的Duox-TLR3 RNAi灭活NF-κB信号通路并下调抗菌肽表达,进而减弱机体对病原体的抑制作用。综上所述,DUOX在无脊椎动物抗菌先天免疫中发挥重要作用。【本研究切入点】已有研究证实,duox1基因在果蝇免疫应答反应中被激活,通过产生H2O2介导Toll信号通路激活,帮助机体抵抗外来病原菌的侵染(Ramond et al.,2021),但关于克氏原螯虾duox1基因抵抗金黄色葡萄球菌侵染的免疫应答作用机制至今鲜见报道。【拟解决的关键问题】采用实时荧光定量PCR检测克氏原螯虾感染金黄色葡萄球菌后血细胞、肝胰腺、肠道及鳃组织中的duox1基因表达情况,并通过RNA干扰(RNAi)降低duox1基因表达后再以金黄色葡萄球菌进行刺激,统计克氏原螯虾存活率、检测H2O2含量及观察血淋巴黑化现象,并检测Toll信号通路上经典抗菌肽基因的表达变化,探究duox1基因在克氏原螯虾抗细菌感染先天免疫应答中的作用机制,为确保克氏原螯虾产业的持续健康发展提供技术支撑。
1材料与方法
1.1试验材料
克氏原螯虾购自内蒙古包头市友谊福瑞水产市场,挑选体积较小、活力好的个体。正式试验前,克氏原螯虾在恒温水池中避光暂养2周。金黄色葡萄球菌由内蒙古科技大学分子免疫实验室保存提供,试验前用LB液体培养基进行活化培养。动物试验获得内蒙古大学机构动物护理与使用委员会许可,许可证号为SCXK(内蒙古)2016-0001。
1.2试验方法
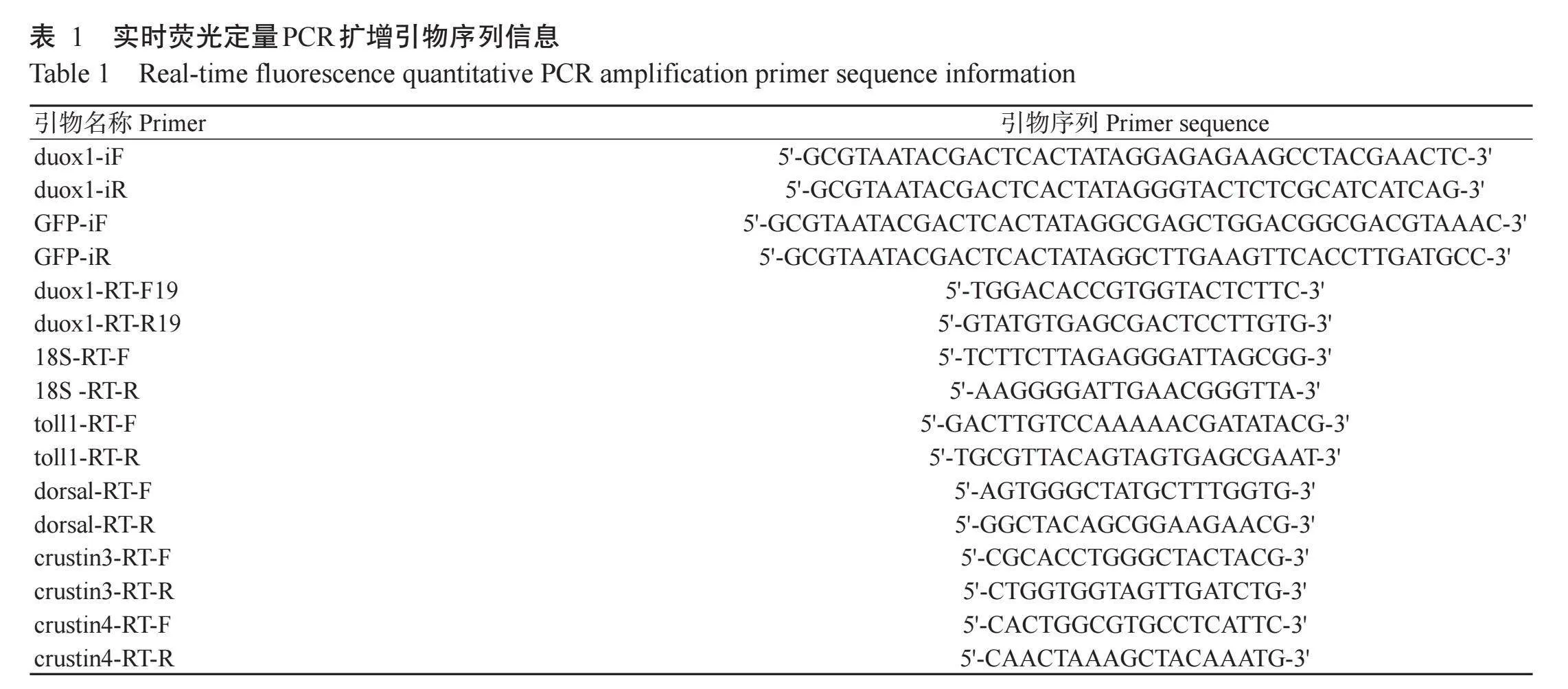
1.2.1金黄色葡萄球菌刺激及表达模式研究试验分别设金黄色葡萄球菌刺激组及磷酸盐缓冲液(PBS)刺激组,每组30只健康的克氏原螯虾。金黄色葡萄球菌刺激组在克氏原螯虾第二腹节处注射金黄色葡萄球菌悬液(以PBS稀释,2×107 CFU/mL),注射剂量100.0μL;PBS刺激组克氏原螯虾注射等体积的PBS。注射后0、2、6、12、24、36和48 h,无菌条件下采集克氏原螯虾的血淋巴、肝胰腺、肠道和鳃组织,每个时间点取3只克氏原螯虾。其中,血淋巴在4℃下2900 r/min离心10 min,以获得血细胞(Huang et al.,2023)。分别提取各时间点不同组织总RNA,并反转录成cDNA,利用实时荧光定量PCR检测金黄色葡萄球菌刺激后克氏原螯虾duox1基因的表达情况。以18S rRNA为内参基因,扩增程序:95℃预变性30 s;95℃5 s,60℃34 s,进行40个循环。设3个生物学重复,采用2-△△Ct法计算目的基因相对表达量。实时荧光定量PCR扩增引物如表1所示。
1.2.2克氏原螯虾duox1基因双链RNA体外转录合成基于前期的转录组测序结果,使用Primer 5.0设计干扰引物(duox1-iF和duox1-iR)(表1)。扩增克氏原螯虾duox1基因干扰片段,扩增程序:94℃预变性3 min;94℃30 s,60℃45 s,72℃40 s,进行35个循环;72℃延伸10min。富集纯化后进行1%琼脂糖凝胶电泳,回收目的条带,-20℃保存备用。使用T7体外转录试剂盒(日本TaKaRa公司)进行体外转录,制备克氏原螯虾duox1基因双链RNA,反应体系25.0μL:10×Transcription Buffer 4.0μL,ATP/UTP/CTP/GTP 4.0μL,RNase Inhibitor 1.0μL,T7 RNA聚合酶1.0μL,DNA模板10.0μL,RNase Free H2O 5.0μL。混匀后,42℃水浴2h。加入RNase free DNaseI 4.0μL,37℃继续水浴1h以去除残留的DNA。体外转录合成的克氏原螯虾duox1基因双链RNA置于-80℃冰箱保存备用,并以相同方法制备GFP双链RNA作为对照。
