高原IBS与肠屏障损伤、炎性介质及神经递质的相关性研究
张兴光 王邦茂 刘涛涛 张文成 董艳美 李小青 牛海艳 夏时海
作者单位:1武警特色医学中心消化内科(邮编300162);2天津医科大学总医院消化内科
作者简介:张兴光(1984),男,主治医师,主要从事内镜下消化道早癌及高原肠道菌群方面研究。E-mail:zhangxingguang1024@163.com
△通信作者 E-mail:xshhcx@sina.com
摘要:目的 分析高原环境下肠易激综合征(IBS)患者的肠屏障损伤指标、神经递质及炎性介质变化,探讨IBS的潛在发病机制。方法 81例男性汉族健康志愿者自平原进入西藏拉萨并完成1年前瞻性队列观察,其中诊断IBS(罗马Ⅳ)13例(IBS组),并随机抽取11例健康者为对照组(NC组),另随机抽取从平原进入拉萨1周内且未发生急性高山病者11例为NC早期组。采用酶联免疫吸附试验(ELISA)检测血清二胺氧化酶(DAO)和肠脂肪酸结合蛋白(I-FABP)、5羟色胺(5-HT)、白细胞介素(IL)-6、IL-17A及脂多糖(LPS)水平;随访末月,IBS中9例行肠镜检查及肠黏膜病理活检,NC组中4例行肠镜检查。结果 高原环境下IBS患者肠镜及病理结果提示肠黏膜轻度炎症表现。与NC早期组比较,NC组进入拉萨后3个月时5-HT、DAO、I-FABP、IL-6、IL-17A和LPS水平及6个月时5-HT、I-FABP、IL-6和LPS水平均升高,IBS组3、6个月时6项血清学指标均升高(P<0.05);与NC组比较,IBS组3个月时5-HT和LPS水平降低,6个月时DAO、I-FABP、IL-6、IL-17A和LPS水平升高(P<0.05)。NC组6个月时6项血清学指标均较3个月时降低(P<0.05);IBS组3个月与6个月时血清学指标差异均无统计学意义。结论 肠黏膜屏障损伤指标、神经递质及炎性介质可能参与高原环境下IBS的发生。
关键词:肠易激综合征;血清素;脂肪酸结合蛋白质类;白细胞介素6;高原环境
中图分类号:R574.4文献标志码:ADOI:10.11958/20230882
Correlation study between high altitude IBS and intestinal barrier damage, inflammatory mediators and neurotransmitters
ZHANG Xingguang1, WANG Bangmao2, LIU Taotao1, ZHANG Wencheng1, DONG Yanmei1,
LI Xiaoqing1, NIU Haiyan1, XIA Shihai1△
1 Department of Gastroenterology, Characteristic Medical Center of Chinese Peoples Armed Police Force, Tianjin 300162, China; 2 Department of Gastroenterology, Tianjin Medical University General Hospital
△Corresponding Author E-mail: xshhcx@sina.com
Abstract: Objective To analyzing changes of indicators related to intestinal barrier damage, neurotransmitters and inflammatory mediators in patients with irritable bowel syndrome (IBS) in the plateau environment, and explore the underlying pathogenesis of IBS. Methods A prospective cohort observation was conducted on 81 healthy male Han Chinese volunteers who were admitted to Lhasa of Tibe from plain. The study lasted for one year. During the one-year follow-up, 13 patients who developed IBS (Rome Ⅳ) were selected as the study subjects. Additionally 11 participants who remained healthy were randomly selected as the control group (named NC). Eleven participants who entered Lhasa from plain within one week and did not develop acute mountain sickness were randomly selected as the NC early group. Serum levels of diamine oxidase (DAO) and intestinal fatty acid-binding protein (I-FABP), 5-hydroxytryptamine (5-HT), interleukin (IL)-6, IL-17A and lipopolysaccharide (LPS) were measured using enzyme-linked immunosorbent assay (ELISA). At the end of the follow-up period, colonoscopy and intestinal mucosal biopsy were performed in 9 cases of IBS, while colonoscopy was performed in 4 cases of NC. Results Colonoscopy and pathological results of IBS patients in plateau environment suggested mild inflammation of intestinal mucosa. Compared to the NC early group, the NC group showed increased levels of 5-HT, DAO, I-FABP, IL-6, IL-17A and LPS at 3 months after entering Lassa, and increased levels of 5-HT, I-FABP, IL-6, and LPS at 6 months (P<0.05). Compared with the NC group, the IBS group showed decreased levels of 5-HT and LPS at 3 months after entering Lhasa (P<0.05). Serological indexes of NC group at 6 months were lower than those at 3 months in the NC group (P<0.05). There were no significant differences in serological indexes at 3 and 6 months of the IBS group. Conclusion Indicators of intestinal mucosal barrier damage, neurotransmitters and inflammatory mediators might be involved in the development of IBS in plateau environment.
