谷氨酸铁螯合物对缺铁性贫血小鼠的补铁效果评价


DOI:10.3969/j.issn.10001565.2024.04.004
摘要:为评价自制谷氨酸铁(Glu-Fe(Ⅲ))和谷氨酸亚铁(Glu-Fe(Ⅱ))的补铁效果,采用低铁饲料联合放血法建立缺铁性贫血(IDA)小鼠模型,并将IDA小鼠随机分成硫酸亚铁组、市售右旋糖酐铁组、Glu-Fe(Ⅱ)组和Glu-Fe(Ⅲ)组,分别灌胃给予同等铁剂量的不同补铁剂4周,并以IDA小鼠为阴性对照、正常小鼠为空白对照组进行比较.用全自动血液分析仪监测小鼠血中血红蛋白(HGB)、红细胞(RBC)和血细胞比容(HCT)变化,采用血清铁(SI)、总铁结合力(TIBC)和转铁蛋白(TRF)试剂盒测定小鼠体内铁状况并测定小鼠组织(心、肝、脾和肾)铁含量,利用总超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和丙二醛(MDA)试剂盒检测各种补铁剂对小鼠体内抗氧化的影响.结果显示:IDA小鼠建模成功,Glu-Fe(Ⅲ)和Glu-Fe(Ⅱ)可提高IDA小鼠的HGB、RBC和HCT值,降低TIBC和TRF水平并提升SI含量,而Glu-Fe(Ⅲ)补铁剂效果更佳.此外,相比硫酸亚铁和右旋糖酐铁,饲喂Glu-Fe(Ⅲ)在改善IDA小鼠内脏器官(心、脾和肾)肿大、促进肝恢复、提升肝脾储铁、清除组织活性氧(ROS)且增强抗氧化活性等方面表现更佳.
关键词:谷氨酸亚铁螯合物;谷氨酸铁螯合物;缺铁性贫血;补铁剂
中图分类号:R973文献标志码:A文章编号:10001565(2024)04036508
Evaluation the iron supplementation effect of glutamate chelated
iron on iron deficiency anemia of mice
REN Zhiyuan1, LIU Xinshuo1, LU Shijin2, YANG Wenzhi1, LI Haiying1
(1. Key Laboratory of Pharmaceutical Quality Control of Hebei Province, College of Pharmaceutical
Sciences, Hebei University, Baoding 071002, China;2. Department of Pharmacy, Chinese 967th Hospital of
the Joint Logistics Support Force of the Peoples Liberation Army, Dalian 116021, China)
Abstract: Glutamate chelated ferric iron (Glu-Fe (Ⅲ)) and glutamate chelated ferrous iron (Glu-Fe (Ⅱ)) were prepared and their iron supplementation effects were evaluated. The iron deficiency anemia (IDA) mouse model was established by combining low iron feed and bloodletting. IDA mice were randomly divided into five groups (n=5): ferrous sulfate group, commercially available iron dextran group, Glu-Fe (Ⅲ) group, Glu-Fe (Ⅱ) group and negative controls group, using normal mice as blank controls. Different iron supplements at equal iron doses were given by intragastric administration for 4 weeks. The changes of hemoglobin (HGB), red blood cells (RBC) and hematocrit (HCT) in blood were monitored by automatic hematology analyzer. The iron status of IDA mice were determined by serum iron (SI), total iron
收稿日期:20230504;修回日期:20240119
基金项目:河北省自然科学基金资助项目(C2021201026)
第一作者:任梽源(2000—),男,河北大学在读硕士研究生,主要从事药物制剂方向研究.E-mail:17835420887@163.com
通信作者:李海鹰(1973—),女,河北大学副教授,博士,主要从事药物制剂与质量控制方面研究.E-mail:lihylihy@163.com
binding capacity (TIBC) and transferrin (TRF) kits and the iron contents in mouse tissues (heart, liver, spleen and kidney) were measured. Superoxide dismutase (SOD), catalase (CAT) and malondialdehyde (MDA) kits were used to detect the effects of various iron supplements on antioxidant activity of IDA mice in vivo. The results showed that IDA mouse model was successfully established. Glu-Fe (Ⅲ) and Glu-Fe (Ⅱ) could improve the HGB, RBC and HCT values of IDA mice, meanwhile, reduce TIBC and TRF content and increase SI level for administrated IDA mice, especially Glu-Fe (Ⅲ). Compared with ferrous sulfate and iron dextran, Glu-Fe (Ⅲ) was more effective in reducing the swollen of organs (heart, spleen and kidney), improving liver recovery, enhancing iron storage in liver and spleen, scavenging tissue ROS and enhancing antioxidant activity in IDA mice.
