Nrf2/HO-1通路在银屑病中作用的研究进展
黎敏 龚坚 吴伟伟 刘巧
基金项目:国家自然科学基金资助项目(81860848);江西省自然科学基金青年基金项目(20232BAB216108);江西省卫生健康委科技计划项目(202311334)
作者单位:1江西中医药大学(邮编330004);2江西省皮肤病专科医院;3海南省第五人民医院整形与皮肤外科
作者简介:黎敏(1996),女,博士在读,主要从事中西医结合诊疗皮肤病相关研究。E-mail:limin6816@yeah.net
△通信作者 E-mail:drliuqiao@163.com
摘要:银屑病的发病机制复杂,探索其发病机制对于治疗十分重要。核因子E2相关因子2(Nrf2)/血红素加氧酶1(HO-1)通路对细胞具有保护作用,与氧化应激、免疫、血管异常增生、表皮失衡以及细胞死亡等生理病理过程密切相关。目前已有研究证实Nrf2/HO-1通路对银屑病皮损有抑制作用,可能通过多种途径参与银屑病发病。就Nrf2/HO-1通路在银屑病中的作用机制及中医药通过干预该通路治疗银屑病的研究进展进行综述。
关键词:NF-E2相关因子2;血红素加氧酶-1;银屑病;中医药学;氧化性应激;细胞死亡
中图分类号:R758.63文献标志码:ADOI:10.11958/20231583
Research progress on the role of Nrf2/HO-1 pathway in psoriasis
LI Min1, GONG Jian2, WU Weiwei3, LIU Qiao1△
1 Jiangxi University of Traditional Chinese Medicine, Nanchang 330004, China; 2 Jiangxi Provincial Clinical Research Center for Skin Diseases; 3 Department of Plastic and Dermatological Surgery, the Fifth Peoples Hospital of Hainan Province
△Corresponding Author E-mail: drliuqiao@163.com
Abstract: The pathogenesis of psoriasis is complex, so it is very important to explore the pathogenesis for the treatment of psoriasis. The Nrf2/HO-1 pathway has a protective effect on cells and is closely related to physiological and pathological aspects such as oxidative stress, immunity, abnormal proliferation of blood vessels, epidermal imbalance and cell death. Currently, some studies have confirmed that Nrf2/HO-1 pathway may have an inhibitory effect on psoriatic lesion, and it may be involved in psoriasis pathogenesis through multiple pathways. This paper reviews the mechanism of Nrf2/HO-1 pathway in psoriasis and the research progress in the treatment of psoriasis through the intervention of traditional Chinese medicine.