Key words: irritable bowel syndrome; serotonin; fatty acid-binding proteins; interleukin 6; plateau environment
肠易激综合征(irritable bowel syndrome,IBS)为常见的功能性胃肠道疾病,高原环境能够导致IBS發生率升高及肠道菌群失调,但其机制不明[1]。高原环境暴露会导致小鼠结肠腔内氧浓度下降,进而损伤小鼠结肠组织屏障的完整性,同时炎性介质干扰素(IFN)-γ、肿瘤坏死因子(TNF)-α和白细胞介素(IL)-6水平升高[2]。一项模拟高原环境并采用粪便菌群移植方法和抗生素鸡尾酒疗法的动物实验证实,肠道菌群失调破坏了肠黏膜屏障,最终诱发并加剧了肠道损伤[3]。另一组模拟高原动物实验显示,低氧能促进抗菌肽血管生成素-4的分泌,增加脱硫弧菌属(Desulfovibrio)的丰度;而脱硫弧菌属的磷脂代谢产物可被肠上皮细胞的CD1d呈递,诱导产生IL-17A的γδT细胞增殖,加重肠损伤[4]。高原环境下的IBS发生率较高,与肠道菌群失调相关,肠屏障损伤、神经递质及炎性介质是否影响其发生机制目前尚未明确。本文通过前瞻性队列研究,从神经递质、炎性介质及肠屏障损伤等方面对高原环境下IBS发生的可能机制进行初步探讨,为高原IBS的预防及治疗提供参考。
1 对象与方法
1.1 研究对象 按照团体编制系列,选择2020年9月自平原(海拔<500 m)进入西藏拉萨(海拔>3 600 m)的92例男性汉族健康志愿者,年龄(20.20±1.61)岁,对其进行为期1年的观察。81例完成随访,其中诊断IBS(罗马Ⅳ)13例,其他非特异性胃肠道症状者12例,健康者56例。将13例IBS患者作为IBS组,从健康者中随机抽取11例作为对照组(NC组)。由于IBS组和NC组未采集到进入拉萨时的早期数据,另外从2021年3月自平原进入拉萨1周内且未发生急性高山病(acute mountain sickness,AMS)的男性汉族健康志愿者中随机抽取11例作为NC早期组一同纳入研究。本研究通过武警特色医学中心伦理委员会审核批准(编号2021-0015.1),获得参与者知情同意并签署知情同意书。