Key words: glutamate chelated ferrous iron; glutamate chelated ferric iron; iron deficiency anemia; iron supplements
铁元素在维持大脑功能中起着至关重要的作用,哺乳动物中缺铁会降低树突的细度,干扰突触功能,导致发育性认知缺陷.铁也是细胞和嘌呤代谢、DNA和神经递质合成的重要辅酶因子,对细胞生长与分化至关重要[1].体内血红蛋白(HGB)和肌红蛋白(MB)中铁不可或缺[2],体内约2/3铁储于血红蛋白,少量存于肌红蛋白、代谢酶和铁蛋白中[3].铁摄入受限会发生铁缺乏症,导致铁减少(ID)、缺铁性红细胞生成(IDE)和缺铁性贫血(IDA),严重程度依次增加,服用铁营养强化剂是治疗和预防铁缺乏症的常用办法[4].氨基酸是维持生命的基本组成部分,参与众多细胞代谢活动[5],其分子富含氨基和羧基,基团孤对电子可与铁离子形成配位键,生成稳定的具环状结构的螯合铁[6].氨基酸铁可改善无机铁对胃肠道的刺激性[7],具有更佳的生物利用度[8].氨基酸铁螯合物已被开发用于食品添加剂和缺铁性贫血的预防和治疗[9].含谷氨酸的肽显示出更好的亚铁螯合能力,可有效改善IDA症状,但补铁剂需加抗氧剂以避免亚铁氧化[10].而铁(Ⅲ)螯合物可避免铁氧化和水解,在生理pH条件下补铁,是治疗IDA常见补铁剂[11].谷氨酸(Glu)属人体非必需氨基酸,是中枢神经系统的兴奋性神经递质,参与兴奋性突触活动[12]、神经元发育和神经内分泌控制[13],也是一种重要代谢燃料,可作为体内能量来源[14].本实验室已报道Glu-Fe(Ⅲ)螯合物的制备,但对其补铁效果未加评价[15].因此,本文自制谷氨酸铁(Glu-Fe(Ⅲ))和谷氨酸亚铁(Glu-Fe(Ⅱ)),并建立缺铁性贫血(IDA)模型,对IDA小鼠灌胃治疗,考察其补铁效果.
1实验部分
1.1仪器与试药
TEK-Ⅱ系列全自动三分群血液分析仪(江西特康科技有限公司);Synergy HT酶标仪(美国伯腾仪器有限公司);HWS-80B恒温恒湿培养箱(天津市宏诺仪器有限公司);KZ-Ⅲ-F高速低温组织研磨仪(武汉塞维尔生物科技有限公司).
谷氨酸(分析纯,天津福晨化学试剂有限公司);四水氯化亚铁(国药集团化学试剂);六水氯化铁(上海麦克林生化科技有限公司);小鼠组织铁测定试剂盒、血清铁(SI)测定试剂盒、总铁结合力(TIBC)测定试剂盒、转铁蛋白(TRF)测定试剂盒、过氧化氢酶(CAT)测定试剂盒、丙二醛(MDA)测定试剂盒和总超氧化物歧化酶(SOD)测试盒均购自南京建成生物工程研究所;其他试剂均为分析纯,实验用水为二次蒸馏水.SPF级三周龄雄性ICR小鼠,体质量18~22 g,斯贝福(北京)生物技术有限公司提供(许可证号:SCXK(京)2019-0010,合格证编号:110324220103742158).1.2实验方法
1.2.1谷氨酸铁和谷氨酸亚铁的制备
Glu-Fe(Ⅱ)的制备[16]:称取1.5 g的Glu溶于30 mL蒸馏水中,70 ℃恒温水浴条件下,加热搅拌0.5 h,使Glu完全溶解.取2.0 g的四水氯化亚铁配成1 mol/L的水溶液,将其缓慢滴入Glu溶液中,边加边搅拌,用1 mol/L的NaOH溶液调节反应pH至6,加适量还原铁粉,通入N2,继续恒温水浴反应1.5 h.反应结束后,趁热过滤,在旋转蒸发仪上浓缩滤液,放冷至室温.浓缩滤液加入9倍体积的无水乙醇,醇沉静置24 h,离心所得沉淀即为Glu-Fe(Ⅱ)粗品.用少量的蒸馏水溶解,真空冷冻干燥即得Glu-Fe(Ⅱ).
Glu-Fe(Ⅲ)的制备[15]:称取1.5 g的Glu完全溶解于30 mL蒸馏水中.取4.6 g的六水氯化铁配成1 mol/L的水溶液,将其缓慢滴入Glu溶液中,边加边搅拌,用1 mol/L的NaOH溶液调节反应pH至4.8,50 ℃恒温水浴反应3 h.反应结束后,取滤液浓缩,放冷至室温,醇沉静置24 h,离心所得沉淀即为Glu-Fe(Ⅲ)粗品,用少量蒸馏水溶解,真空冷冻干燥即得Glu-Fe(Ⅲ).