1.2.3 RNA干扰试验RNA干扰试验分为3组,分别为dsduox1+金黄色葡萄球菌组、dsGFP+金黄色葡萄球菌组和PBS组。将制备的双链RNA稀释至3000 ng/μL。每组随机挑选30只健康的克氏原螯虾,于血窦处注射25.0μL双链RNA,同时在第二腹节处注射金黄色葡萄球菌悬液(2×107 CFU/mL),注射剂量100.0μL。PBS组注射等体积的无菌PBS。
1.2.4 RNA干扰后克氏原螯虾存活率统计经RNA干扰及金黄色葡萄球菌刺激后,对克氏原螯虾存活率进行统计;同时,在注射后0、24和48 h无菌解剖克氏原螯虾并采集肝胰腺组织,每个时间点至少解剖3只克氏原螯虾,肝胰腺组织样品-80℃冰箱保存备用。
1.2.5 RNA干扰后克氏原螯虾肝胰腺H2O2含量检测取上述RNA干扰及金黄色葡萄球菌刺激后0、24和48 h的克氏原螯虾肝胰腺组织(3只克氏原螯虾的混合样品),置于无菌匀浆器中加入1 mL预冷丙酮,冰浴匀浆后,4℃下12000 r/min离心10 min,取上清液,按H2O2含量检测试剂盒(苏州格锐思生物科技有限公司)说明在415nm处检测吸光度(A),并换算为克氏原螯虾肝胰腺H2O2含量。
1.2.6 RNA干扰后克氏原螯虾血淋巴黑化反应情况观察在RNA干扰及金黄色葡萄球菌刺激后0、24和48h分别进行克氏原螯虾血淋巴黑化反应情况观察。每个时间点抽取3只克氏原螯虾的血淋巴,将2 mL克氏原螯虾血淋巴与1 mL抗凝剂(表2)混合后,立即滴一滴混合液于载玻片上,轻轻盖上盖玻片,电子显微镜下观察拍摄。
1.2.7干扰后克氏原螯虾肝胰腺抗菌肽基因表达检测取RNA干扰及金黄色葡萄球菌刺激后0、24和48 h的克氏原螯虾肝胰腺组织样品50 mg(3只克氏原螯虾的混合样品),使用RNAiso Plus试剂盒(日本TaKaRa公司)提取总RNA,以NanoDrop 2000超微量分光光度计检测RNA浓度,并通过1%琼脂糖凝胶电泳测定其完整性。采用Prime Script TR Reagent Kit试剂盒(日本TaKaRa公司)反转录合成cDNA,反应体系10.0μL:5×Prime Script TR Master Mix 2.0μL,RNA模板1.0μL,RNase freedH2O 7.0μL。反转录程序:37℃15 min,85℃5 s。以制备获得的cDNA为模板,通过实时荧光定量PCR检测抗菌肽基因(toll1、dorsal、crustin3和crustin4)的表达情况,以18S rRNA为内参基因,采用2-ΔΔCt法计算目的基因相对表达量。扩增引物序列如表1所示,扩增程序同1.2. 每个样品设3个生物学重复。
1.3统计分析
试验数据采用Excel 2020进行统计分析,并通过GraphPad Prism 8.0进行双因素方差分析(Two-way ANOVA)。
2结果与分析
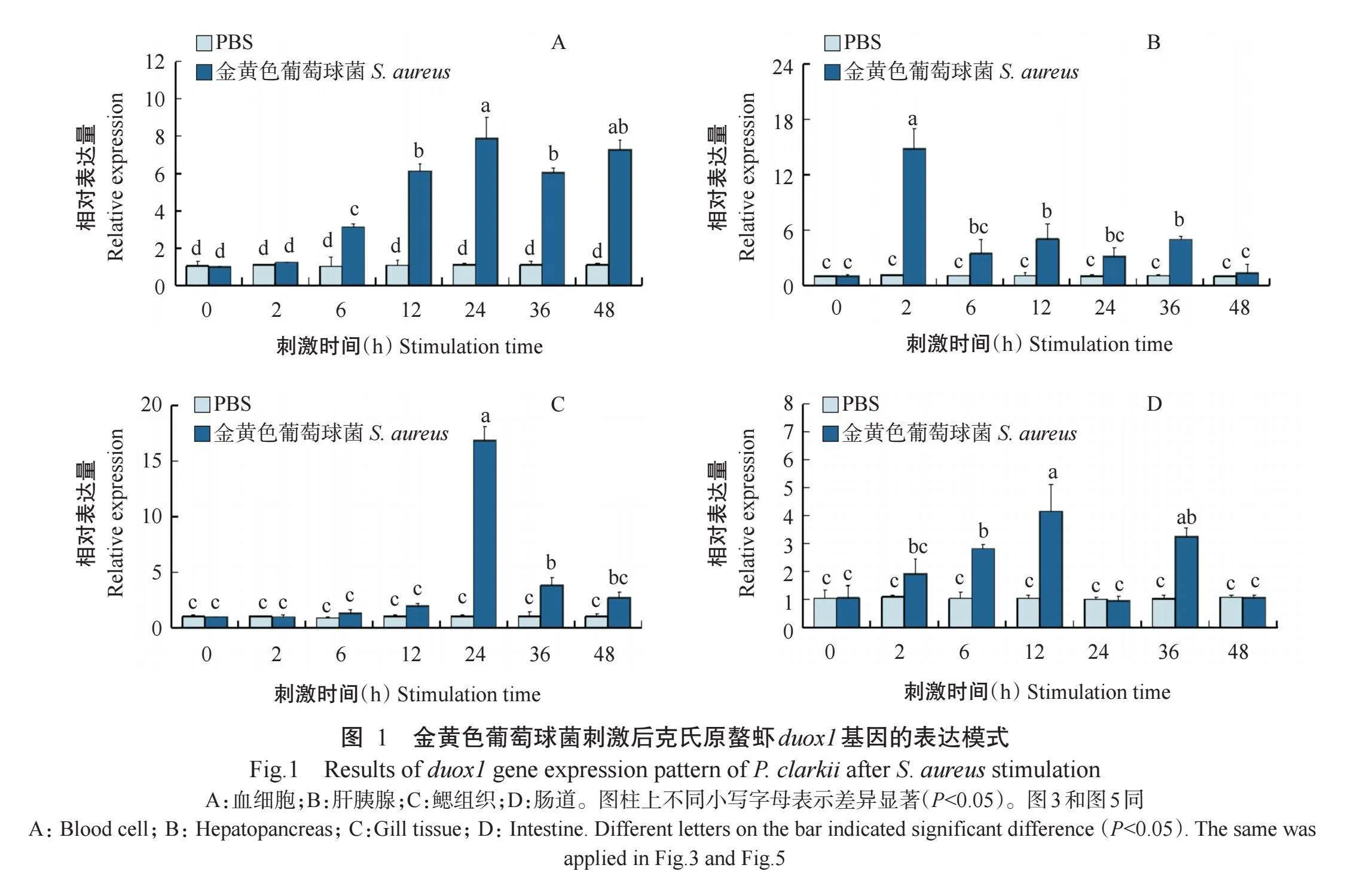
2.1金黄色葡萄球菌刺激后克氏原螯虾duox1基因表达模式
通过实时荧光定量PCR检测金黄色葡萄球菌刺激后duox1基因在克氏原螯虾不同组织中的表达模式,结果如图1所示。经金黄色葡萄球菌刺激后,克氏原螯虾duox1基因在血细胞、肝胰腺、肠道及鳃组织中的相对表达量较PBS组整体上呈上升趋势。在血细胞中,duox1基因相对表达量在金黄色葡萄球菌刺激后6~48 h呈显著上调趋势(P<0.05,下同),于感染后24 h达最高值(图1-A);金黄色葡萄球菌刺激后2 h,肝胰腺中的duox1基因相对表达量达最高值,随后呈显著下降趋势(图1-B);在鳃组织中,duox1基因在金黄色葡萄球菌刺激6h后开始上调表达,其相对表达量在刺激后24h达最高值(图1-C);金黄色葡萄球菌刺激后12h,肠道中的duox1基因相对表达量达最高值(图1-D)。由此推测,duox1基因参与克氏原螯虾的抗菌先天免疫应答,具有潜在的抵抗细菌侵染作用。