1.2 诊断、纳入及排除标准 IBS诊断标准:症状出现至少6个月,近3个月平均每周至少发作1次腹痛;伴有以下2项及以上:(1)与排便相关。(2)排便频率有改变。(3)粪便性状外观改变。纳入标准:(1)团体编制系列人员。(2)参与集体管理,生活作息、工作、学习、训练强度及时间相同。(3)调查资料完整及血清学标本合格者。排除标准:(1)近3个月内有益生菌或抗生素药物史。(2)研究前既往做过结肠镜检查,间断使用泻药、大便软化剂或止泻药等。
1.3 指标检测 (1)血清学指标检测。IBS组和NC组在进入拉萨后的第3、6个月及NC早期组在入拉萨后1周内,清晨空腹采集受检者静脉血3~5 mL,3 000 r/min离心10 min,收集上清液,采用酶联免疫吸附试验(ELISA)检测患者血清肠黏膜屏障损伤指标[二胺氧化酶(diamine oxidase,DAO)和肠脂肪酸结合蛋白(intestinal fatty acid binding protein,I-FABP)]、神经递质5羟色胺(5-Hydroxytryptamine,5-HT)及炎性介质IL-6、IL-17A及脂多糖(LPS)水平,ELISA试剂盒均购自江苏酶免实业有限公司,酶标分析仪型号为Rayto RT-6100,按照各试剂盒说明书进行操作。(2)肠镜检查及肠黏膜病理活检。在第12个月的随访中,IBS中有9例参与者完成肠镜检查并行肠黏膜病理活检,NC组中有4例行肠镜检查,未行病理活检。肠道清肠采用3 L聚乙二醇电解质散的分次剂量方案,肠道清洁质量达到波士顿肠道准备评分量表(Boston bowel preparation scale,BBPS)6分以上方可进行肠镜检查[5]。设备采用奥林巴斯CF-Q1501电子结肠镜进行检查,采用一次性活检钳取肠黏膜标本送检。
1.4 统计学方法 采用SPSS 22.0软件进行数据分析。符合正态分布的计量资料以均数±标准差([x] ±s)表示,多组间比较采用单因素方差分析,方差齐者组间多重比较行LSD-t检验,方差不齐者组间多重比较行Tamhane检验。组内不同时点比较采用配对t检验;计数资料以例表示,组间比较采用χ2检验。P<0.05为差异有统计学意义。
2 结果
2.1 各组人群基线特征比较 3组间年龄、体质量指数(BMI)及学历的差异无统计学意义,NC早期组睡眠时间长于IBS组和NC组(P<0.05),见表1。
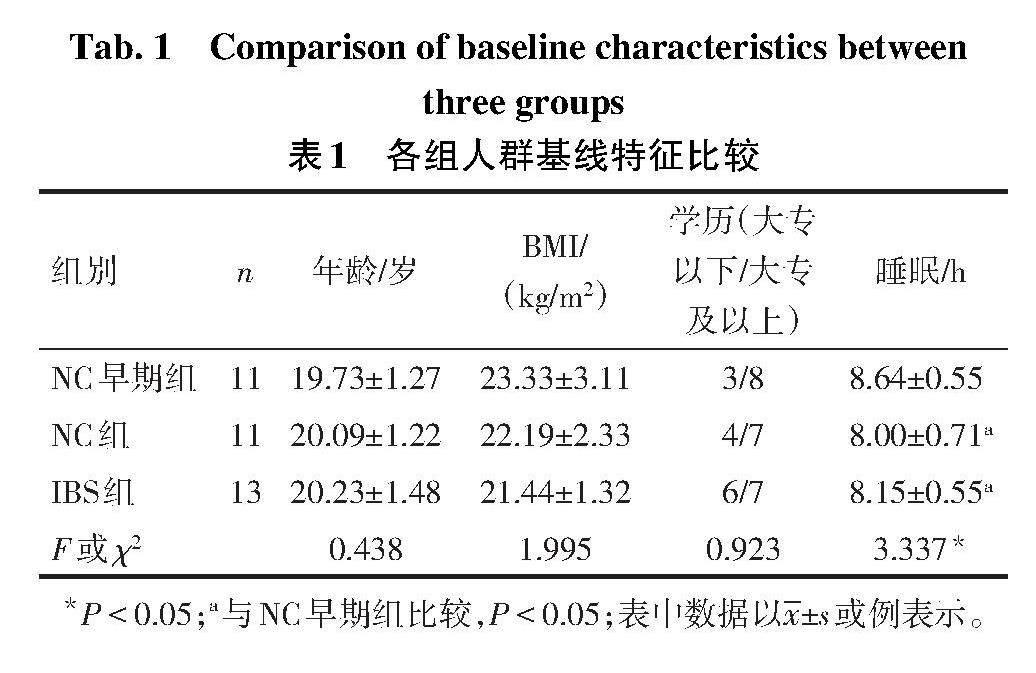
2.2 IBS组和NC组肠镜下黏膜表现 IBS组肠镜下表现为肠黏膜轻度炎症(图1A、B),而NC组肠黏膜表现大致正常(图1C、D)。从整体上来看,高原缺氧环境下,参与者的肠黏膜均可见细微的水肿表现,而相对于NC组,IBS组的肠黏膜炎症表现更加严重。
