阿尔茨海默病病变相关脑区Pgc-1alpha敲除小鼠模型的构建



[摘要]" 目的: 构建在阿尔茨海默病(Alzheimer′s disease,AD)病变相关脑区过氧化物酶体增殖物激活受体γ辅激活因子-1α(peroxisome proliferator-activated receptor γ coactivator-1 alpha,Pgc-1alpha)敲除小鼠。方法: 引入Pgc-1alpha条件性敲除杂合子小鼠(Pgc-1alphafl/+),通过自交获得Pgc-1alpha条件性敲除纯合子小鼠(Pgc-1alphafl/fl)。将Pgc-1alphafl/fl纯合子小鼠和在AD病变脑区(皮层、海马)特异性表达Cre重组酶的Cre工具鼠(Calb1-Cre)杂交,提取子代转基因小鼠基因组DNA,行PCR鉴定,确定其基因型;采用蛋白免疫印迹技术检测转基因小鼠与野生型小鼠脑区及肾脏PGC-1α蛋白表达。结果: 双重PCR鉴定结果显示,同时扩增出400 bp及444 bp条带的小鼠为AD病变脑区Pgc-1alpha敲除纯合子小鼠(Pgc-1alphafl/fl::Calb1-Cre,Pgc-1alpha-/-)。蛋白免疫印迹结果显示,与野生型小鼠相比,条件性敲除小鼠脑区PGC-1α蛋白表达明显减少(Plt;0.05)。结论: 成功构建在AD病变相关脑区Pgc-1alpha敲除小鼠。
[关键词]" 过氧化物酶体增殖物激活受体γ辅激活因子-1α(Pgc-1alpha);条件性敲除;小鼠;阿尔茨海默病;皮层;海马
[中图分类号]" R749.16" [文献标志码]" A" [文章编号]" 1671-7783(2024)05-0423-05
DOI: 10.13312/j.issn.1671-7783.y230109
[引用格式]史厚珍,张维君,翟鸿儒,等. 阿尔茨海默病病变相关脑区Pgc-1alpha敲除小鼠模型的构建[J]. 江苏大学学报(医学版), 2024, 34(5): 423-427,450.
[基金项目]江苏省重点研发社会发展面上项目(BE2022782);镇江市政策引导类计划项目(GJ2021010);镇江“金山英才”高层次领军人才培养计划(第六期“169”工程)科研资助项目;镇江市重点研发产业前瞻与共性关键技术项目(SH2022019)
[作者简介]史厚珍(1998—),女,硕士研究生;王佳(通讯作者),教授,博士生导师,E-mail: wangjia@ujs.edu.cn
Construction of Pgc-1alpha knockout mouse model in Alzheimer′s disease related pathological brain regions
SHI Houzhen1, ZHANG Weijun1, ZHAI Hongru1, WANG Yijie1,WANG Yuxin1, LIU Zizhong2, WANG Jia1,2,3
(1. School of Medicine, Jiangsu University, Zhenjiang Jiangsu 212013; 2. Department of Neurology, the Fourth Affiliated Hospital of Jiangsu University, Zhenjiang Jiangsu 212001; 3. Zhenjiang Jieshengrui Biotechonology Co., Ltd, Zhenjiang Jiangsu 212013, China)
[Abstract]" Objective: To constuct peroxisome proliferator-activated receptor γ coactivator-1 alpha (Pgc-1alpha) knockout mouse model in Alzheimer′s disease (AD)-related pathologic regions. Methods: Pgc-1alphafl/fl homozygous mice were obtained by crossing Pgc-1alphafl/+ heterozygous offspring. Pgc-1alphafl/fl homozygous mice were hybridized with Cre tool mice (Calb1-Cre), which specifically expressed Cre recombinase in AD lesion brain regions (cortex and hippocampus), and the genetic DNA of progeny transgenic mice was extracted and identified by PCR. The expression of PGC-1α protein in brain and kidney of transgenic mice and wild-type mice was detected by Western blotting. Results: Dual PCR analysis revealed that mice exhibiting simultaneous amplification of 400 bp and 444 bp bands were characterized by Pgc-1alpha deletion specific to AD-related brain regions (Pgc-1alphafl/fl::Calb1-Cre, Pgc-1alpha-/-). The results of Western blotting showed that the expression of PGC-1α protein in the brain region of conditioned knockout mice was significantly decreased compared with that of wild-type mice (Plt;0.05). Conclusion: Conditional Pgc-1alpha knockout mice in AD-related pathological brain regions were successfully constructed.