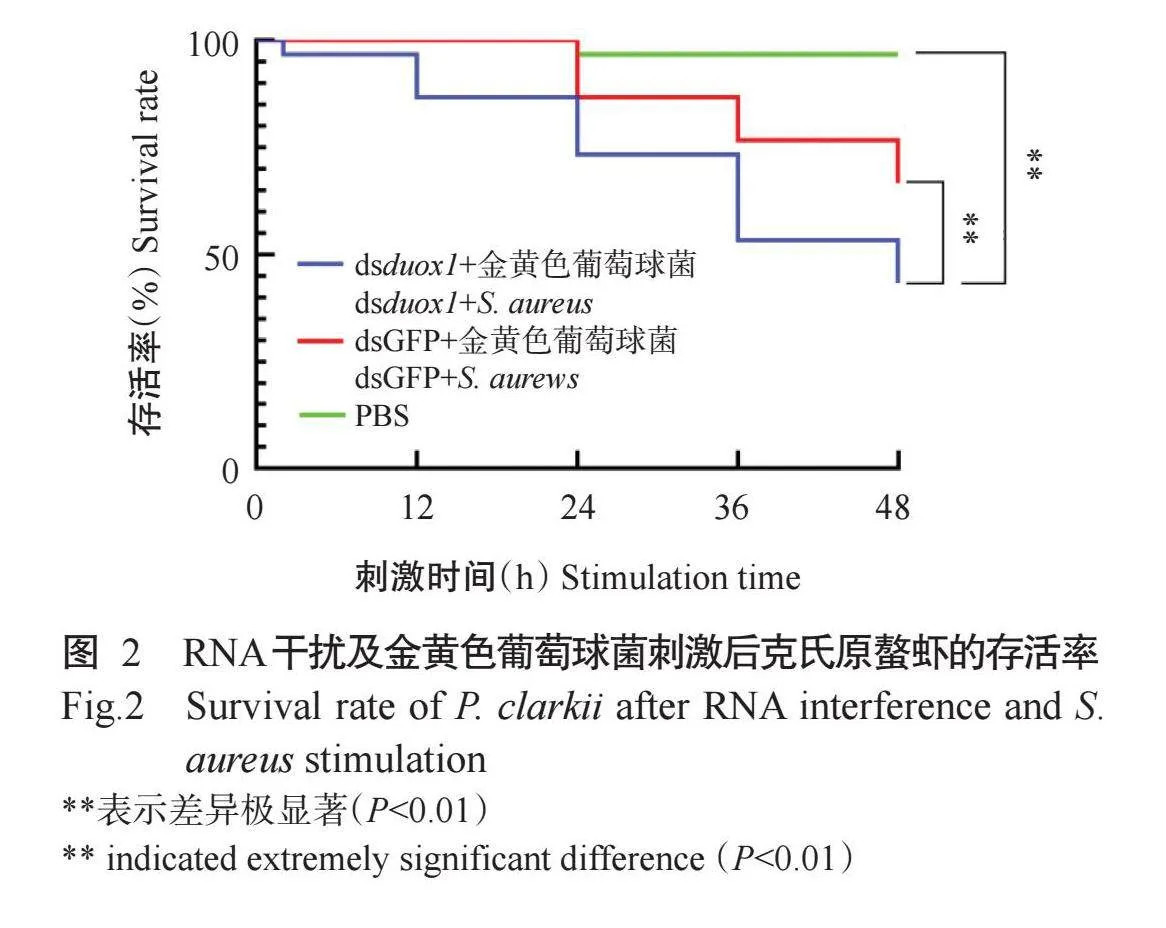
2.2 RNA干扰及金黄色葡萄球菌刺激后克氏原螯虾的存活率
经RNA干扰及金黄色葡萄球菌刺激后,克氏原螯虾的存活率如图2所示。与dsGFP+金黄色葡萄球菌组和PBS组相比,dsduox1+金黄色葡萄球菌组克氏原螯虾存活率呈明显下降趋势,在刺激后36和48 h的差异达极显著水平(P<0.0 下同)。
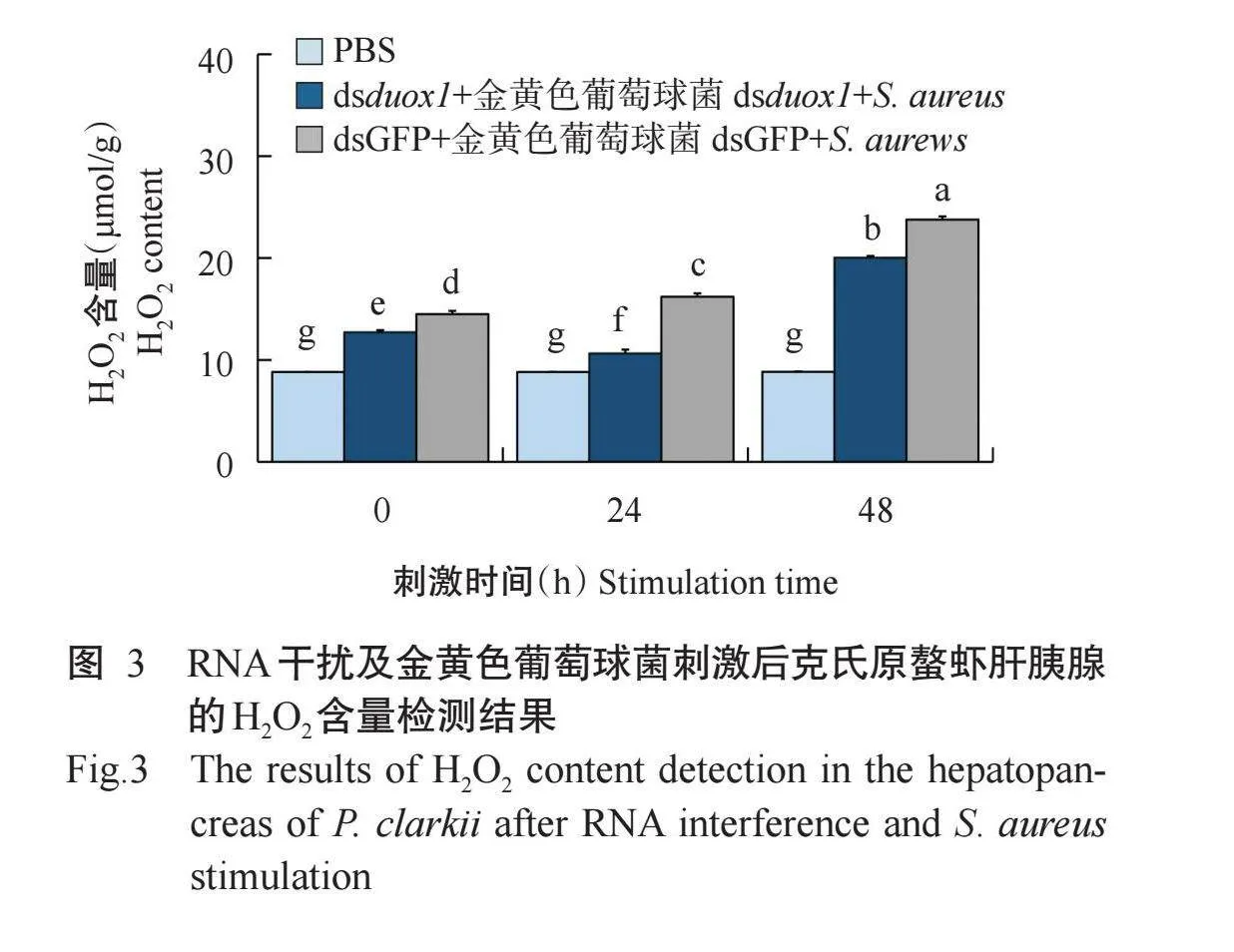
2.3 RNA干扰及金黄色葡萄球菌刺激后克氏原螯虾肝胰腺的H2O2含量
为进一步探究duox1基因对克氏原螯虾抗金黄色葡萄球菌侵染的作用机制,使用H2O2含量检测试剂盒检测克氏原螯虾肝胰腺的H2O2含量,结果如图3所示。与dsGFP+金黄色葡萄球菌组相比,dsduox1+金黄色葡萄球菌组克氏原螯虾肝胰腺的H2O2含量呈先降低后回升的变化趋势,在刺激后24 h达最低值,H2O2含量被显著抑制。
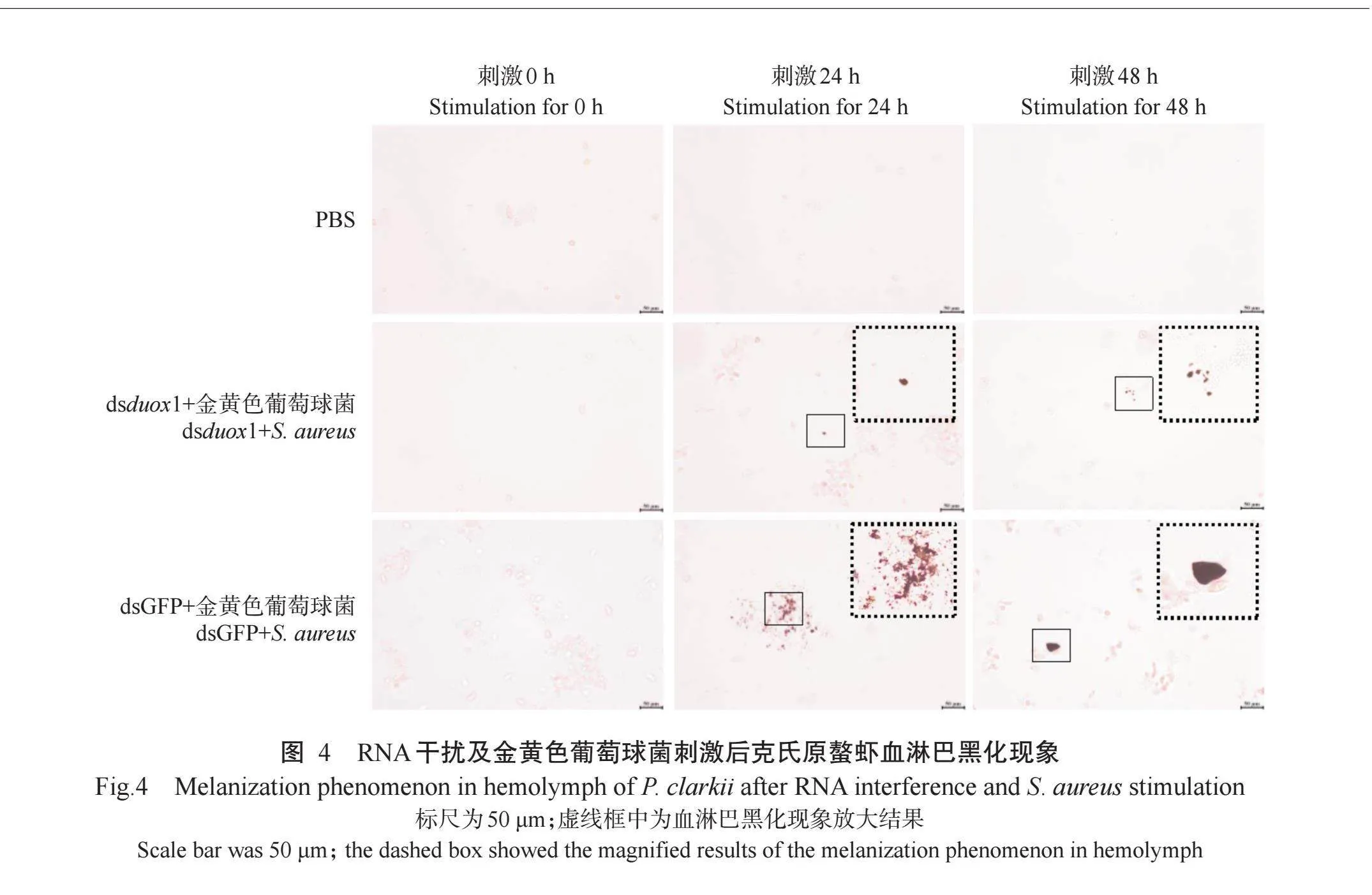
2.4 RNA干扰及金黄色葡萄球菌刺激后克氏原螯虾血淋巴黑化现象
经RNA干扰及金黄色葡萄球菌刺激后,分别于刺激后0、24和48 h对克氏原螯虾血淋巴黑化反应进行观察,结果如图4所示。与dsGFP+金黄色葡萄球菌组相比,dsduox1+金黄色葡萄球菌组克氏原螯虾血淋巴黑化反应被抑制。在刺激后24和48 h,dsGFP+金黄色葡萄球菌组克氏原螯虾血淋巴出现明显的黑化现象,dsduox1+金黄色葡萄球菌组克氏原螯虾虽然存在血淋巴黑化现象,但黑化反应程度明显减弱。
2.5 RNA干扰及金黄色葡萄球菌刺激后克氏原螯虾肝胰腺抗菌肽基因表达情况
经RNA干扰及金黄色葡萄球菌刺激后,分别在刺激后0、24和48 h通过实时荧光定量PCR检测克氏原螯虾肝胰腺抗菌肽基因表达情况,结果如图5所示。与PBS组相比,dsGFP+金黄色葡萄球菌组克氏原螯虾肝胰腺中toll1、dorsal、crustin3和crustin4基因的表达整体上呈不同程度的上调趋势。与dsGFP+金黄色葡萄球菌组相比,dsduox1+金黄色葡萄球菌组克氏原螯虾肝胰腺中toll1、dorsal、crustin3和crustin4基因呈显著下调表达趋势。可见,干扰duox1基因表达并感染金黄色葡萄球菌后,克氏原螯虾肝胰腺Toll信号通路上的toll1、dorsal、crustin3和crustin4基因表达被抑制,导致参与抗细菌反应的先天免疫能力下降,最终引起克氏原螯虾存活率下降。
3讨论
DUOX是NOX家族的主要成员之一。NOX家族包括5个产生超氧阴离子的Noxes和2个产生H2O2的Duoxes(Inada et al.,2013)。DUOX主要介导H2O2的产生,进而产生低硫氰酸根离子,在宿主的防御反应或促炎反应中发挥重要作用(Rada and Leto,2008)。在脊椎动物中,duox基因与黏膜免疫相关;在无脊椎动物中,duox基因的主要功能是维持机体肠道菌群平衡(Li etal.,2024)。细菌通过自身产生的尿嘧啶,促使膜上的G蛋白偶联受体激活2条信号通路[激活转录因子2(ATF2)和内质网膜应激]。其中,内质网膜应激致使内质网膜上的通道打开,钙离子外泄形成钙波,作为第二信使激活duox基因表达,从而产生ROS(Hong and Qin,2023)。