1.2.2小鼠缺铁性贫血模型的建立和治疗
所有小鼠均饲养于不锈钢笼中,温度23~25 ℃,相对湿度50%~70%,自由摄食和饮水.将小鼠随机分为正常对照组和贫血模型组.正常对照组小鼠用含铁200 mg/kg的饲料喂养,贫血模型组将饲料铁含量降低到7 mg/kg,以低铁饲料喂养联合放血法,造模IDA小鼠.实验过程中每天记录食物摄入量.每周监测HGB和体质量,并观察小鼠的精神状态.小鼠HGB含量低于100 g/L认为成功建立缺铁性贫血模型.
将IDA模型组小鼠随机分为5组,每组6只,依次分为贫血模型对照组、Glu-Fe(Ⅱ)组、Glu-Fe(Ⅲ)组、FeSO4组和市售右旋糖酐铁组.除正常对照组以正常饮食外,IDA小鼠均以低铁饲料喂养.以含铁量为45.5 mg/(kg·d)的剂量分别灌胃给予IDA模型组小鼠Glu-Fe(Ⅱ)、Glu-Fe(Ⅲ)、硫酸亚铁和市售右旋糖酐铁溶液,正常对照组和贫血模型组灌胃等体积生理盐水,每天固定时间灌胃小鼠,持续4周,每周监测HGB含量和体质量.
1.2.3小鼠血常规指标测定
小鼠眼眶取血,通过全自动三分群血液分析仪测定小鼠的HGB、RBC和HCT.
1.2.4小鼠血清指标、器官指数、组织铁含量和氧化应激反应测定
小鼠灌胃4周,摘眼球取血于离心管中,室温静置30 min,2 500~4 000 r/min离心15 min分离血清,采用小鼠SI、TIBC和TRF测试盒测定血清指标.
将小鼠解剖,摘取心、肝、脾和肾,用生理盐水冲洗,用滤纸吸干并称重,按式(1)计算器官指数.分别称取0.1 g心、肝、肾和脾,以1∶9(g∶mL)的比例加入生理盐水,制备质量分数为10%的组织匀浆液,4 ℃ 2 500 r/min离心10 min,弃去沉淀取上清液检测.采用组织铁测试盒测定小鼠各组织铁含量,SOD、CAT和MDA试剂盒测定小鼠肝脏的抗氧化活性.器官指数=器官质量(g)活体质量(g)×100%.(1)1.2.5数据处理方法
实验数据用x±s表示,采用SPSS软件对数据进行单因素方差分析(ANOVA),并且进行多重比较检验,以P<0.05为有显著性差异.
2结果与讨论
2.1小鼠缺铁性贫血模型的建立和体质量差异分析
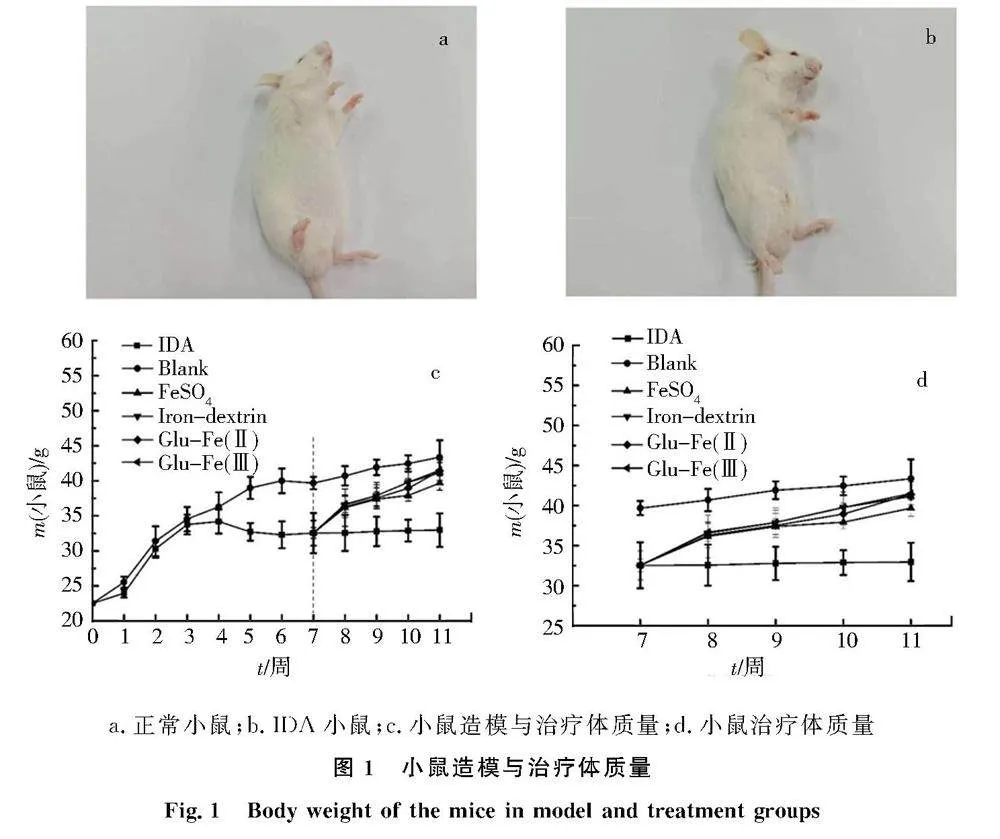