[Key words]" peroxisome proliferator-activated receptor γ coactivator-1α (Pgc-1alpha); conditional knockout; mouse; Alzheimer′s disease; cortex; hippocampus
过氧化物酶体增殖物激活受体γ辅激活因子-1α(peroxisome proliferator-activated receptor γ coactivator-1 alpha,Pgc-1alpha),编码基因为PPARGC1A,1998年由Spiegelman课题组在小鼠棕色脂肪中首次发现[1]。作为一种转录辅激活因子,Pgc-1alpha不仅可以调控线粒体的生物合成,而且在调控代谢稳态方面起重要作用[2]。PGC-1α主要表达于线粒体含量丰富及氧化代谢活跃的组织,如脂肪、骨骼肌、心脏和大脑等[3-4]。多种神经疾病的发生与脑组织中PGC-1α表达降低、抗氧化酶减少及活性氧诱导的线粒体功能障碍密切相关[5],如γ-氨基丁酸(γ-aminobutyric acid,GABA)能神经元敲除Pgc-1alpha可模拟精神分裂症的行为学表型[6]。Pgc-1alpha通过调控基因和环境的相互作用影响神经突触的可塑性[6]。帕金森病患者尸检报告结果显示,其黑质脑区PGC-1α表达显著降低,同时伴随线粒体融合蛋白表达降低[7]。
本课题组前期在对阿尔茨海默病(Alzheimer′s disease,AD)患者脑组织、AD模型动物(APP/PS1)脑组织及APPswe转染的AD细胞模型的研究中发现,AD的发生伴随PGC-1α表达降低、磷酸化Tau蛋白表达增加以及自噬异常等[8-9]。通过脑区定点微注射技术及腺相关病毒介导的基因重组技术诱导PGC-1α在AD病变脑区过表达,结果发现PGC-1α活化可改善AD模型小鼠的学习、认知障碍以及空间记忆能力下降[10]。由此推测,Pgc-1alpha可能为AD治疗的潜在靶点。因此,本研究拟构建AD病变脑区(皮层、海马、纹状体、丘脑)Pgc-1alpha条件性敲除小鼠,以便后续探讨Pgc-1alpha对于AD靶向药物的研发。
1" 材料与方法
1.1" 材料
1.1.1" 抗体" 兔抗小鼠PGC-1α多克隆抗体(武汉爱博泰克生物科技有限公司);小鼠抗小鼠GAPDH单克隆抗体(武汉博士德生物工程有限公司);HRP标记的山羊抗小鼠二抗、山羊抗兔二抗均购自苏州ImmunoWay Biotechnology公司。