DUOX主要与氧化应激、能量代谢及炎症反应等有关(Lee et al.,2018;Grasberger et al.,2021;Baek et al.,2022),但至今有关无脊椎动物DUOX的研究报道较少。Yang等(2016)以日本囊对虾(Marsu-penaeus japonicus)为研究对象,发现在鳗弧菌(V.anguillarum)侵染机体后,duox1基因呈明显上调表达趋势;Sun等(2018)研究发现,以LPS模拟细菌刺激及副溶血性弧菌感染拟穴青蟹后,其体内的duox1基因表现出明显的免疫响应,故推测拟穴青蟹duox1基因参与机体抵抗细菌侵袭的免疫过程。可见,duox1基因在无脊椎动物抵御病原菌侵染的过程中发挥重要作用。本研究以金黄色葡萄球菌为病原菌感染刺激克氏原螯虾,分别检测血细胞、肝胰腺、肠道和鳃组织中的duox1基因表达情况,结果发现duox1基因在各组织中的表达整体上呈上升趋势。值得注意的是,鳃组织中duox1基因的上调表达主要集中在感染后期,可能是鳃组织作为主要的呼吸器官,其免疫响应相对较弱,而出现滞后现象(Burgos-Aceves et al.,2021)。为进一步验证duox1基因在抵御金黄色葡萄球菌侵染的作用机制,本研究通过RNA干扰技术敲低duox1基因表达,再以金黄色葡萄球菌进行刺激,结果显示,与dsGFP+金黄色葡萄球菌组相比,克氏原螯虾存活率极显著下降,与Inada等(2013)的研究结果相似,即敲低duox基因表达后以WSSV进行刺激,日本囊对虾的存活率显著下降。说明duox1基因在克氏原螯虾抵抗病原菌感染的过程中对于维持机体生存起重要作用。
DUOX主要介导H2O2的产生而发挥杀菌作用,同时可作为第二信使激活或募集血细胞执行免疫效应(Cheon et al.,2020;Giusti et al.,2020;To et al.,2020)。Huang等(2020)以黑水牤(Hermetiaillucens)为模式生物,敲低duox基因表达后发现,相对于dsGFP组,其H2O2含量显著下降;Jia等(2023)以中华绒螯蟹(Eriocheir sinensis)为研究对象,同样发现敲低duox基因表达后,机体内的ROS含量显著降低,表明duox基因介导ROS产生的主要类型是H2O2;Zhang等(2023)对同家族的NOX基因进行RNA干扰,结果发现拟穴青蟹H2O2含量也出现明显的抑制现象,其体内细菌含量则显著增加。本研究结果表明,经RNA干扰及金黄色葡萄球菌刺激后,克氏原螯虾肝胰腺H2O2含量较dsGFP+金黄色葡萄球菌组呈先降低后回升的变化趋势,在刺激后24 h达最低值。此外,Li等(2019)在以大肠杆菌(Escherichia coli)刺激的家蚕(Bombyx mori)中发现血淋巴黑化现象增加,表明血淋巴黑化作用有可能具有清除病原体的功能。在本研究中,敲低duox1基因表达后以金黄色葡萄球菌进行刺激,克氏原螯虾血淋巴黑化反应程度明显减弱,与H2O2含量检测结果相互验证。