Key words: NF-E2-related factor 2; heme oxygenase-1; psoriasis; traditional Chinese medicine and pharmacy; oxidative stress; cell death
银屑病是一种慢性炎症性皮肤病,临床表现以鳞屑性红斑为主,病因不明,发病机制复杂[1]。银屑病是由角质形成细胞(keratinocytes,KCs)异常增殖,树突状细胞(dendritic cells,DCs)、中性粒细胞、肥大细胞和T细胞之间的相互作用而诱导发生的,与白细胞介素(IL)-21、IL-22、IL-17、肿瘤坏死因子(TNF)-α和γ-干擾素(IFN-γ)等细胞因子相关[2]。核因子E2相关因子2(nuclear factor erythroid 2-related factor 2,Nrf2)是协调细胞中外源物质和氧化应激反应的主要转录因子,激活后能与其他转录因子和辅助因子相互作用,调控其靶基因和下游蛋白,参与机体抗氧化、解毒、代谢和炎症等过程[3]。Nrf2能通过调节其下游蛋白血红素加氧酶1(heme oxygenase 1,HO-1)参与动脉粥样硬化[4]、帕金森病[5]、骨关节炎[6]和急性肺损伤[7]等的发病。此外,Nrf2/HO-1通路能够通过调节机体氧化应激和免疫功能,抑制机体的炎症反应、细胞异常增殖以及调控细胞死亡等过程,参与银屑病的发病[8]。本文对Nrf2/HO-1通路在银屑病中的潜在作用以及中医药通过干预该通路治疗银屑病的研究进展进行简要综述,以期为银屑病的治疗提出新思路。
1 Nrf2/HO-1通路概述
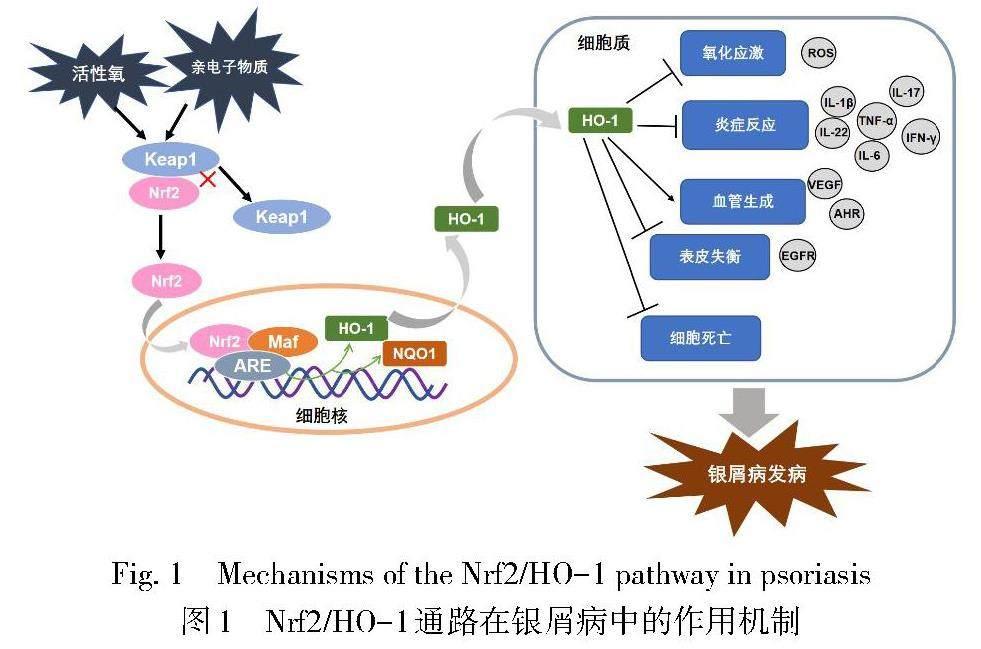
Nrf2属碱性亮氨酸拉链(basic leucine zipper,bZIP)转录因子CNC亚家族。在正常生理状态下,Nrf2通过与Kelch样ech相关蛋白1(Kelch-like ECH-associated protein 1,Keap1)结合在细胞质内保持低活性状态,当机体暴露于亲电子物质、活性氧(ROS)或其他活性物质时,Nrf2发生磷酸化并与Keap1解离,同时进入细胞核与小肌肉筋膜纤维肉瘤蛋白(small musculoaponeurotic fibrosarcoma proteins,sMAF)形成异二聚体,随后与抗氧化反应元件(antioxidant response elements,ARE)序列结合,诱导HO-1、磷酸酰胺腺嘌呤二核苷酸醌氧化还原酶-1(NADPH quinone oxidoreductase 1,NQO1)等下游蛋白的表达[3,9-10]。研究发现,Nrf2能够参与机体的蛋白酶体合成、细胞自噬、细胞凋亡、氧化还原、血红素代谢和铁稳态等病理生理过程,并调控相关转录因子以及DNA修复[11-12]。Nrf2依赖性细胞反应的主要效應因子HO-1是血红素加氧酶的亚型之一,是血红素降解为游离铁、一氧化碳(CO)和胆绿素的限速酶[13]。在HO-1参与血红素降解的过程中,胆绿素还原产生的胆红素能够调节炎症反应、免疫功能和内皮细胞活性;CO能够参与血管发育和血管内皮生长因子(vascular endothelial growth factor,VEGF)的合成,促进内皮细胞增殖,还能作用于T细胞和抗原提呈细胞以减轻炎症[14-15]。同时,HO-1及其代谢产物能够参与机体的氧化还原、细胞凋亡和炎症反应等病理生理过程。综上,Nrf2/HO-1通路是在机体应激条件下通过Nrf2诱导HO-1及其代谢产物表达,从而发挥细胞保护作用的重要通路,在抗氧化、抗炎、抗凋亡和调节免疫方面均发挥重要的作用[16-17],可参与机体某些病理生理过程,且与许多疾病密切相关。该通路在银屑病中的作用机制见图1。