1.1.2" 实验动物" Pgc-1alphafl/+杂合子小鼠,品系名称B6N.129(FVB)-Ppargc1atm2.1Brsp/J,从美国Jackson Lab引进,雌、雄各2只。Calb1-Cre工具鼠,品系名称B6/JGpt-Calb1em1Cin(P2A-iCre)/Gpt,购自江苏集萃药康有限公司(#T006202),雌、雄各2只。遗传背景均为C57BL/6J小鼠。C57BL/6J野生型小鼠购自江苏大学实验动物中心,许可证编号:SCXK 2018-0012。
1.2" 方法
1.2.1" 小鼠饲养" 按照SPF动物饲养标准执行,温度(22±1)℃,湿度(55±5)%,小鼠自由摄食和饮水。所有实验程序均严格按照SPF动物管理相关规定进行。本实验通过江苏大学实验动物管理委员会和伦理委员会批准(UJS-IACUC-AP-2022022525)。
1.2.2" Pgc-1alphafl/+、Pgc-1alphafl/fl小鼠鉴定
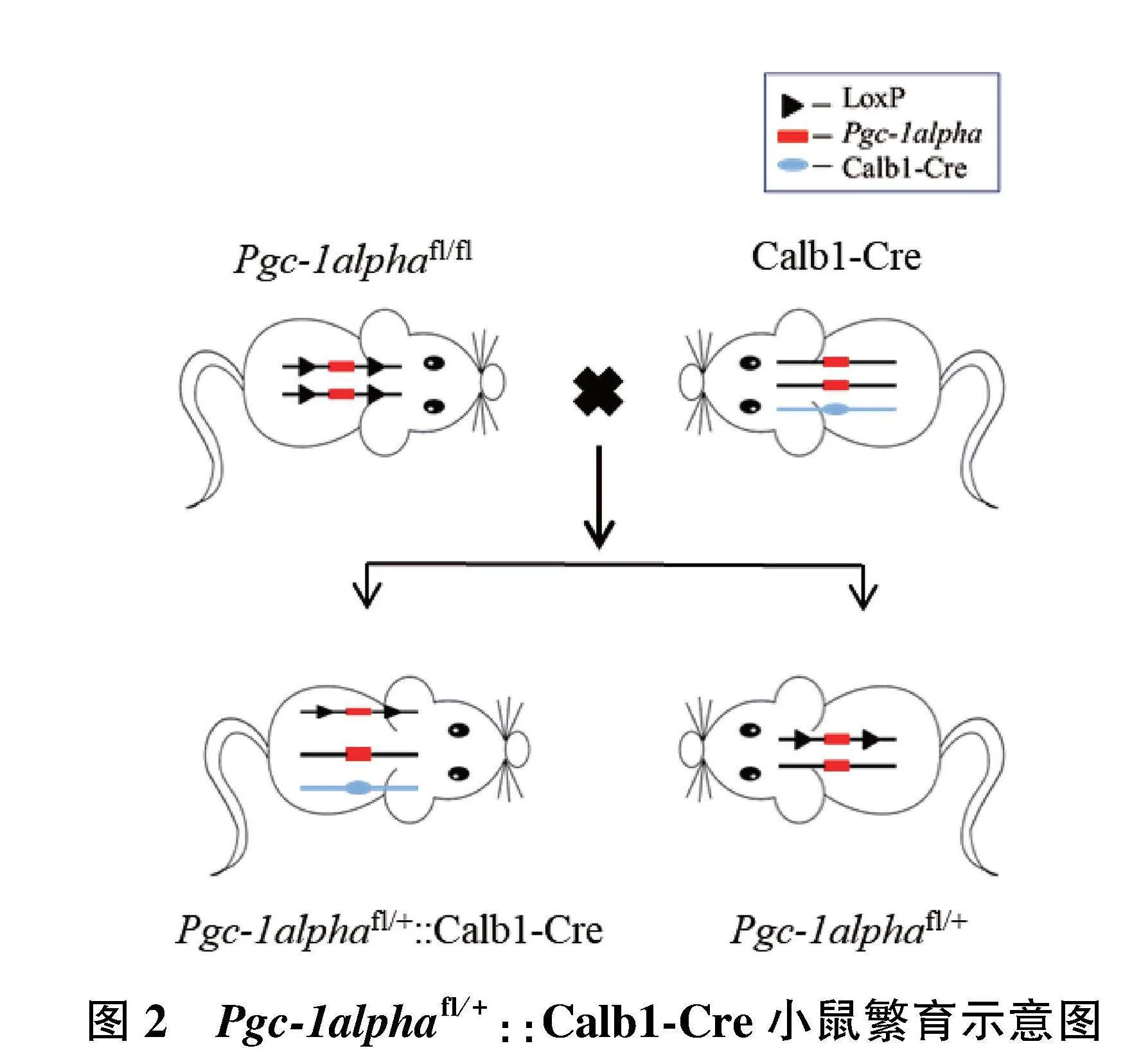
1.2.2.1" Pgc-1alphafl/fl小鼠的获得" Pgc-1alphafl/+小鼠与Pgc-1alphafl/+杂交获得Pgc-1alphafl/fl纯合子、Pgc-1alphafl/+杂合子以及Pgc-1alpha+/+野生型小鼠(图1)。
1.2.2.2" Pgc-1alpha条件性敲除小鼠基因组DNA提取" 杂交Pgc-1alphafl/+小鼠,取其繁育后出生10 d的子代,提取其基因组DNA:通过剪取1~3 mm脚趾的方式获得小鼠组织样本并按一定顺序进行编号,从后肢到前肢,从左到右。将小鼠脚趾按照编号依次放入1.5 mL EP管中,待其沉于管底,吸取27 μL组织裂解液和3 μL蛋白酶K至EP管中并混匀。将EP管放置于金属浴恒温器中,55 ℃消化3~5 h,12 000 r/min离心30 s,95 ℃金属浴30 min灭活蛋白酶K,12 000 r/min离心30 s。取上清液,-20 ℃保存,制备PCR的DNA模板。
1.2.2.3" Pgc-1alpha条件性敲除小鼠基因型鉴定" Pgc-1alpha条件性敲除小鼠的3~5号外显子两端具有2个LoxP序列,其鉴定引物8041和8491的序列分别为5′-TCCAGTAGGCAGAGATTTATGAC-3′和5′-TGTCTGGTTTGACAATCTGCTAGGTC-3′。PCR条件:95 ℃ 5 min;94 ℃ 30 s,58 ℃ 35 s,72 ℃ 45 s,35个循环;72 ℃ 3 min;10 ℃保存。PCR产物行2%琼脂糖凝胶电泳,BioRad凝胶电泳成像仪分析。