抗菌肽作为一种广谱抗菌活性物质,是无脊椎动物杀菌的主要途径(李博等,2020;梁子莹等,2023;Guryanova et al.,2023)。Lipinski等(2009)在敲低duox基因表达的基础上,发现NOD2基因和duox基因协同发挥抗菌作用;Huang等(2020)以黑水虻为研究对象,通过干扰体内duox基因信号级联,发现其体内抵抗病原菌侵染的相关基因显著下调表达,推测duox基因通过影响下游效应基因表达而发挥作用。此外,Chakrabarti和Visweswariah(2020)研究表明,机体损伤部位的H2O2水平决定着DUOX活性,H2O2在血细胞内的产生与积累对JAK/STAT信号通路和Toll信号通路的激活至关重要。为进一步探究引起克氏原螯虾存活率下降的原因,本研究通过RNA干扰敲低duox1基因表达,再进行金黄色葡萄球菌刺激,结果发现克氏原螯虾肝胰腺中的toll1、dorsal、crustin3和crustin4基因均呈显著下调表达,无法及时发挥杀菌作用。综上所述,在克氏原螯虾针对金黄色葡萄球菌感染的免疫响应中duox1基因可能通过影响抗菌肽相关基因表达而发挥免疫调控功能。
4结论
克氏原螯虾duox1基因通过调控H2O2产生、影响血淋巴黑化现象及调控toll1、dorsal、crustin3和crustin4等抗菌肽基因的表达,参与机体的先天免疫应答,进而协助机体抵御金黄色葡萄球菌的侵染。
参考文献(References):
陈卫军.2019.中国虾蟹养殖现状[J].科学养鱼,(2):1-2.[Chen W J.2019.Status of shrimp and crab farming in China[J].Scientific Fish Farming,(2):1-2.]doi:10.14184/j.cnki.issn 1004-843x.2019.02.001.
兰培利,戴蕾,赵瑞臻,卫少华,周鹏,陈彦哲,程春荣.2024.一起由金黄色葡萄球菌感染引起的食物中毒疫情的病原检测[J].现代疾病预防控制,35(2):138-141.[Lan P L,Dai L,Zhao R Z,Wei S H,Zhou P,Chen Y Z,Cheng C R.2024.Pathogenic detection of food poisoning caused by Staphylococcus aureus[J].Modern Disease Control and Prevention,35(2):138-141.]doi:10.13515/j.cnki.hnjpm.1006-8414.2024.02.017.
李博,王凯,于晓东,刘睿哲,蔺思函,商静,沈秀丽,杜志强.2020.克氏原螯虾抗菌肽crustin5基因克隆及其表达分析[J].南方农业学报,51(4):953-960.[Li B,Wang K,Yu X D,Liu R Z,Lin S H,Shang J,Shen X L,Du Z Q.2020.Gene cloning and expression analysis of crustin5 gene in Procambarus clarkii[J].Journal of Southern Agri-culture,51(4):953-960.]doi:10.3969/j.issn.2095-1191.2020.04.028.
梁子莹,邱丽华,王鹏飞,张博,闫路路,乔秀亭,赵超.2023.斑节对虾新型抗菌肽ALF-like基因克隆表达及其抗菌功能分析[J].南方农业学报,54(9):2654-2664.[Liang ZY,Qiu L H,Wang P F,Zhang B,Yan L L,Qiao X T,Zhao C.2023.Cloning,expression and antibacterial function analy-sis of novel antibacterial peptide gene ALF-like in Penaeus monodon[J].Journal of Southern Agriculture,54(9):2654-2664.]doi:10.3969/j.issn.2095-1191.2023.09.016.