造模42 d后,36只小鼠造模成功,造模成功率64%.与正常对照组小鼠相比,IDA小鼠体形偏瘦,毛发稀疏,耳朵和爪子苍白,见图1a和图1b.在造模初期,正常对照组与IDA组的体质量无统计学意义.而造模3周,IDA组小鼠体质量增长停滞,4周后IDA组小鼠体质量明显下降,见图1c.IDA小鼠饲喂补铁剂后体质量变化见图1d,治疗恢复初期,正常对照组小鼠体质量明显高于其他组;治疗4周,硫酸亚铁组、市售右旋糖酐铁组、Glu-Fe(Ⅱ)组和Glu-Fe(Ⅲ)组的小鼠体质量,均逐渐上升.a.正常小鼠;b.IDA小鼠;c.小鼠造模与治疗体质量;d.小鼠治疗体质量
2.2小鼠血中HGB、RBC和HCT指标测定
造模与治疗过程IDA小鼠HGB变化,见图2a与图2b.红细胞的HGB负责运输氧气,铁是HGB重要组成成分,缺铁会降低HGB含量[17].正常对照组HGB含量稳定在约135 g/L,造模小鼠的HGB逐渐降低至57.2 g/L.饲喂补铁剂后,在7、14、21、28 d分别测定HGB含量,各组HGB含量均随时间增加而增大,治疗前2周增加显著.补铁4周,所有补铁组的HGB含量均得到改善,HGB含量大小依次为Glu-Fe(Ⅲ)组、市售右旋糖酐铁组、Glu-Fe(Ⅱ)组和硫酸亚铁组.
图2c和图2d是小鼠RBC变化图,RBC代表红细胞数,约占血量的50%,负责CO2和O2的运输[18].相比正常组RBC稳定在约8.70×1012/L,造模小鼠RBC含量逐渐降低至4.27×1012/L.饲喂补铁剂4周后,补铁组IDA小鼠的RBC含量大小依次为Glu-Fe(Ⅲ)组、市售右旋糖酐铁组、Glu-Fe(Ⅱ)组和硫酸亚铁组.
图2e和图2f是小鼠HCT变化图.HCT代表计算出的红细胞在血液中的体积百分比[19],取决于红细胞中的HGB浓度,IDA症状会导致HCT水平偏低.正常对照组HCT体积分数稳定在46%左右,造模IDA组小鼠HCT体积分数逐渐降低至21%.给予补铁剂4周,补铁组小鼠HCT含量均得到改善,各补铁组HCT含量大小依次为Glu-Fe(Ⅲ)组、市售右旋糖酐铁组、Glu-Fe(Ⅱ)组和硫酸亚铁组.
综上,饲喂补铁剂后各组IDA小鼠血中HGB、RBC和HCT指标都得到改善,其中Glu-Fe(Ⅲ)组效果最佳,补铁剂可以在一定程度上改善贫血症状.