1.2.3" Calb1-Cre小鼠基因型鉴定" 通过Cre-LoxP介导的基因重组技术可以特异性删除两个LoxP之间的基因片段,在Calb1启动子作用下,使Cre重组酶在Calb1阳性细胞,如皮层、海马、纹状体和丘脑等特异性移除Pgc-1alpha基因片段。Cre鉴定引物为T006202-F1和T006202-R1,二者序列分别为5′-CTTAATGGACTGGTGTAGCAAGCAGT-3′和5′-CTG-CACACAGACAGGAGCATCTTC-3′。PCR条件:95 ℃ 5 min;98 ℃ 30 s,58 ℃ 30 s,72 ℃ 45 s,35个循环;72 ℃ 5 min;10 ℃保存。PCR产物行2%琼脂糖凝胶电泳,BioRad凝胶电泳成像仪分析。
1.2.4" 皮层、海马条件性敲除Pgc-1alpha基因小鼠的繁育
1.2.4.1" Pgc-1alphafl/+::Calb1-Cre(Pgc-1alpha+/-)小鼠的获得" 通过Pgc-1alphafl/fl小鼠与Calb1-Cre杂交,获得Pgc-1alpha基因敲除杂合子小鼠(Pgc-1alphafl/+::Calb1-Cre)以及Pgc-1alphafl/+杂合子小鼠(图2)。
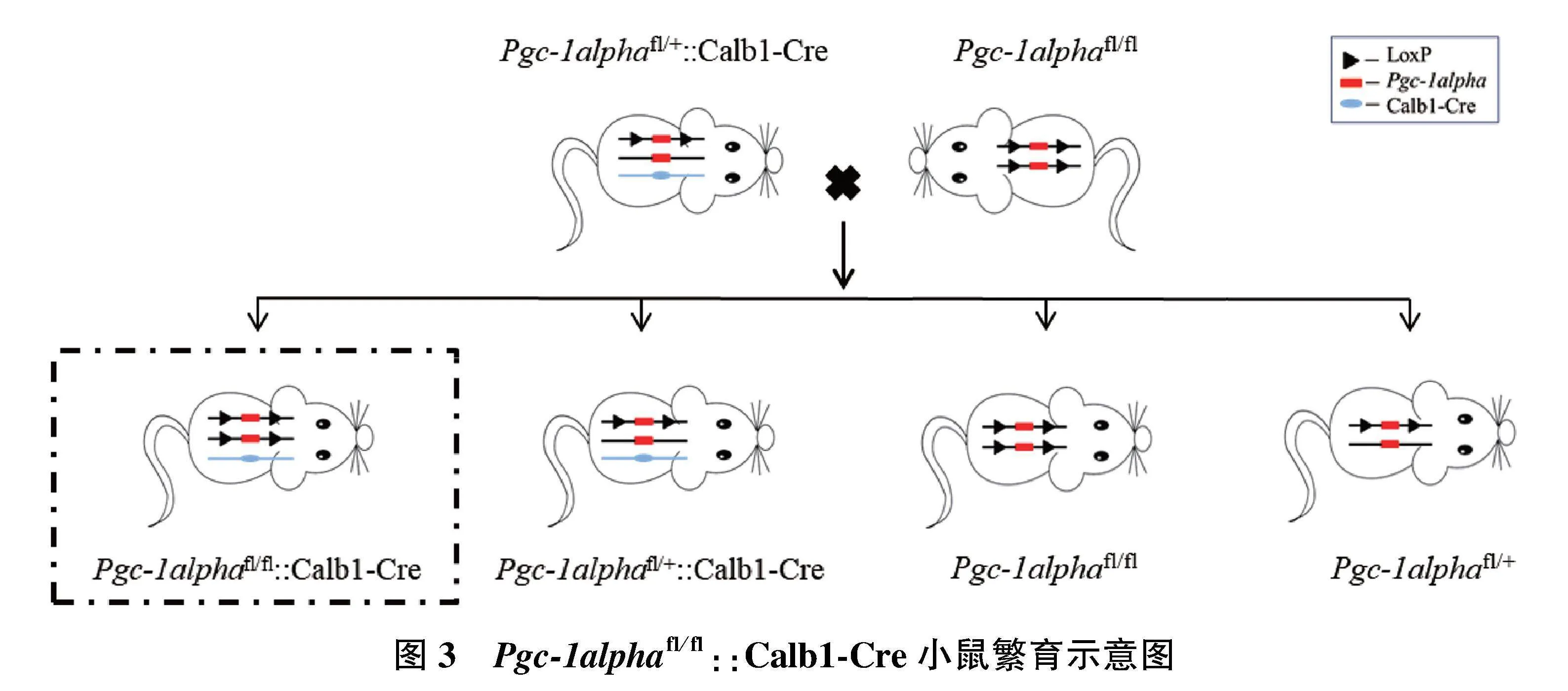
1.2.4.2" Pgc-1alphafl/fl::Calb1-Cre(Pgc-1alpha-/-)小鼠的获得" 通过Pgc-1alphafl/fl与Pgc-1alphafl/+::Calb1-Cre小鼠杂交,获得Pgc-1alpha基因敲除纯合子小鼠(Pgc-1alphafl/fl::Calb1-Cre)、Pgc-1alpha基因敲除杂合子小鼠(Pgc-1alphafl/+::Calb1-Cre)、Pgc-1alphafl/fl小鼠以及Pgc-1alphafl/+小鼠(图3)。