2 Nrf2/HO-1通路在银屑病中的作用
2.1 抑制氧化应激 银屑病的发病与氧化应激密切相关,其发病受到内源性和外源性因素的影响,中性粒细胞增加是银屑病的特征性表现,中性粒细胞的长期聚集可引起周围组织损伤,此时机体出现的全身炎症反应会提高机体的氧化活性,破坏氧化还原稳态,导致氧化应激[18-20]。目前,IL-17和IL-23已被证明是银屑病的强相关因子[1]。Medovic等[20]研究发现氧化应激在辅助性T(T helper,Th)17细胞相关银屑病样皮肤炎症的IL-23/IL-17轴中起着至关重要的作用。有研究发现,寻常型银屑病出现的氧化应激状态与Nrf2和HO-1水平升高相关[18]。在动物实验中,间充质干细胞来源的外泌体(MSC-Exo)能够通过调节Nrf2/HO-1轴降低过氧化氢(H2O2)刺激的KCs和紫外线照射的小鼠皮肤中ROS的产生,减轻炎症和氧化应激,改善氧化应激诱导的皮肤损伤[21]。由此推测,在银屑病中Nrf2/HO-1通路能够作用于氧化损伤的KCs和过量的ROS来抑制氧化应激。此外,Sangaraju等[22]发现在咪喹莫特诱导的银屑病小鼠模型中利用高良姜能够上调HO-1的表达,增强Nrf2的核转位,改善银屑病皮损,提示高良姜能够通过Nrf2/HO-1通路促进抗氧化酶的防御系统来改善咪喹莫特诱导的银屑病。
2.2 调节免疫 银屑病是由T细胞介导的炎症性免疫性疾病,包括淋巴细胞、巨噬细胞、中性粒细胞等在内的多种免疫细胞均参与其发病[2,23]。目前研究发现,HO-1不仅可以调节ROS的产生,还可以通过极化巨噬细胞中具有抗炎作用的M2表型,调节IL-1β、TNF-α和IL-6等炎性因子,发挥细胞保护作用[24]。Rajendiran等[25]研究发现,Nrf2能够激活CD4+ T细胞,减少细胞中IFN-γ的分泌。Van Nguyen等[26]研究发现,通过激活Nrf2/HO-1信号通路能够减少促炎细胞因子TNF-α、IL-1β、IL-6、IL-8的分泌。由此可知,Nrf2和HO-1能够调节机体免疫,调控部分炎性因子的释放。在银屑病中,1型免疫与3型免疫可以共同发生,其中3型免疫主要由CD8+细胞毒性T(CD8+ cytotoxic T,TC)17细胞和Th17细胞介导,主要产生IL-17和IL-22,以保护皮肤免受细胞外细菌和真菌的侵袭[27]。Ogawa等[28]研究发现,Nrf2能通过抑制IL-1β调控3型免疫反应。另一项研究发现激活Nrf2通路能促进Nrf2和HO-1的核内转移,从而在一定程度上抑制IL-1β诱导的炎症反应[29]。此外,Chen等[30]发现Nrf2/HO-1通路能够逆转Th17细胞向Treg细胞的分化,从而抑制炎性细胞浸润和炎性因子的产生。
2.3 抑制血管生成 血管异常增生与银屑病发病密切相关,真皮血管扩张突出是银屑病组织病理特征之一[31]。朱玉婷等[32]研究发现,川芎嗪能够通过下调银屑病小鼠皮损组织中TNF-α、IL-17和VEGF的表达水平,改善小鼠银屑病样皮损的炎症表现。多项研究发现Nrf2/HO-1轴能够通过靶向VEGF,调控机体的血管异常增生[16,33]。此外,新证据表明皮肤血管内皮细胞(vascular endothelial cells,VECs)通过调节炎性细胞浸润参与银屑病的发生发展[34]。VECs中的芳香族碳氢受体(AHR)能够通过调节中性粒细胞募集参与银屑病的发病,然而进一步研究发现AHR的配体能够激活Nrf2的转录因子,上调HO-1和NQO1等多种抗氧化酶的基因表达来发挥细胞保护作用[34-35]。可见Nrf2、HO-1与导致银屑病血管扩张和增殖的因素密切相关,推测Nrf2/HO-1通路能调节VECs和KCs中的生长因子、细胞因子和转录因子,抑制血管异常增生和炎症反应,从而对银屑病皮损起到改善作用。