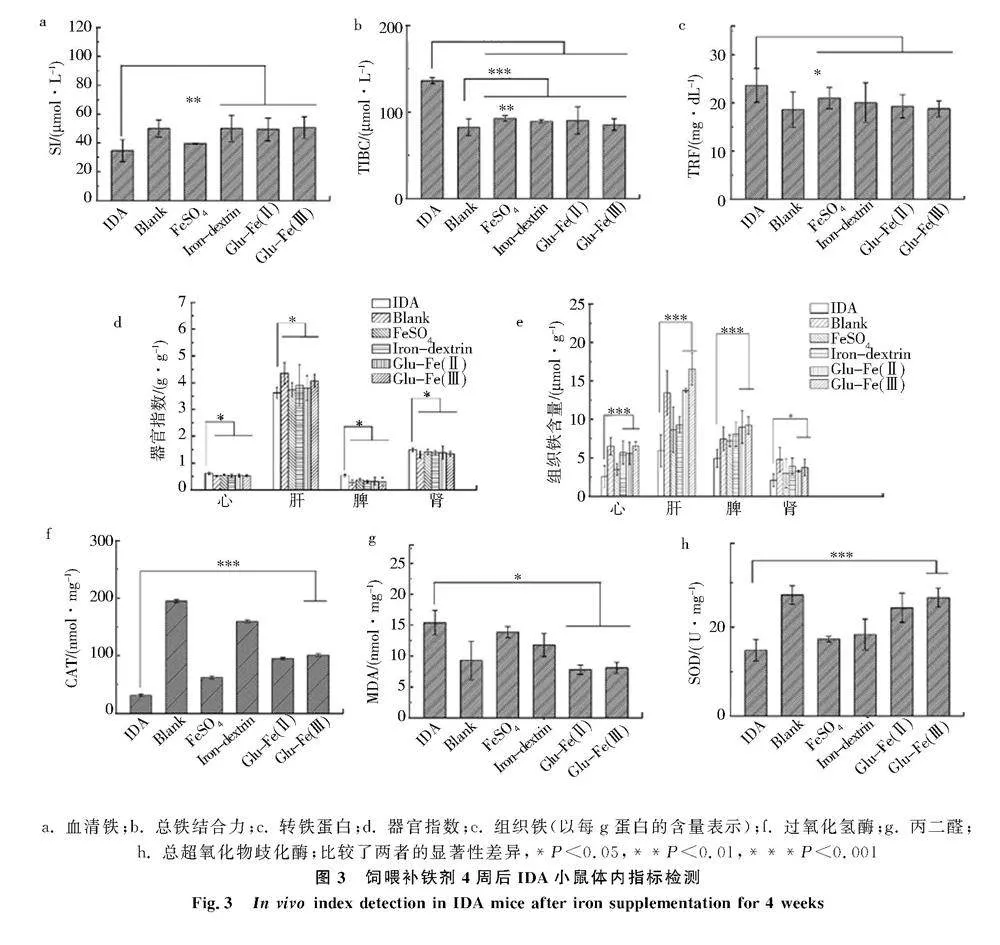
2.3给予补铁剂后IDA小鼠体内指标评价
血清生化指标是评价机体健康状况的重要指标,SI指标有助于判断体内铁含量状况[20],TIBC是铁状态的生物标志物[21],TRF是负责铁运输的蛋白质[22],SI、TIBC和TRF可反映体内铁代谢的情况.如图3a-3c,正常组小鼠的SI、TIBC和TRF分别为50.00 μmol/L、82.31 μmol/L、18.61 mg/dL,而IDA小鼠分别为34.62 μmol/L、136.20 μmol/L和23.66 mg/dL.给予补铁剂4周,相比IDA组小鼠,各补铁组小鼠SI含量明显提高,TIBC水平和TRF含量降低,3种指标参数均有所改善.SI含量大小依次为Glu-Fe(Ⅲ)组、市售右旋糖酐铁组、Glu-Fe(Ⅱ)组和硫酸亚铁组;TIBC含量大小依次为硫酸亚铁组、Glu-Fe(Ⅱ)组、市售右旋糖酐铁组、Glu-Fe(Ⅲ)组;TRF含量大小依次为硫酸亚铁组、市售右旋糖酐铁组、Glu-Fe(Ⅱ)组和Glu-Fe(Ⅲ)组.此外,市售右旋糖酐铁组、Glu-Fe(Ⅱ)组和Glu-Fe(Ⅲ)组的SI、TIBC和TRF含量之间无显著性差异,Glu-Fe(Ⅲ)组的3个指标更接近正常组.
小鼠器官质量和指数是研究药物毒性的指标,IDA组小鼠的心、肝、脾和肾器官指数与正常组小鼠均具有显著差异(图3d).贫血会导致血液携氧能力下降,限制红细胞生成,导致铁消耗和铁储存量减少[23].铁缺乏会导致心脏肥厚和心肌细胞增大[24],IDA组小鼠的心脏指数明显高于正常对照组.肝和脾是储铁的主要器官,体内多余的铁主要以铁蛋白和血红蛋白的形式沉积在肝、脾和骨髓中[25].IDA组小鼠肝脏较小,文献[26]认为缺铁导致肝脏生长和DNA合成受损.缺铁会引起免疫功能变化,且贫血引起的血红蛋白改变导致脾肿大[27],缺铁会导致肾脏损伤发生水肿[28],这与IDA组小鼠的脾和肾指数明显高于正常对照组的结果一致,表明IDA组小鼠发生了器官损伤.饲喂补铁剂4周,各组小鼠器官指数均有改善,与IDA组小鼠具有显著性差异.补铁组IDA小鼠的肝脏指数大小依次为Glu-Fe(Ⅲ)组、市售右旋糖酐铁组、Glu-Fe(Ⅱ)组和硫酸亚铁组;而IDA小鼠的心、脾和肾脏指数明显下降,心、脾和肾指数大小依次为硫酸亚铁组、Glu-Fe(Ⅱ)组、市售右旋糖酐铁组和Glu-Fe(Ⅲ)组.