1.2.5" 蛋白免疫印迹检测PGC-1α表达" 提取野生型及Pgc-1alpha-/-小鼠(脑、肾)组织蛋白并测定浓度;上样量为100 μg/孔,行8% SDS-PAGE;先80 V,跑出浓缩胶升至120 V;300 mA转膜90 min,将蛋白转至0.45 μm PVDF膜;5%脱脂奶粉封闭90 min;TBST洗膜3次,5 min/次;兔抗PGC-1α抗体(稀释比1∶1 000)、小鼠抗GAPDH抗体(稀释比1∶5 000)于4 ℃孵育过夜;TBST洗膜5次,5 min/次;HRP标记的山羊抗兔、山羊抗小鼠二抗常温孵育1 h(稀释比1∶5 000);TBST洗膜5次,5 min/次;化学发光显色,Image J软件进行灰度扫描分析。
1.3" 统计分析
采用SPSS 22.0统计软件进行数据分析,计量资料用均数±标准差(x±s)表示。两组间均数比较采用独立样本t检验,Plt;0.05为差异有统计学意义。
2" 结果
2.1" Pgc-1alphafl/+、Pgc-1alphafl/fl小鼠PCR鉴定结果
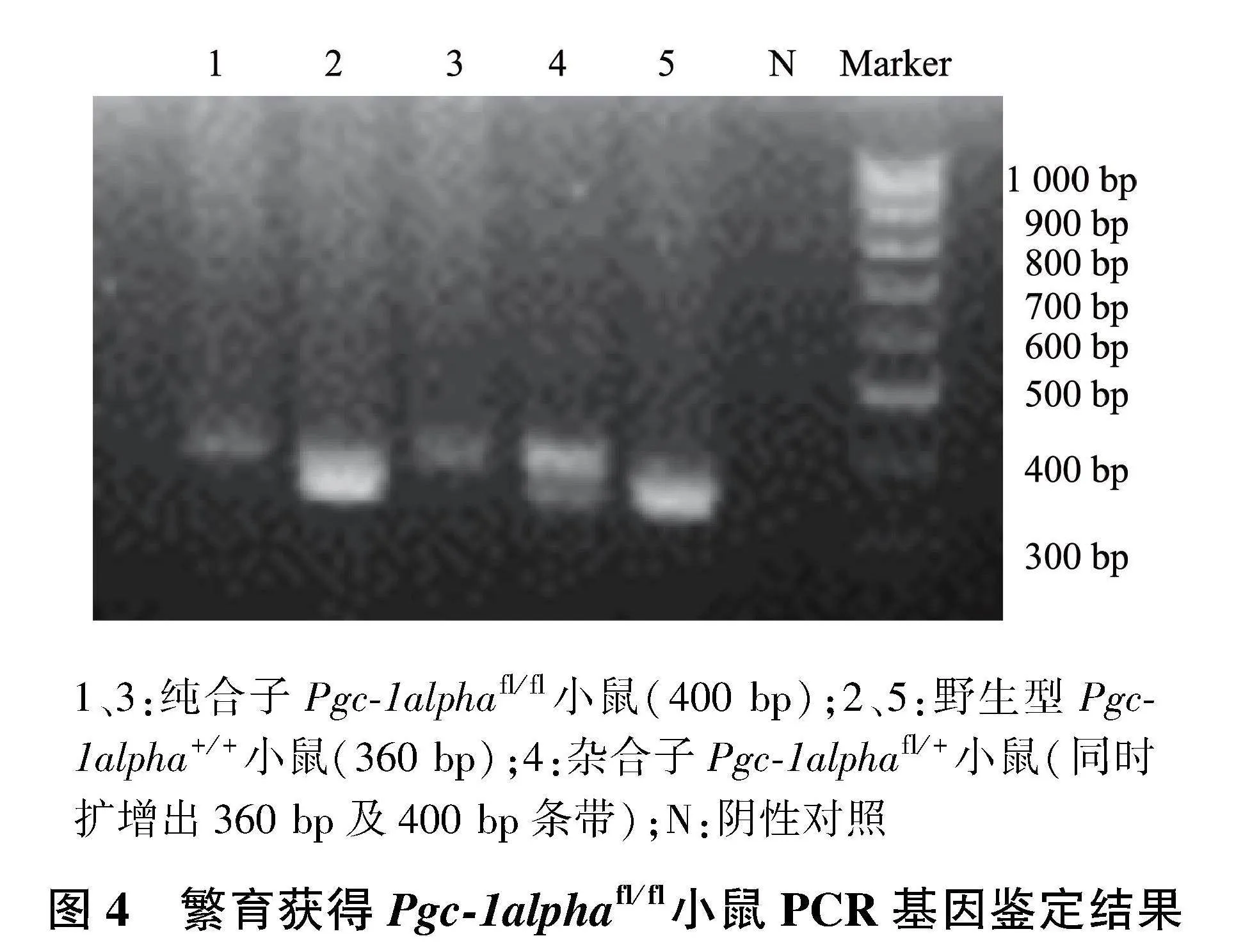
PCR鉴定结果显示,仅扩增得到一条400 bp条带的为纯合子Pgc-1alphafl/fl小鼠;同时具有360 bp和400 bp两条带的为杂合子Pgc-1alphafl/+小鼠,而仅扩增出一条360 bp条带的为野生型Pgc-1alpha+/+小鼠(图4)。
1、3:纯合子Pgc-1alphafl/fl小鼠(400 bp);2、5:野生型Pgc-1alpha+/+小鼠(360 bp);4:杂合子Pgc-1alphafl/+小鼠(同时扩增出360 bp及400 bp条带);N:阴性对照
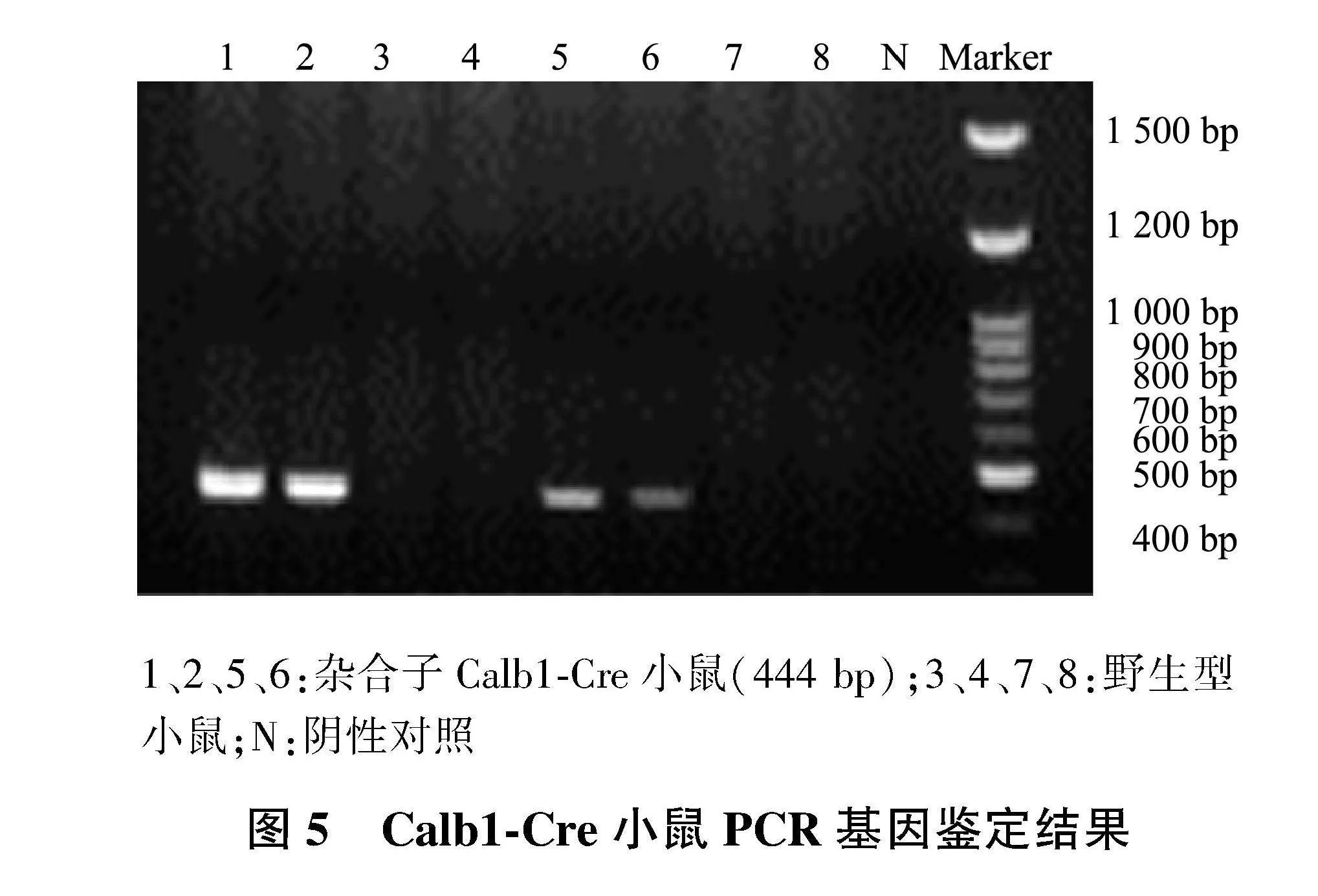
2.2" Calb1-Cre小鼠PCR鉴定结果
提取Calb1-Cre小鼠的基因组DNA,通过引物T006202-F1和T006202-R1鉴定其基因型,PCR鉴定结果显示,扩增出一条444 bp条带的为Calb1-Cre小鼠,而未扩增出条带的为野生型小鼠(图5)。
1、2、5、6:杂合子Calb1-Cre小鼠(444 bp);3、4、7、8:野生型小鼠;N:阴性对照
2.3" AD病变脑区Pgc-1alpha基因条件性敲除小鼠的鉴定
提取小鼠基因组DNA,通过引物8041和8491以及引物T006202-F1和T006202-R1鉴定其基因型。双重PCR鉴定显示,同时扩增出444 bp(Cre)及400 bp(目的基因)两条带的小鼠为Pgc-1alphafl/fl::Calb1-Cre,记为Pgc-1alpha-/-;同时扩增出444 bp(Cre)及360 bp和400 bp(目的基因)三条带的小鼠为Pgc-1alphafl/+::Calb1-Cre,记为Pgc-1alpha+/-;仅扩增出400 bp(目的基因)一条带的小鼠为Pgc-1alphafl/fl;仅扩增出360 bp和400 bp(目的基因)两条带的小鼠为Pgc-1alphafl/+(图6)。