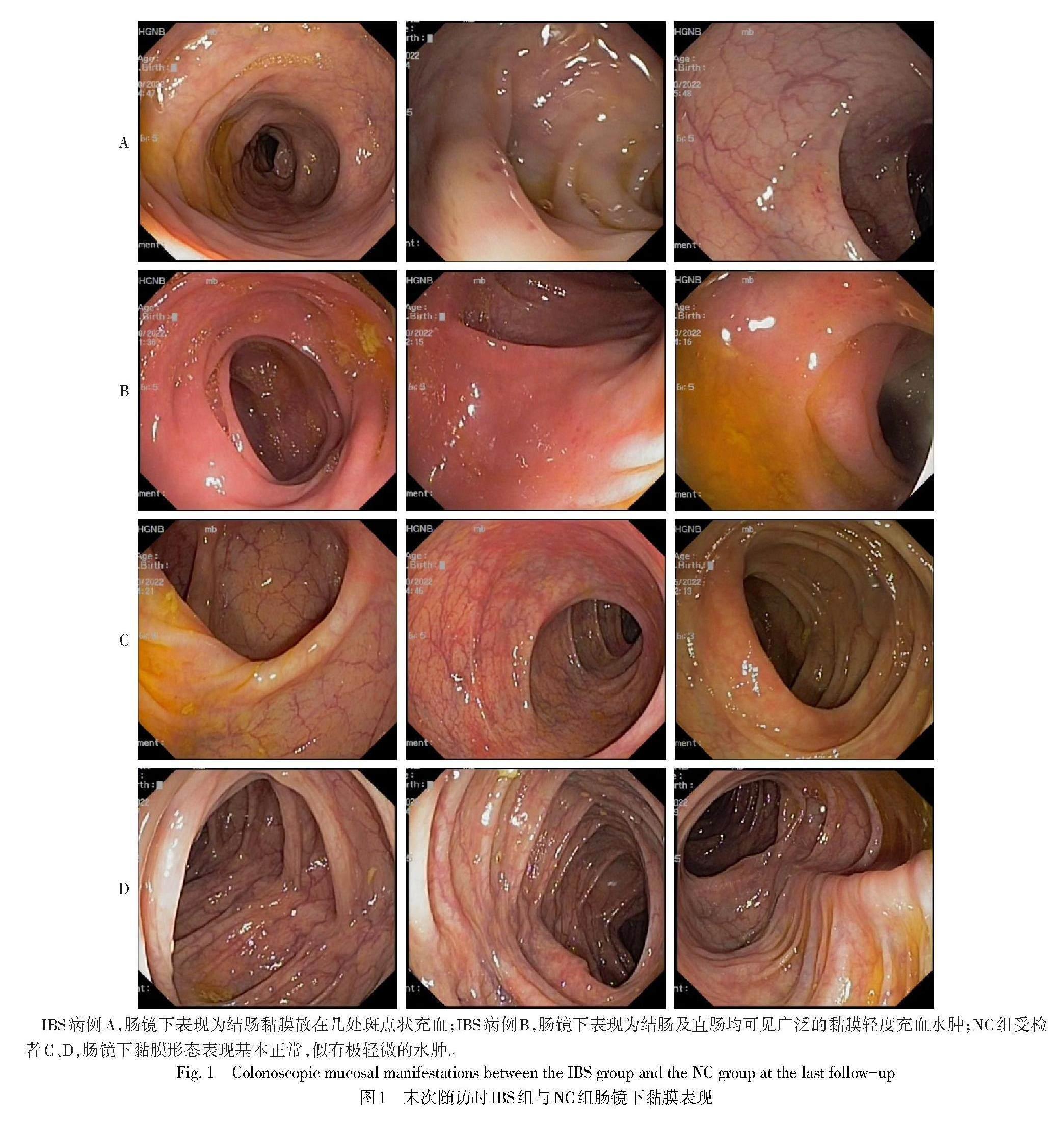
2.3 IBS组肠黏膜病理表现 镜下可见腺体呈管状,间质可见淋巴细胞,局部淋巴细胞呈团状,提示慢性炎症表现,见图2。
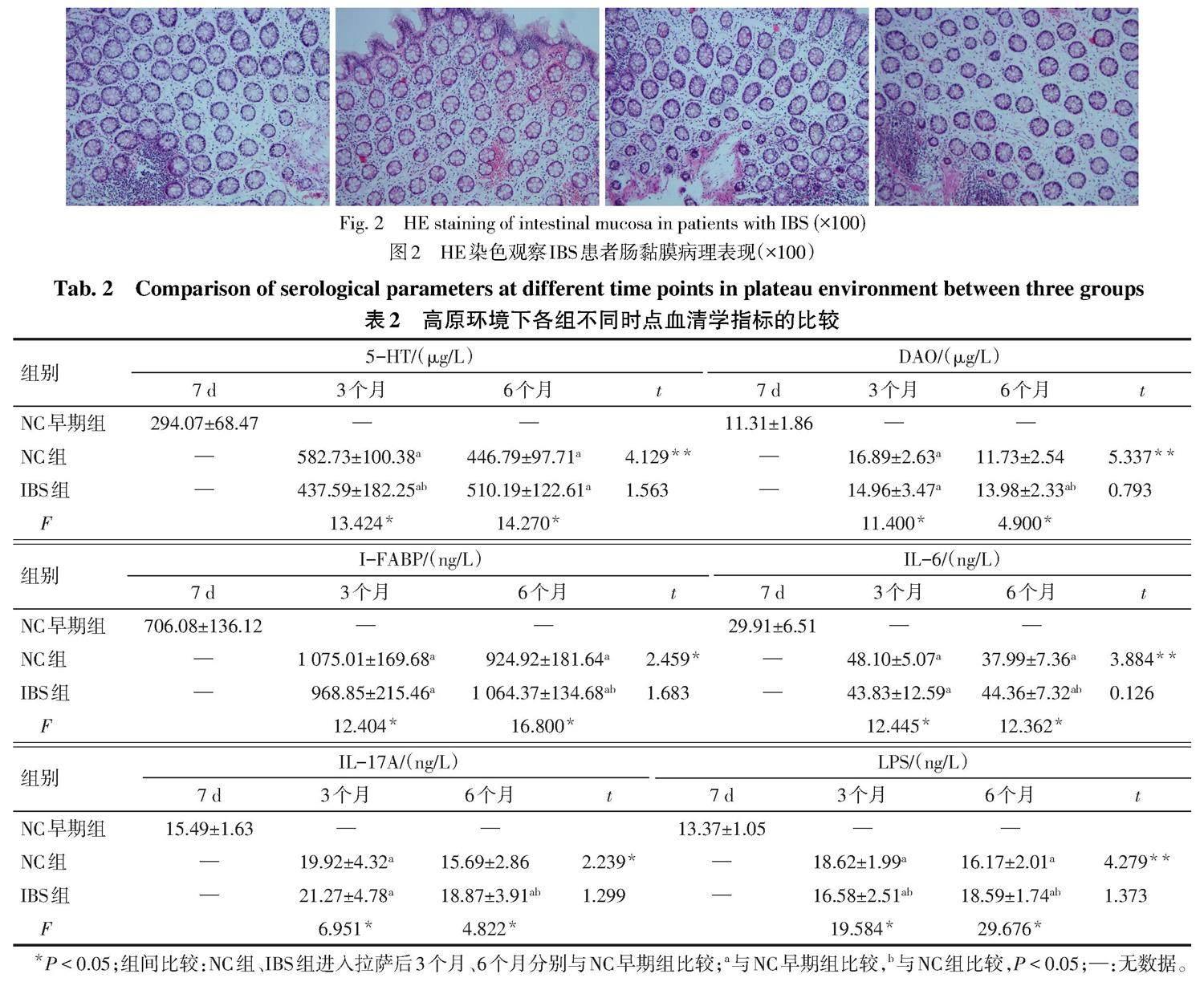
2.4 IBS组和NC组不同时点与NC早期组血清学指标的比较 (1)组间比较。与NC早期组比较,NC组进入拉萨后3个月时5-HT、DAO、I-FABP、IL-6、IL-17A和LPS水平及6个月时5-HT、I-FABP、IL-6和LPS水平均升高,IBS组进入拉萨后3、6个月6项血清学指标均升高(P<0.05);与NC组比较,IBS组进入拉萨后3个月时5-HT和LPS水平降低(P<0.05),DAO、I-FABP、IL-6和IL-17A水平差异无统计学意义,IBS组进入拉萨后6个月时DAO、I-FABP、IL-6、IL-17A和LPS水平均升高(P<0.05),5-HT水平差异无统计学意义。(2)组内比较。NC组进入拉萨后6个月时6项血清学指标均较3个月时降低(P<0.05);IBS组进入拉萨后3个月与6个月时6项血清学指标差异均无统计学意义。见表2。
3 讨论
高原地区炎症性肠病肠镜下表现多以炎症为主,91.9%的患者镜下可见黏膜充血水肿,与缺氧环境对肠黏膜屏障损伤相关[6]。研究认为,IBS患者结肠镜下除结肠袋消失、肠腔扭曲及粘合的表现外,肠黏膜基本正常[7]。而另有研究发现,结肠轻中度慢性炎症与IBS的发生相关,病理多表现为以淋巴细胞为主的慢性炎性细胞浸润[8]。本研究中,高原环境下IBS患者肠镜下表现为黏膜充血、水肿,病理提示慢性炎症表现,进一步表明高原环境可诱发IBS患者的肠黏膜炎症损伤。
本研究结果显示,NC组和IBS组进入拉萨后3、6个月时,其LPS水平均高于NC早期组;IBS组进入拉萨后3个月时的LPS水平低于NC组,进入拉萨后6个月时的LPS水平高于NC组;NC组进入拉萨后6个月时LPS水平较3个月时降低,提示LPS水平受到高原环境的影响,LPS在高原IBS的发生中可能发挥重要作用。有研究发现,IBS患者血清中可检测到抗鞭毛蛋白抗体和抗脂质A抗体,可能与肠屏障损伤和通透性改变相关[9]。一项纳入了45项研究、包括21 421例肠炎患者的荟萃分析[10]发现,患感染性肠炎后的12个月内IBS的总患病率为10.1%(95%CI:7.2~14.1),为未患感染性肠炎者的4.2倍(95%CI:3.1~5.7)。