机体铁稳态需要多个器官的协同,小鼠器官组织中的铁含量可反映铁稳态[29].图3e是各组小鼠的心、肝、脾和肾的铁含量(以每g蛋白中的含量表示),IDA组小鼠的各器官组织铁含量与正常组小鼠均具有显著差异.经过4周的补铁剂饲喂,各补铁组心脏铁含量大小依次为Glu-Fe(Ⅲ)组、市售右旋糖酐铁组、Glu-Fe(Ⅱ)组和硫酸亚铁组;肝和脾中铁含量大小依次为Glu-Fe(Ⅲ)组、Glu-Fe(Ⅱ)组、市售右旋糖酐铁组和硫酸亚铁组;肾脏铁含量大小依次为市售右旋糖酐铁组、Glu-Fe(Ⅲ)组、Glu-Fe(Ⅱ)组和硫酸亚铁组.饲喂Glu-Fe(Ⅲ)的IDA小鼠心、肝和脾中铁含量均高于其他补铁剂的IDA小鼠.a. 血清铁;b. 总铁结合力;c. 转铁蛋白;d. 器官指数;e. 组织铁(以每g蛋白的含量表示);f. 过氧化氢酶;g. 丙二醛;
h. 总超氧化物歧化酶;比较了两者的显著性差异,*P<0.05,**P<0.01,***P<0.001
图3饲喂补铁剂4周后IDA小鼠体内指标检测
Fig.3In vivo index detection in IDA mice after iron supplementation for 4 weeks氧化应激是体内活性氧失衡[30],IDA症状可导致氧化应激[31],降低抗氧化酶的活性.体内抗氧化是清除活性氧(ROS),防止氧化损伤,而酶系统SOD和CAT变化是评价细胞氧化还原态重要指标[32].体内自由基作用于脂质发生过氧化反应,产生的MDA会导致蛋白质或核酸等大分子交联,产生细胞毒性[33].饲喂补铁剂的IDA小鼠氧化应激结果见图3f-3h,正常组小鼠每mg蛋白中CAT、SOD和MDA含量为194.65 nmol、27.24 U和9.28 nmol,而IDA组小鼠分别为31.36 nmol、14.79 U和15.39 nmol,CAT值和SOD值降低,MDA值升高.由于IDA症状导致体内氧化损伤,使得抗氧化标志物下降和脂质过氧化增加[34].相比IDA组,各补铁组小鼠的CAT值和SOD值升高,而MDA值降低.各补铁组CAT大小依次为市售右旋糖酐铁组、Glu-Fe(Ⅲ)组、Glu-Fe(Ⅱ)组和硫酸亚铁组;SOD含量大小依次为Glu-Fe(Ⅲ)组、Glu-Fe(Ⅱ)组、市售右旋糖酐铁组和硫酸亚铁组;MDA含量大小依次为硫酸亚铁组、市售右旋糖酐铁组、Glu-Fe(Ⅲ)组和Glu-Fe(Ⅱ)组.通过3项指标检测发现饲喂FeSO4补铁剂IDA小鼠体内的抗氧化能力最差,可能与无机铁体内吸收相对较差有关;Glu-Fe(Ⅱ)和Glu-Fe(Ⅲ)补铁剂在对抗体内脂质氧化(图3g)和催化超氧化物阴离子歧化作用上(图3h)表现较优;市售右旋糖酐铁组小鼠的体内清除过氧化氢显示出高能力(图3f);此外,饲喂Glu-Fe(Ⅲ)IDA小鼠的MDA和SOD值更接近对照的正常组小鼠.
3结论
本文建立IDA小鼠模型,评价Glu-Fe(Ⅱ)和Glu-Fe(Ⅲ)的补铁效果.结果表明2种补铁剂均可提升IDA小鼠HGB、RBC和HCT值,使得小鼠体质量、主要脏器获得恢复,血中SI、TIBC和TRF指标趋向正常.此外,2种补铁剂均可提升IDA小鼠的SOD和CAT,并抑制MDA产生,显示出自由基清除能力.相比市售硫酸亚铁和右旋糖酐铁,饲喂Glu-Fe(Ⅲ)改善IDA小鼠贫血相关的HGB、RBC和HCT血常规指标及SI、TIBC和TRF血生化指标、减轻内脏器官(心、脾和肾)肿大、促进肝恢复、提升肝脾储铁、清除组织ROS且增强抗氧化活性等表现更佳.而Glu-Fe(Ⅱ)制剂在改善IDA小鼠贫血相关的血常规指标、减轻内脏器官(心、脾和肾)肿大并增加肝脾储铁上仅比市售硫酸亚铁好.综上,Glu-Fe(Ⅲ)制剂可作为同时补充氨基酸和铁的营养强化剂.