孟思妤,孟长明,陈昌福.2017.人工养殖淡水螯虾的传染性病害问题(1)[J].渔业致富指南,(14):67-68.[Meng S Y,Meng C M,Chen C F.2017.Infectious disease prob-lems of freshwater crayfish in captivity(1)[J].Fishery Guide to Be Rich,(14):67-68.]
张庆利.2007.中国明对虾免疫系统中抗氧化相关基因的克隆与表达分析[D].北京:中国科学院研究生院.[Zhang Q L.2007.Cloning and expression analysis of antioxidantgenes involving in the immune system in Chinese shrimp Fenneropenaeus chinensis[D].Beijing:University of Chi-nese Academy of Sciences.]
Baek M,Jang W,Kim C.2022.Dual oxidase,a hydrogen-peroxide-producing enzyme,regulates neuronal oxidative damage and animal lifespan in Drosophila melanogaster[J].Cells,11(13):2059.doi:10.3390/cells 11132059.
Bedard K,Krause K H.2007.The NOX family of ROS-generating NADPH oxidases:Physiology and pathophysio-logy[J].Physiological Reviews,87(1):245-313.doi:10.1152/physrev.00044.2005.
Burgos-Aceves M A,Abo-Al-Ela H G,Faggio C.2021.Impact of phthalates and bisphenols plasticizers on haemocyte immune function of aquatic invertebrates:A review on physiological,biochemical,and genomic aspects[J].Jour-nal of Hazardous Materials,419:126426.doi:10.1016/j.jhazmat.2021.126426.
Caillou B,Dupuy C,Lacroix L,Nocera M,Talbot M,Ohayon R,Dème D,Bidart J M,Schlumberger M,Virion A.2001.Expression of reduced nicotinamide adenine dinucleotide phosphate oxidase(ThoX,LNOX,Duox)genes and pro-teins in human thyroid tissues[J].The Journal of Clinical Endocrinology&Metabolism,86(7):3351-3358.doi:10.1210/jcem.86.7.7646.