2.4 维持表皮平衡 表皮增殖和失衡与银屑病关系密切,也是银屑病组织病理的典型特征之一。表皮生长因子受体(epidermal growth factor receptor,EGFR)与银屑病发病密切相关。一方面,EGFR活化能介导KCs增殖,参与银屑病中的表皮棘层增生[36];另一方面,EGFR是参与银屑病表皮平衡的主要因素。有学者发现非典型转录因子IκBζ能通过影响IL-17介导的Th17细胞的发育参与银屑病的发病[37]。此外,IκBζ的表达受转录因子Nrf2的调控[38]。EGFR配体可通过促进KCs中IκBζ和B-细胞淋巴瘤因子3(Bcl-3)的表达,增强局部银屑病标志性基因的生成,从而参与银屑病的进展[39]。此外,Kim等[40]通过沉默EGFR基因表达,证实了人参皂苷对HO-1的作用是EGFR介导的。
2.5 调控细胞死亡 近年来,坏死性凋亡、细胞焦亡和铁死亡等调节性细胞死亡在银屑病中的研究日渐增多。Duan等[41]通过使用坏死性凋亡相关的相互作用蛋白激酶(RIPK)-1抑制剂(R-7-Cl-O-Necrostatin-1,Nec-1s)和混交激酶域蛋白(MLKL)抑制劑(MLKL-inhibitor necrosulfonamide,NSA)有效地阻断咪喹莫特诱导的银屑病炎症反应,并显著降低IL-1β、IL-6、IL-17A等炎性因子的产生。Zhao等[42]研究发现激活Nrf2/HO-1通路能够抑制小胶质细胞的氧化应激和坏死性凋亡水平。另外,Deng等[43]研究发现环黄芪醇能通过抑制核苷酸结合寡聚化结构域样受体蛋白3(NLRP3)介导的细胞焦亡,调节巨噬细胞功能,从而改善银屑病小鼠的皮肤炎症。此外,Shou等[44]发现KCs中TNF-α、IL-6、IL-1α、IL-1β、IL-17、IL-22和IL-23的表达在铁死亡激动剂(erastin)刺激后显著增加,而在铁死亡抑制剂(Fer-1)处理后减少,且在进一步实验中发现Fer-1减轻了银屑病小鼠的皮损。Dong等[45]研究发现Nrf2可通过调控溶质载体家族7成员11(solute carrier family 7 member 11,SLC7A11)和HO-1抑制铁死亡。
3 中药干预Nrf2/HO-1通路在银屑病中的研究
近年来,临床上中医药治疗银屑病取得了一定的成效。一些中药的抗氧化、抗炎和调节免疫等特性对治疗银屑病具有一定疗效,但其作用靶点尚不清楚。现尝试从Nrf2/HO-1通路出发,解释部分中药及其有效成分治疗银屑病的潜在作用靶点。
3.1 高良姜素 高良姜具有抗凝血、改善微循环、抗氧化、抗菌和抗炎等作用,其主要成分是高良姜素[46]。目前研究发现高良姜素能激活Nrf2-ARE信号,拮抗蓝光诱导的KCs损伤和凋亡,进而发挥抗氧化作用[47-48]。此外,有研究表明高良姜能通过调控Nrf2/HO-1通路上调银屑病相关的抗氧化标志物,从而减轻咪喹莫特诱导的银屑病[22]。
3.2 没食子酸 五倍子和山茱萸在皮肤科中运用广泛,二者有效成分中均有没食子酸,该成分具有抗氧化[49]、抗炎[49]、抗微生物[50]等作用。张金卫[51]研究发现没食子酸能通过激活Nrf2抑制角蛋白6、16、17的表达来治疗银屑病。