参考文献:
[1]GUO X, JIN X, HAN K, et al. Iron promotes neurological function recovery in mice with ischemic stroke through endogenous repair mechanisms[J]. Free Radic Biol Med, 2022, 182: 59-72. DOI:10.1016/j.freeradbiomed.2022.02.017.
[2]JING J L, NING T C Y, NATALI F, et al. Iron supplementation delays aging and extends cellular lifespan through potentiation of mitochondrial function[J]. Cells, 2022, 11(5): 862. DOI:10.3390/ cells11050862.
[3]ZORODDU M A, AASETH J, CRISPONI G, et al. The essential metals for humans: a brief overview[J]. J Inorg Biochem, 2019, 195: 120-129. DOI: 10.1016/j.jinorgbio.2019.03.013.
[4]韦晓丽,周吉超,张晓伟.铁代谢及其在心力衰竭治疗中的作用机制研究进展[J].药学学报,2022,57(6):1584-1592.DOI: 10.16438/j.0513-4870.2022-0268.
[5]KELLY B, PEARCE E L. Amino assets: how amino acids support immunity[J]. Cell Metab, 2020, 32(2): 154-175. DOI: 10.1016/j.cmet.2020.06.010.
[6]SANCHEZ J, VILLADA O A, ROJAS M L, et al. Effect of zinc amino acid chelate and zinc sulfate in the incidence of respiratory infection and diarrhea among preschool children in child daycare centers[J]. Biomedica, 2014, 34(1): 79-91. DOI:10.1590/S0120-41572014000100011.
[7]MOETY G A F A, ALI A M, FOUAD R, et al. Amino acid chelated iron versus an iron salt in the treatment of iron deficiency anemia with pregnancy: A randomized controlled study[J]. Eur J of Obst Gynecol Reprod Biol, 2017, 210: 242-246. DOI: 10.1016/j.ejogrb.2017.01.003.
[8]FISCHER J A J, CHERIAN A M, Bone J N, et al. The effects of oral ferrous bisglycinate supplementation on hemoglobin and ferritin concentrations in adults and children: a systematic review and meta-analysis of randomized controlled trials[J]. Nutr Rev, 2023,81(8):904-920. DOI: 10.1093/nutrit/nuac106.
[9]HERTRQMPFE, OLIVARES M. Iron qmino acid chelates[J]. International Journal for Vitamin and Nutrition Research, 2004,74(6):435-443. DOI: 10.1024/0300-9831.74.6.635.
[10]JIANG S, DONG W, ZHANG Z, et al. A new iron supplement: The chelate of pig skin collagen peptide and Fe2+ can treat iron-deficiency anemia by modulating intestinal flora[J]. Front Nutr, 2022, 9: 1055725. DOI: 10.3389/fnut.2022.1055725.
[11]LU Q, XU L, MENG Y, et al. Preparation and characterization of a novel astragalus membranaceus polysaccharide-iron (Ⅲ) complex[J]. Int J Biol Macromol, 2016, 93: 208-216. DOI: 10.1016/j.ijbiomac.2016.08.049.
[12]RODRIGUEZ-CAMPUZANO A G, ORTEGA A. Glutamate transporters: Critical components of glutamatergic transmission[J]. Neuropharmacology, 2021, 192: 108602. DOI: 10.1016/j.neuropharm.2021.108602.
[13]代毅聪,陈凤容,王昆华.谷氨酸转运体的结构、功能及其在神经精神疾病中的作用[J].昆明医科大学学报,2020,41(9):142-148.
[14]MORTENSEN A, AGUILAR F, CREBELLI R, et al. Re-evaluation of glutamic acid (E 620), sodium glutamate (E 621), potassium glutamate (E 622), calcium glutamate (E 623), ammonium glutamate (E 624) and magnesium glutamate (E 625) as food additives[J]. EFSA J, 2017, 15(7): e04910. DOI: 10.2903/j.efsa.2017.4910.
[15]刘新硕,王真,李晴,等.谷氨酸铁的制备及表征[J].河北大学学报(自然科学版),2023,43(5):476-483. DOI:10.3969/j.issn.1000 1565.2023.05.005.
[16]黄杰,于新,刁丽婷,等.响应面法优化谷氨酸亚铁的制备工艺[J].食品科学,2015,36(10):81-85.DOI: 10.7506/spkx1002-6630-201510016.
[17]BAHDILA D, MARKOWITZ K, PAWAR S, et al. The effect of iron deficiency anemia on experimental dental caries in mice[J]. Arch Oral Biol, 2019, 105: 13-19. DOI: 10.1016/j.archoralbio.2019.05.002.