王晓辉等[11]调查发现,既往有痢疾及急性胃肠炎病史的人群发展为IBS的相对危险度分别为2.567和1.361。急性胃肠炎患者8年后发生IBS的风险仍高于未患病者(OR=3.12,95%CI:1.99~5.04)[12]。感染后IBS(post-infectious irritable bowel syndrome,PI-IBS)的病因可能涉及神经递质释放、淋巴细胞浸润、炎性介质释放及肠道菌群失调等[13]。
有研究发现,与健康对照组相比,IBS组IL-6水平升高,表明IBS可能存在低度的炎症反应[14]。PI-IBS患者的血清及回盲部和直肠黏膜中IL-17A水平升高[15]。另有研究发现IBS患者有直肠黏膜炎症异常及IL-17水平的升高[16]。一项采用旋毛虫感染C57BL/6小鼠建立PI-IBS的模型显示,回肠中IL-17水平显著升高[17]。以上研究提示IL-17在黏膜炎症和免疫相关疾病中发挥重要作用[18]。IL-17A是IL-17的一个亚型,主要由Th17细胞分泌,可刺激上皮细胞产生IL-6,从而发挥促炎效应[19]。有研究显示,IBS-D患者IL-6水平高于正常组,其大量释放從而激活免疫反应可能与IBS患者的症状和焦虑程度相关[20]。本研究显示,与NC早期组比较,NC组进入拉萨后3个月时的IL-6和IL-17A及6个月时的IL-6水平均升高,IBS组进入拉萨后3、6个月时的IL-6和IL-17A水平均升高;IBS组进入拉萨后6个月时的IL-6和IL-17A水平均高于NC组;NC组进入拉萨后6个月时的IL-6和IL-17A水平较3个月时降低,提示高原环境会导致炎性因子水平升高,且在IBS中更为显著,可能原因是高原环境导致肠道损伤,出现肠道炎症及免疫激活,继而释放炎性因子,诱导肠道炎症及黏膜免疫。因此,炎性因子IL-6及IL-17A可能在高原IBS的病因机制中发挥重要作用。
I-FABP为小肠上皮细胞中分子质量为15 ku的胞浆蛋白,参与摄取和运输极性脂类,并与肠黏膜损伤相关。研究发现,I-FABP与肠黏膜损伤程度呈正相关,并在肠黏膜缺血早期即可检出[21]。IBS存在肠屏障通透性改变[22],与上皮结构异常、黏膜免疫激活和微生物失调等有关[23]。IBS不同亚型中I-FABP存在差异,研究发现I-FABP在PI-IBS中浓度最高[24]。DAO是一种含脱氨腐胺和组胺的细胞内酶,主要分布在哺乳动物的小肠黏膜/纤毛上皮细胞中。