Chakrabarti S,Visweswariah S S.2020.Intramacrophage ROS primes the innate immuGpTyYQBFOPhhTlxhLZQ8RWEIvYNE8OdBjYg1zzsKGI8=ne system via JAK/STAT and Toll activation[J].Cell Reports,3(6):108368.doi:10.1016/j.celrep.2020.108368.
Cheon Y H,Lee C H,Jeong D H,Kwak S C,Kim S,Lee M S,Kim J Y.2020.Dual oxidase maturation factor 1 positivelyregulates RANKL-induced osteoclastogenesis via activa-ting reactive oxygen species and TRAF6-mediated signa-ling[J].International Journal of Molecular Sciences,21(17):6416.doi:10.3390/ijms21176416.
Cheung G Y C,Bae J S,Otto M.2021.Pathogenicity and viru-lence of Staphylococcus aureus[J].Virulence,12(1):547-569.doi:10.1080/21505594.2021.1878688.
Chopra K,Ishibashi S,Amaya E.2019.Zebrafish duoxmuta-tions provide a model for human congenital hypothyroi-dism[J].Biology Open,8(2):bio037655.doi:10.1242/bio.037655.
García J G,Ansorena E,Izal I,Zalba G,de Miguel C,Milagro F I.2023.Structure,regulation,and physiological func-tions of NADPH oxidase 5(NOX5)[J].Journal of Physio-logy and Biochemistry,79(2):383-395.doi:10.1007/s13105-023-00955-3.
Giusti N,Gillotay P,Trubiroha A,Opitz R,Dumont J E,Costa-gliola S,de Deken X.2020.Inhibition of the thyroid hor-monogenic H2O2 production by Duox/DuoxA in zebrafish reveals VAS2870 as a new goitrogenic compound[J].Mo-lecular and Cellular Endocrinology,500:110635.doi:10.1016/j.mce.2019.110635.
Grasberger H,Magis A T,Sheng E,Conomos M P,Zhang M,Garzotto L S,Hou G P,Bishu S,Nagao-Kitamoto H,El-Zaatari M,Kitamoto S,Kamada N,Stidham R W,Akiba Y,Kaunitz J,Haberman Y,Kugathasan S,Denson L A,Omenn G S,Kao J Y.2021.DUOX2 variants associate with preclinical disturbances in microbiota-immune ho-meostasis and increased inflammatory bowel disease risk[J].The Journal of Clinical Investigation,131(9):e141676.doi:10.1172/JCI141676.
Gu F,Krüger A,Roggenkamp H G,Alpers R,Lodygin D,Jaquet V,Möckl F,Hernandez C L C,Winterberg K,BaucheA,Rosche A,Grasberger H,Kao J Y,Schetelig D,Werner R,Schröder K,Carty M,Bowie A G,Huber S,Meier C,Mittrücker H W,Heeren J,Krause K H,Flügel A,Diercks B P,Guse A H.2021.Dual NADPH oxidases DUOX1 and DUOX2 synthesize NAADP and are neces-sary for Ca2+signaling during T cell activation[J].Science Signaling,14(709):eabe3800.doi:10.1126/scisignal.abe 3800.
Guan R,Wen X Y,Leung C H,Ciano-Oliveira C D,Lam S,Dai S Y,Karbassi F,Mauro A,Wang Y D,Rotstein O.2021.Plasma obtained following murine hindlimb ische-mic conditioning protects againstPTKG5otGWt/GG4Iv+Zk0kA== oxidative stress in zebrafish models through activation of nrf2a and down-regulation of duox[J].PLoS One,16(11):e0260442.doi:10.1371/journal.pone.0260442.
Guryanova S V,Balandin S V,Belogurova-Ovchinnikova O Y,Ovchinnikova T V.2023.Marine invertebrate antimicro-bial peptides and their potential as novel peptide antibiotics[J].Marine Drugs,21(10):503.doi:10.3390/md21100503.
Hong S Y,Qin B L.2023.The protective role of dietary poly-phenols in urolithiasis:Insights into antioxidant effects and mechanisms of action[J].Nutrients,15(17):3753.doi:10.3390/nu 15173753.
Huang Y Q,Yu Y Q,Zhan S,Tomberlin J K,Huang D,Cai M M,Zheng L Y,Yu Z N,Zhang J B.2020.Dual oxidase Duox and Toll-like receptor 3 TLR3 in the Toll pathway suppress zoonotic pathogens through regulating the intesti-nal bacterial community homeostasis in Hermetiaillucens L.[J].PLoS One,15(4):e0225873.doi:10.1371/journal.pone.0225873.
Huang Z H,Guan W L,Wei X B,Chen R C,Lyu X M,Zheng G H,Mao L C.2023.Examination of the role of hypoxia-inducible factor-1α(HIF-1α)in preventing hemocyte apo-ptosis in whiteleg shrimp(Litopenaeusvannamei)[J].Aquaculture,563:738905.doi:10.1016/j.aquaculture.2022.738905.
Inada M,Kihara K,Kono T,Sudhakaran R,Mekata T,Sakai M,Yoshida T,Itami T.2013.Deciphering of the Dual oxi-dase(Nox family)gene from kuruma shrimp,Marsu-penaeus japonicus:Full-length cDNA cloning and charac-terization[J].Fish&Shellfish Immunology,34(2):471-485.doi:10.1016/j.fsi.2012.11.026.
Ji Y J,Gao B,Zhao D,Wang Y,Zhang L,Wu H,Xie Y F,ShiQ Y,Guo W.2024.Involvement of Sep38βin the insecti-cidal activity of Bacillus thuringiensis against beet army-worm,Spodoptera exigua(Lepidoptera)[J].Journal of Agricultural and Food Chemistry,72(4):2321-2333.doi:10.1021/acs.jafc.3c06667.
Jia R,Dai X L,Li Y F,Yang X T,Min X W,Quan D R,Liu P,Huang X,Ge J C,Ren Q.2023.Duox mediated ROS pro-duction inhibited WSSV replication in Eriocheir sinensis under short-term nitrite stress[J].Aquatic Toxicology,260:106575.doi:10.1016/j.aquatox.2023.106575.
Kim S H,Lee W J.2014.Role of DUOX in gut inflammation:Lessons from Drosophila model of gut-microbiota interac-tions[J].Frontiers in Cellular and Infection Microbiology,3:116.doi:10.3389/fcimb.2013.00116.