[18]TOBIAN A, HEDDLE N M, WIEGMANN T L, et al. Red blood cell transfusion: 2016 clinical practice guidelines from AABB[J]. Transfusion, 2016, 56(10): 2627-2630. DOI: 10.1111/trf.13735.
[19]谢晓恬.儿科血常规检查的意义与解读[J].临床儿科杂志,2013,31(4):398-400.DOI: 10.3969/j.issn.1000-3606.2013.04.029.
[20]NGUYEN L T, BUSE J D, BASKIN L, et al. Influence of diurnal variation and fasting on serum iron concentrations in a community-based population[J]. Clin Biochem, 2017, 50(18): 1237-1242. DOI: 10.1016/j.clinbiochem.2017.09.018.
[21]IKEDA-TANIGUCHI M, TAKAHASHI K, SHISHIDO K, et al. Total iron binding capacity is a predictor for muscle loss in maintenance hemodialysis patients[J]. Clini Exp Nephrol, 2022, 26(6): 583-592. DOI: 10.1007/s10157-022-02193-1.
[22]KAWABATA H. Transferrin and transferrin receptors update[J]. Free Radic Biol and Med, 2019, 133: 46-54. DOI: 10.1016/j.freeradbiomed.2018.06.037.
[23]TANGEDA P R, PATIL S, SHASTRI N, et al. Maternal myocardial performance in second trimester of pregnancy with iron deficiency anaemia[J]. J Clin Diagn Res, 2016, 10(3): CC16-18. DOI: 10.7860/JCDR/2016/17774.7507.
[24]KOBAK K A, RADWANSKA M, DZIEGALA M, et al. Structural and functional abnormalities in iron-depleted heart[J]. Heart Fail Rev, 2019, 24: 269-277. DOI: 10.1007/s10741-018-9738-4.
[25]REGENBOOG M, BOHTE A E, AKKERMAN E M, et al. Iron storage in liver, bone marrow and splenic Gaucheroma reflects residual disease in type 1 Gaucher disease patients on treatment[J]. Br J Haematol, 2017, 179(4): 635-647. DOI: 10.1111/bjh.14915.
[26]JANA F, ROGALSKY D W, TRUKSA J, et al. Effect of stimulated erythropoiesis on liver SMAD signaling pathway in iron-overloaded and iron-deficient mice[J]. PLoS One, 2019, 14(4): e0215028. DOI: 10.1371/journal.pone.0215028.
[27]ANGERMEIER E, DOMES K, LUKOWSKI R, et al. Iron deficiency anemia in cyclic GMP kinase knockout mice[J]. Haematologica, 2016, 101(2): e4851. DOI: 10.3324/haematol.2015.137026.
[28]DRAKE K A, SAUERBRY M J, BLOHOWIAK S E, et al. Iron deficiency and renal development in the newborn rat[J]. Pediatr Res, 2009, 66(6): 619-624. DOI: 10.1203/PDR.0b013e3181be79c2.
[29]PARK K T, SIM I, KO H S, et al. Gamma aminobutyric acid increases absorption of glycine-bound iron in mice with iron deficiency anemia[J]. Biol Trace Elem Res, 2020, 197(2): 628-638. DOI: 10.1007/s12011-020-02027-9.
[30]蔺冬冬,侯亚璐,唐婷,等.白藜芦醇对紫外线诱导家蝇氧化损伤的保护作用[J].河北大学学报(自然科学版),2019,39(5):510-515.DOI: 10.3969/j.issn.1000-1565.2019.05.011.
[31]SCHWARTZ A J, CONVERSO-BARAN K, MICHELE D E, et al. A genetic mouse model of severe iron deficiency anemia reveals tissue-specific transcriptional stress responses and cardiac remodeling[J]. J Biol Chem, 2019, 294(41): 14991-15002. DOI: 10.1074/jbc.RA119.009578.
[32]NAGABABU E, GULYANI S, EARLEY C J, et al. Iron-deficiency anaemia enhances red blood cell oxidative stress[J]. Free Radic Res, 2008, 42(9): 824-829. DOI: 10.1080/10715760802459879.
[33]ZHANG C, JIN Y, YU Y, et al. Cadmium-induced oxidative stress, metabolic dysfunction and metal bioaccumulation in adult palaemonid shrimp Palaemon macrodactylus (Rathbun, 1902)[J]. Ecotoxicol Environ Saf, 2021, 208: 111591. DOI: 10.1016/j.ecoenv.2020.111591.
[34]USMAN S S, DAHIRU M, ABDULLAHI B, et al. Status of malondialdehyde, catalase and superoxide dismutase levels/activities in schoolchildren with iron deficiency and iron-deficiency anemia of Kashere and its environs in Gombe State, Nigeria[J]. Heliyon, 2019, 5(8): e02214. DOI: 10.1016/j.heliyon.2019.e02214.
(责任编辑:梁俊红)