研究发现血清DAO和I-FABP在胃肠道早期损伤时出现升高,且水平高低与损伤程度相关[25]。与对照组相比,IBS中DAO水平升高[26]。本研究中,IBS组和NC组中I-FABP均高于NC早期组,NC组进入拉萨后6个月时I-FABP水平较3个月时降低,IBS组进入拉萨后6个月时I-FABP水平高于NC组。NC组DAO在进入拉萨3个月时最高,在6个月时降至接近NC早期组水平;IBS组进入拉萨后3、6个月DAO均高于NC早期组。因此提示高原环境能够导致肠黏膜屏障损伤,且在IBS中更为明显,推测肠黏膜屏障损伤可能在高原环境下IBS发病中有重要作用。
脑-肠-微生物轴的失调为IBS的一个主要病因,而5-HT作为连接肠道微生物和大脑的关键神经递质,能够影响胃肠动力、痛觉、黏膜炎症、免疫反应和大脑活动[27],这可能解释了IBS的部分症状[28]。与健康对照组相比,IBS组中5-HT水平显著增高[14]。结肠5-HT水平升高可导致IBS的腹泻和内脏的超敏反应[29]。特异性5-HT受体拮抗剂作为心理应激的药物已在IBS中表现出临床效果[30]。本研究中,IBS组和NC组中5-HT水平均高于NC早期组;NC组5-HT水平在进入拉萨6个月时相比3个月时降低,提示高原环境下神经递质5-HT的变化可能在高原IBS的发病中发挥重要作用。
综上所述,高原缺氧环境对神经递质、炎性介质和肠黏膜屏障损伤具有显著影响,其在高原IBS病因机制中可能发挥重要作用。
参考文献
[1] ZHANG X G,XU W,ZHONG W L,et al. Exploring the links between gut microbiome changes and irritable bowel syndrome in Han populations in the Tibetan Plateau[J]. J Zhejiang Univ Sci B,2023,24(9):823-838. doi:10.1631/jzus.B2200509.
[2] CHENG J F,SUN Y M,HE J X,et al. The mechanism of colon tissue damage mediated by HIF-1alpha/NF-kappaB/STAT1 in high-altitude environment[J]. Front Physiol,2022,13:933659. doi:10.3389/fphys.2022.933659.