3.3 款冬花酮 款冬花具有抗炎、神经保护和抗氧化作用,其有效成分为款冬花酮[52]。Lee等[53]研究发现款冬花酮可通过激活Nrf2进而抑制核因子(NF)-κB和转录活化因子3(STAT3),同时款冬花酮能降低咪喹莫特诱导的银屑病相关炎症细胞因子和抗菌肽的mRNA水平,并有效减少表皮的过度增殖。
3.4 黄芩提取物 黄芩是治疗银屑病的常用药物,其成分有黄芩苷、黄芩素等。Ibrahim等[54]研究发现黄芩苷能诱导Nrf2、HO-1和VEGF表达下降,推测其可能是通过调控银屑病的氧化应激和血管生成从而改善银屑病样皮损。Wang等[55]发现黄芩提取物能激活Nrf2/HO-1信号级联反应,激活其抗氧化和细胞保护能力,阻止KCs的增殖,从而改善银屑病患者的皮损。
3.5 靛玉红和芍药苷 青黛和赤芍是治疗银屑病常用的清热凉血药物,二者的有效成分为靛玉红和芍药苷。荣光莉[56]研究发现靛玉红和芍药苷均能通过抑制Nrf2、HO-1和IL-6的表达,减轻银屑病小鼠的皮损。
3.6 白藜芦醇 虎杖、覆盆子、大黄中的有效成分白藜芦醇已被证明具有抗癌、抗微生物和抗炎的作用[57]。有研究发现白藜芦醇可通过调节HO-1的表达,保护H2O2诱导的HaCaT细胞免受氧化损伤[58]。
4 小结
综上,Nrf2/HO-1通路在银屑病的发病机制中发挥了重要作用,该通路能够通过抑制氧化应激、调节免疫、抑制血管生成、维持表皮平衡以及调控细胞死亡等参与银屑病的发病进程;同时,部分中药也能通过介导Nrf2/HO-1通路作用于银屑病。但是,Nrf2/HO-1通路对银屑病具体的作用机制以及在各种银屑病相关治疗方法中的干预作用还需深入探索。此外,目前Nrf2/HO-1通路在银屑病中的研究多局限于基础研究阶段,仍需开展更多相关临床研究为银屑病的精准治疗提供参考依据。
参考文献
[1] GRIFFITHS C E M,ARMSTRONG A W,GUDJONSSON J E,et al. Psoriasis[J]. Lancet,2021,397(10281):1301-1315. doi:10.1016/S0140-6736(20)32549-6.
[2] RENDON A,SCHAKEL K. Psoriasis pathogenesis and treatment[J]. Int J Mol Sci,2019,20(6):1475. doi:10.3390/ijms20061475.
[3] HE F,RU X,WEN T. NRF2,a transcription factor for stress response and beyond[J]. Int J Mol Sci,2020,21(13):4777. doi:10.3390/ijms21134777.
[4] ZHANG Q,LIU J,DUAN H,et al. Activation of Nrf2/HO-1 signaling: an important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress[J]. J Adv Res,2021,34:43-63. doi:10.1016/j.jare.2021.06.023.
[5] WANG Y,GAO L,CHEN J,et al. Pharmacological modulation of Nrf2/HO-1 signaling pathway as a therapeutic target of Parkinsons disease[J]. Front Pharmacol,2021,12:757161. doi:10.3389/fphar.2021.757161.