Lee K A,Cho K C,Kim B,Jang I H,Nam K,Kwon Y E,Kim M,Hyeon D Y,Hwang D,Seol J H,Lee W J.2018.Inflammation-modulated metabolic reprogramming is re-quired for DUOX-dependent gut immunity in Drosophila[J].Cell Host&Microbe,23(3):338-352.doi:10.1016/j.chom.2018.01.011.
Li C S,Kausar S,Gul I,Yao X X,Li M Y,Chen C C,Abbas M N,Dai L S.2020.Heat shock protein 20 from Procam-barus clarkii is involved in the innate immune responses against microbial infection[J].Developmental&Compa-rative Immunology,106:103638.doi:10.1016/j.dci.2020.103638.
Li Q Q,Zhang M D,Qin S Y,Wen J,Shen X L,Du Z Q.2024.Dual oxidase 2(duox 2)participates in the intestinal anti-bacterial innate immune responses of Procambarus clarkii by regulating ROS levels[J].Developmental&Compara-tive Immunology,153:105116.doi:10.1016/j.dci.2023.105116.
Li T,Yan D F,Wang X H,Zhang L,Chen P.2019.Hemocyte changes during immune melanization in Bombyx mori infected with Escherichia coli[J].Insects,10(9):301.doi:10.3390/insects 10090301.
Lipinski S,Till A,Sina C,Arlt A,Grasberger H,Schreiber S,Rosenstiel P.2009.DUOX2-derived reactive oxygen spe-cies are effectors of NOD2-mediated antibacterial res-ponses[J].Journal of Cell Science,122(19):3522-3530.doi:10.1242/jcs.050690.
Nocella C,D'Amico A,Cammisotto V,Bartimoccia S,Castel-lani V,Loffredo L,Marini L,Ferrara G,Testa M,Motta G,Benazzi B,Zara F,Frati G,Sciarretta S,Pignatelli P,Violi F,Carnevale R,Group S.2023.Structure,activation,and regulation of NOX2:At the crossroad between the innate immunity and oxidative stress-mediated pathologies[J].Antioxidants,12(2):429.doi:10.3390/antiox 12020429.
Qin Z D,Babu V S,Lin H Z,Dai Y,Kou H Y,Chen L H,Li J,Zhao L J,Lin L.2019.The immune function of propheno-loxidase from red swamp crayfish(Procambarus clarkii)in response to bacterial infection[J].Fish&ShellfishIm-munology,92:83-90.doi:10.1016/j.fsi.2019.05.005.
Rada B,Leto T L.2008.Oxidative innate immune defenses by Nox/Duox family NADPH oxidases[J].Trends in Innate Immunity,15:164-187.doi:10.1159/000136357.
Ramond E,JametA,Ding X Q,Euphrasie D,Bouvier C,Lalle-mant L,He X Y,Arbibe L,Coureuil M,Charbit A.2021.Reactive oxygen species-dependent innate immune mecha-nisms control methicillin-resistant Staphylococcus aureus virulence in the drosophila larval model[J].mBio,12(3):e0027621.doi:10.1128/mBio.00276-21.
Sun Z Q,Hao S F,Gong Y,Zhang M,Aweya J J,Tran N T,Zhang Y L,Ma H Y,Li S K.2018.Dual oxidases partici-pate in the regulation of hemolymph microbiota homeosta-sis in mud crab Scylla paramamosain[J].Developmental&Comparative Immunology,89:111-121.doi:10.1016/j.dci.2018.08.009.
Tassanakajon A,Rimphanitchayakit V,Visetnan S,Amparyup P,Somboonwiwat K,Charoensapsri W,Tang S.2018.Shrimp humoral responses against pathogens:Antimicro-bial peptides and melanization[J].Developmental&Com-parative Immunology,80:81-93.doi:10.1016/j.dci.2017.05.009.
To E E,O'Leary J J,O'Neill L A J,Vlahos R,Bozinovski S,Porter C J H,Brooks R D,Brooks D A,Selemidis S.2020.Spatial properties of reactive oxygen species govern pathogen-specific immune system responses[J].Antioxi-dants&Redox Signaling,32(13):982-992.doi:10.1089/ars.2020.8027.
Vasta G R,Wang J X.2020.Galectin-mediated immune recog-nition:Opsonic roles with contrasting outcomes in selected shrimp and bivalve mollusk species[J].Developmental&Comparative Immunology,110:103721.doi:10.1016/j.dci.2020.103721.
Wilson K,Cotter S C,Reeson A F,Pell J K.2001.Melanism and disease resistance in insects[J].Ecology Letters,4(6):637-649.doi:10.1046/j.1461-0248.2001.00279.x.
Yang H T,Yang M C,Sun J J,Shi X Z,Zhao X F,Wang J X.2016.Dual oxidases participate in the regulation of intesti-nalmicrobiotic homeostasis in the kuruma shrimp Marsu-penaeus japonicus[J].Developmental&ComparativeIm-munology,59:153-163.doi:10.1016/j.dci.2016.01.024.
Yang Q H,Sun Z Q,Zhou Y L,Tran N T,Zhang X S,Lin Q,Zhou C,Zhang Y L,Li S K.2020.SpATF2 participates in maintaining the homeostasis of hemolymph microbiota by regulating dual oxidase expression in mud crab[J].Fish&Shellfish Immunology,104:252-261.doi:10.1016/j.fsi.2020.05.049.
Yu J J,Teng S S,Yue X,Wang H X,Liu B Z.2023.The Toll pathway and Duox-ROS system are required for the clam antibacterial immune response in the hepatopancreas[J].Aquaculture,574:739637.doi:10.1016/j.aquaculture.2023.739637.
Zhang D,Dong M R,Song X R,Qiao X,Yang Y,Yu S M,Sun W D,Wang L L,Song L S.2022.ROS function as an inducer of autophagy to promote granulocyte proliferation in Pacific oyster Crassostrea gigas[J].Developmental&Comparative Immunology,135:104479.doi:10.1016/j.dci.2022.104479.
Zhang M,Tran N T,Zhang Y S,Yang Q H,Tang Y,Zhang Y L,Li S K.2023.SpNox regulates the homeostasis in the hemolymph and gut of mud crab(Scylla paramamosain)by generating ROS[J].Aquaculture,575:739760.doi:10.1016/j.aquaculture.2023.739760.
Zhang X J,Li G Y,Jiang H Y,Li L M,Ma J G,Li H M,Chen J P.2019.Full-length transcriptome analysis of Litopenaeusvannamei reveals transcript variants involved in the innate immune system[J].Fish&Shellfish Immunology,87:346-359.doi:10.1016/j.fsi.2019.01.023.
(责任编辑兰宗宝)