[3] WANG Y H,SHI Y,LI W H,et al. Gut microbiota imbalance mediates intestinal barrier damage in high-altitude exposed mice[J]. FEBS J,2022,289(16):4850-4868. doi:10.1111/febs.16409.
[4] LI Y Y,WANG Y C,SHI F,et al. Phospholipid metabolites of the gut microbiota promote hypoxia-induced intestinal injury via CD1d-dependent γδ T cells[J]. Gut Microbes,2022,14(1):2096994. doi:10.1080/19490976.2022.2096994.
[5] CALDERWOOD A H,SCHROY P C 3RD,LIEBERMAN D A,et al. Boston Bowel Preparation Scale scores provide a standardized definition of adequate for describing bowel cleanliness[J]. Gastrointest Endosc,2014,80(2):269-276. doi:10.1016/j.gie.2014.01.031.
[6] 邢征,張根苗,金志,等. 高原地区炎症性肠病124例调查分析[J]. 临床消化病杂志,2009,21(4):245-246. XING Z,ZHANG G M,JIN Z,et al. Investigation and analysis of 124 cases of inflammatory bowel disease in plateau area[J]. Journal of Clinical Gastroenterology,2009,21(4):245-246. doi:1005-541X(2009)04-245-02.
[7] 李易,韩盛玺. 肠易激综合征的结肠镜下表现[J]. 中国内镜杂志,2008,14(6):628-629. LI Y,HAN S X. Manifestation of irritable bowel syndrome under colonscope [J]. China Journal of Endoscopy,2008,14(6):628-629. doi:1007-1989(2008)06-0628-02.
[8] 颜君,周国华,余细球,等. 肠易激综合征与结肠慢性炎症的相关性研究 [J]. 中国实用医药,2017,12(8):47-49. YAN J,ZHOU G H,YU X Q,et al. Study on correlation between irritable bowel syndrome and colon chronic inflammation[J]. China Prac Med,2017,12(8):47-49. doi:10.14163/j.cnki.11-5547/r.2017.08.019.
[9] FUKUI H. Increased intestinal permeability and decreased barrier function:does it really influence the risk of inflammation?[J]. Inflamm Intest Dis,2016,1(3):135-145. doi:10.1159/000447252.
[10] KLEM F,WADHWA A,PROKOP L J,et al. Prevalence,risk factors,and outcomes of irritable bowel syndrome after infectious enteritis:a systematic review and meta-analysis[J]. Gastroenterology,2017,152(5):1042-1054.e1. doi:10.1053/j.gastro.2016.12.039.
[11] 王晓辉,崔立红,闫志辉,等. 肠道感染病史及药物因素对肠易激综合征发病的影响[J]. 解放军医药杂志,2014,26(2):30-33. WANG X H,CUI L H,YAN Z H,et al. Effect of intestinal infection history and drug use factors on irritable bowel syndrome[J]. Med & Pharm J Chin PLA,2014,26(2):30-33. doi:10.3969/j.issn.2095-140X.2014.02.010.
[12] MARSHALL J K,THABANE M,GARG A X,et al. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery[J]. Gut,2010,59(5):605-611. doi:10.1136/gut.2009.202234.
[13] 王子恺,杨云生. 感染后肠易激综合征[J]. 胃肠病学和肝病学杂志,2012,21(10):966-970. WANG Z K,YANG Y S. Post-infectious irritable bowel syndrome [J]. Chin J Gastroenterol Hepatol,2012,21(10):966-970. doi:10.3969/j.issn.1006-5709.2012.10.026.
[14] 李榕娇,郭维龙,孙慧冰,等. 肠易激综合征患者肠道菌群分布和炎症因子及血清NPY、SP、5-HT水平的变化[J]. 中国微生态学杂志,2020,32(9):1060-1064. LI R J,GUO W L,SUN H B,et al. The distribution of intestinal flora,levels of inflammatory factors and serum NPY,SP and 5-HT in patients with irritable bowel syndrome [J]. Chin J Microecol,2020,32(9):1060-1064. doi:10.13381/J.cnki.cjm.202009014.
[15] 代迎欢,蓝程,刘丹,等. 感染后肠易激综合征患者临床特征和细胞因子的表达 [J]. 中山大学学报(医学科学版),2017,38(2):260-266. DAI Y H,LAN C,LIU D,et al. Clinical features and expression of cytokine in post-infectious irritable bowel syndrome patients [J]. Journal of Sun Yat?Sen University(Medical Sciences),2017,38(2):260-266. doi:10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2017.0042.