1、2:Pgc-1alphafl/-(同时扩增出360 bp及400 bp条带);3:Pgc-1alpha基因敲除纯合子小鼠Pgc-1alphafl/fl:: Calb1-Cre(Pgc-1alpha-/-)(同时扩增出400 bp及444 bp条带);4、7:Pgc-1alpha基因敲除杂合子小鼠Pgc-1alphafl/+::Calb1-Cre(Pgc-1alpha+/-)(同时扩增出360 bp、400 bp及444 bp条带);5、6、8:Pgc-1alphafl/fl(400 bp);N:阴性对照
2.4" PGC-1α在野生型小鼠及Pgc-1alpha敲除小鼠脑、肾组织中的蛋白表达
蛋白免疫印迹结果显示,与野生型小鼠相比,Pgc-1alpha-/-小鼠脑中PGC-1α蛋白相对表达水平明显降低(t=4.623,Plt;0.001),而肾组织匀浆中PGC-1α相对表达水平差异无统计学意义(P>0.05)。见图7。
A:PGC-1α蛋白免疫印迹检测结果;B:统计分析(n=6)
3" 讨论
Lucas等[11]选择B6.Tg(Nestin-Cre)工具鼠与Pgc-1alphafl/fl小鼠杂交,获得Pgc-1alpha神经元和胶质前体细胞条件性敲除小鼠;此外,本课题组前期通过杂交Pgc-1alphafl/fl小鼠与Dlx5/6-cre-IRES-EGFP工具鼠,获得GABA能神经元Pgc-1alpha条件性敲除鼠[6,12-13]。本实验通过杂交Calb1-Cre工具鼠与Pgc-1alphafl/fl条件性敲除小鼠,获得Calb1阳性神经元Pgc-1alpha基因敲除小鼠。
研究表明,Pgc-1alpha可通过调控抗氧化酶和解偶联蛋白表达,促进线粒体的生物发生[5,14]。研究发现,AD的发生伴随PGC-1α表达下调,线粒体动力学及形态改变,同时伴随着线粒体肿胀、分布不均、线粒体膜电位下降[8]。本课题组前期研究发现,PGC-1α过表达调控线粒体动力学,改善AD所伴随的神经元凋亡及突触结构损伤[10,15]。然而Pgc-1alpha对AD小鼠线粒体动力学的直接调控机制有待于进一步阐明。本实验成功构建AD病变脑区Pgc-1alpha敲除小鼠,为探索Pgc-1alpha调控线粒体动力学异常,改善AD发生的分子机制提供了可靠的动物模型。