[16] BERG L K,GOLL R,FAGERLI E,et al. Intestinal inflammatory profile shows increase in a diversity of biomarkers in irritable bowel syndrome[J]. Scand J Gastroenterol,2020,55(5):537-542. doi:10.1080/00365521.2020.1754455.
[17] YANG B,ZHOU X C,LAN C. Changes of cytokine levels in a mouse model of post-infectious irritable bowel syndrome [J]. BMC Gastroenterol,2015,15:43. doi:10.1186/s12876-015 -0272-8.
[18] 陈思琪,朱焰,陈敏. 基于国家专利的中药复方治疗肠易激综合征用药规律及核心处方作用机制研究[J]. 中国中西医结合外科杂志,2023,29(2):197-203. CHEN S Q,ZHU Y,CHEN M. Study on the medication rule and mechanism of core prescription of Chinese herbal compound in the treatment of irritable bowel syndrome based on patent database[J]. Chinese Journal of Surgery of Integrated Traditional and Western Medicine,2023,29(2):197-203. doi:10.3969/j.issn.1007-6948.2023.02.012.
[19] ZHU S,QIAN Y C. IL-17/IL-17 receptor system in autoimmune disease:mechanisms and therapeutic potential[J]. Clin Sci (Lond),2012,122(11):487-511. doi:10.1042/CS20110496.
[20] LIEBREGTS T,ADAM B,BREDACK C,et al. Immune activation in patients with irritable bowel syndrome[J]. Gastroenterology,2007,132(3):913-920. doi:10.1053/j.gastro.2007.01.046.
[21] SCHELLEKENS D H,GROOTJANS J,DELLO S A,et al. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model[J]. J Clin Gastroenterol,2014,48(3):253-260. doi:10.1097/MCG.0b013e3182a87e3e.
[22] 張梦,陈超英,胡玥,等. 黏附连接及紧密连接与腹泻型肠易激综合征症状之间的关系[J]. 中华内科杂志,2020,59(1):40-46. ZHANG M,CHEN C Y,HU Y,et al. The relationship between adherens junction and tight junction and clinical symptoms in patients with diarrhea predominant irritable bowel syndrome[J]. Chin J Intern Med,2020,59(1):40-46. doi:10.3760/cma.j.issn.0578-1426.2020.01.007.
[23] GONZALEZ-CASTRO A M,MARTINEZ C,SALVO-ROMERO E,et al. Mucosal pathobiology and molecular signature of epithelial barrier dysfunction in the small intestine in irritable bowel syndrome[J]. J Gastroenterol Hepatol,2017,32(1):53-63. doi:10.1111/jgh.13417.
[24] WANG J B,LU S H,ZHAO S J. Post-infectious and non post-infectious irritable bowel syndrome:a comparative study[J]. Pak J Med Sci,2016,32(1):116-119. doi:10.12669/pjms.321.8628.
[25] 杨敏,程雁. 血清二胺氧化酶和肠脂肪酸结合蛋白测定评价窒息新生儿胃肠功能障碍的临床研究 [J]. 中国临床药理学杂志,2022,38(23):2803-2806. YANG M,CHENG Y. Clinical trial of serum diamine oxidase and intestinal fatty acid binding protein in evaluating gastrointestinal dysfunction in neonates with asphyxia [J]. Chin J Clin Pharmacol,2022,38(23):2803-2806. doi:10.13699/j.cnki.1001-6821.2022.23.003.
[26] JI M,HUANG H,LAN X M. Correlation between intestinal microflora in irritable bowel syndrome and severity[J]. Dis Markers,2022,2022:1031844. doi:10.1155/2022/1031844.
[27] MISHIMA Y,ISHIHARA S. Enteric microbiota-mediated serotonergic signaling in pathogenesis of irritable bowel syndrome[J]. Int J Mol Sci,2021,22(19):10235. doi:10.3390/ijms221910235.
[28] NG Q X,SOH A Y S,LOKE W,et al. The role of inflammation in irritable bowel syndrome (IBS)[J]. J Inflamm Res,2018,11:345-349. doi:10.2147/JIR.S174982.
[29] GAO J,XIONG T T,GRABAUSKAS G,et al. Mucosal serotonin reuptake transporter expression in irritable bowel syndrome is modulated by gut microbiota via mast cell-prostaglandin E2[J]. Gastroenterology,2022,162(7):1962. doi:10.1053/j.gastro.2022.02.016.
[30] QIN H Y,CHENG C W,TANG X D,et al. Impact of psychological stress on irritable bowel syndrome[J]. World J Gastroenterol,2014,20(39):14126-14131. doi:10.3748/wjg.v20.i39.14126.
(2023-06-13收稿 2023-09-12修回)
(本文編辑 陈丽洁)