AD患者尸检结果显示,其大脑皮质、海马、某些皮层下核团如杏仁核、前脑基底神经核和丘脑中有大量的淀粉样沉积及神经纤维缠结[16-17]。Calb1蛋白作为调控Ca2+浓度的一种重要的钙结合蛋白,主要分布在与认知、记忆密切相关的脑区—皮层、海马、纹状体和丘脑等[18-19]。这些脑区神经元之所以能够完成认知、记忆功能主要依赖于Ca2+/钙调蛋白激酶Ⅱ(CaMKⅡ)途径,当Ca2+浓度升高时,内流Ca2+诱导CaMKⅡ磷酸化,继而影响神经信号传递,参与长时程记忆的诱导与维持[20-21]。研究发现,在人类背外侧前额叶联合皮层中,tau的病理性改变优先影响新皮层中高表达Calb的椎体细胞[22]。此外,与AD脑相似,在老年猴脑中也发现,随着年龄增加,其椎体细胞Calb表达水平逐渐下降[22];其潜在的机制与钙超载相关,即细胞质中Ca2+浓度增加,Ca2+通过诱导tau蛋白磷酸化,经多重、协同的作用发挥神经毒性作用,且随着钙结合蛋白Calb的大量丢失更为恶化[22]。Calb1作为启动子,特异性诱导AD病理的易感脑区—皮层、海马、纹状体、丘脑Cre重组酶表达,本实验通过基因重组技术成功获得Calb1阳性神经元Pgc-1alpha转基因小鼠。此外,与野生型小鼠相比,Pgc-1alpha基因敲除小鼠脑区PGC-1α蛋白表达明显降低。该Calb1阳性神经元Pgc-1alpha敲除小鼠的构建成功克服了Pgc-1alpha整体敲除子代存活率低(存活率约50%)[23]的缺点。
综上所述,本实验成功构建AD病变脑区(皮层、海马、纹状体、丘脑)Pgc-1alpha条件性敲除小鼠。此外,Pgc-1alpha整体敲除鼠的体重下降干扰了AD相关行为学的评价[23],值得注意的是Calb1作为Cre重组酶的启动子,驱动Pgc-1alpha在具有AD病理易感性的Calb1阳性神经元特异性敲除,可为探讨Pgc-1alpha基因与AD发病机制的相关性及Pgc-1alpha靶向药物的研发提供可靠的动物模型。
[参考文献]
[1]" Puigserver P, Wu Z, Park CW, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis[J]. Cell, 1998, 92(6): 829-839.
[2]" Lindholm D, Eriksson O, Mkel J, et al. PGC-1α: a master gene that is hard to master[J]. Cell Mol Life Sci, 2012, 69(15): 2465-2468.
[3]" Lai L, Wang M, Martin OJ, et al. A role for peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1) in the regulation of cardiac mitochondrial phospholipid biosynthesis[J]. J Biol Chem, 2014, 289(4): 2250-2259.
[4]" Pinho RA, Pinho CA, Tromm CB, et al. Changes in the cardiac oxidative metabolism induced by PGC-1{alpha}: response of different physical training protocols in infarction-induced rats[J]. Int J Cardiol, 2013, 168(4): 4560-4562.
[5]" Lv J, Jiang S, Yang Z, et al. PGC-1α sparks the fire of neuroprotection against neurodegenerative disorders[J]. Ageing Res Rev, 2018, 44: 8-21.
[6]" Wang J, Song HR, Guo MN, et al. Adult conditional knockout of PGC-1α in GABAergic neurons causes exaggerated startle reactivity, impaired short-term habituation and hyperactivity[J]. Brain Res Bull, 2020, 157: 128-139.
[7]" St-Pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators[J]. Cell, 2006, 127(2): 397-408.
[8]" 马思飞, 郭美娜, 刘文君, 等. 自噬调控与阿尔茨海默病相关的分子机制研究进展[J]. 江苏大学学报(医学版), 2021, 31(2): 120-123, 128.
[9]" Wang J, Guo MN, Liu ZZ, et al. PGC-1α reduces Amyloid-β deposition in Alzheimer′s disease: Effect of increased VDR expression[J]. Neurosci Lett, 2021, 744: 135598.
[10]" Wang J, Liu WJ, Shi HZ, et al. A role for PGC-1a in the control of abnormal mitochondrial dynamics in Alzheimer′s disease[J]. Cells, 2022, 11(18): 2849.
[11]" Lucas EK, Dougherty SE, McMeekin LJ, et al. Developmental alterations in motor coordination and medium spiny neuron markers in mice lacking pgc-1α[J]. PLoS One, 2012, 7(8): e42878.
[12]" 姜扬, 王佳, 李国海, 等. γ-氨基丁酸能神经元条件性敲除PGC-1α基因对小鼠焦虑抑郁行为的影响[J]. 江苏大学学报(医学版), 2017, 27(5): 369-373.
[13]" Wang J, Yun Q, Qian JJ, et al. Mice lacking the transcriptional coactivator PGC-1α exhibit hyperactivity[J]. Neuropsychobiology, 2019, 78(4): 182-188.
[14]" Kang C, Li Ji L. Role of PGC-1α signaling in skeletal muscle health and disease[J]. Ann N Y Acad Sci, 2012, 1271(1): 110-117.
[15]" 刘文君, 史厚珍, 翟鸿儒, 等. PGC-1α调控OPA1改善阿尔茨海默病的机制研究[J]. 江苏大学学报(医学版), 2022, 32(6): 489-496.
[16]" Karantzoulis S, Galvin JE. Distinguishing Alzheimer′s disease from other major forms of dementia[J]. Expert Rev Neurother, 2011, 11(11): 1579-1591.
[17]" Deng L, Gupta VK, Wu Y, et al. Mouse model of Alzheimer′s disease demonstrates differential effects of early disease pathology on various brain regions[J]. Proteomics, 2021, 21(7/8): e2000213.
[18]" Hof PR, Glezer II, Condé F, et al. Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns[J]. J Chem Neuroanat, 1999, 16(2): 77-116.
[19]" Yang T, Britt JK, Cintrón-Pérez CJ, et al. Ca2+-binding protein 1 regulates hippocampal-dependent memory and synaptic plasticity[J]. Neuroscience, 2018, 380: 90-102.
[20]" Berridge MJ. Calcium regulation of neural rhythms, memory and Alzheimer′s disease[J]. J Physiol, 2014, 592(2): 281-293.
[21]" Cascella R, Cecchi C. Calcium dyshomeostasis in Alzheimer′s disease pathogenesis[J]. Int J Mol Sci, 2021, 22(9): 4914.
[22]" Datta D, Leslie SN, Wang M, et al. Age-related calcium dysregulation linked with tau pathology and impaired cognition in non-human primates[J]. Alzheimers Dement, 2021, 17(6): 920-932.
[23]" Lin J, Wu PH, Tarr PT, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha 1 mice[J]. Cell, 2004, 119(1): 121-135.
[收稿日期]" 2023-04-07" [编辑]" 刘星星