[6] CHEN Z,ZHONG H,WEI J,et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis[J]. Arthritis Res Ther,2019,21(1):300. doi:10.1186/s13075-019-2085-6.
[7] LI J,LU K,SUN F,et al. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway[J]. J Transl Med,2021,19(1):96. doi:10.1186/s12967-021-02745-1.
[8] ZHANG A,SUZUKI T,ADACHI S,et al. Distinct regulations of HO-1 gene expression for stress response and substrate induction[J]. Mol Cell Biol,2021,41(11):e0023621. doi:10.1128/MCB.00236-21.
[9] MANSOURI A,REINER Z,RUSCICA M,et al. Antioxidant effects of statins by modulating Nrf2 and Nrf2/HO-1 signaling in different diseases[J]. J Clin Med,2022,11(5):1313. doi:10.3390/jcm11051313.
[10] 田亞静,杨雪,汪静,等. 芒柄花素对妊娠期糖尿病大鼠氧化应激损伤的影响[J]. 天津医药,2023,51(7):734-739. TIAN Y J,YANG X,WANG J,et al. Influence of formononetin on oxidative stress injury in gestational diabetes mellitus rats[J]. Tianjin Med J,2023,51(7):734-739. doi:10.11958/20221450.
[11] DODSON M,DE LA VEGA M R,CHOLANIANS A B,et al. Modulating NRF2 in disease:timing is everything[J]. Annu Rev Pharmacol Toxicol,2019,59(2019):555-575. doi:10.1146/annurev-pharmtox-010818-021856.
[12] SCHMIDLIN C J,SHAKYA A,DODSON M,et al. The intricacies of NRF2 regulation in cancer[J]. Semin Cancer Biol,2021,76:110-119. doi:10.1016/j.semcancer.2021.05.016.
[13] FACCHINETTI M M. Heme-oxygenase-1[J]. Antioxid redox signal,2020,32(17):1239-1242. doi:10.1089/ars.2020.8065.
[14] NITTI M,IVALDO C,TRAVERSO N,et al. Clinical significance of heme oxygenase 1 in tumor progression[J]. Antioxidants(Basel),2021,10(5):789. doi:10.3390/antiox10050789.
[15] CHEN S,WANG X,NISAR M F,et al. Heme oxygenases: cellular multifunctional and protective molecules against UV-Induced oxidative stress[J]. Oxid Med Cell Longev,2019,2019:5416728. doi:10.1155/2019/5416728.
[16] HUANG Y,YANG Y,XU Y,et al. Nrf2/HO-1 axis regulates the angiogenesis of gastric cancer via targeting VEGF[J]. Cancer Manag Res,2021,13:3155-3169. doi:10.2147/CMAR.S292461.
[17] LI B,NASSER M I,MASOOD M,et al. Efficiency of traditional Chinese medicine targeting the Nrf2/HO-1 signaling pathway[J]. Biomed Pharmacother,2020,26:110074. doi:10.1016/j.biopha.2020.110074.
[18] AMBROZEWICZ E,WOJCIK P,WRONSKI A,et al. Pathophysiological alterations of redox signaling and endocannabinoid system in granulocytes and plasma of psoriatic patients[J]. Cells,2018,7(10):159. doi:10.3390/cells7100159.
[19] JAGANJAC M,CIPAK A,SCHAUR R J,et al. Pathophysiology of neutrophil-mediated extracellular redox reactions[J]. Front Biosci(Landmark Ed),2016,21(4):839-855. doi:10.2741/4423.
[20] MEDOVIC M V,JAKOVLJEVIC V L,ZIVKOVIC V I,et al. Psoriasis between autoimmunity and oxidative stress:changes induced by different therapeutic approaches[J]. Oxid Med Cell Longev,2022,2022:2249834. doi:10.1155/2022/2249834.
[21] WANG T,JIAN Z,BASKYS A,et al. MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system[J]. Biomaterials,2020,257:120264. doi:10.1016/j.biomaterials.2020.120264.
[22] SANGARAJU R,ALAVALA S,NALBAN N,et al. Galangin ameliorates imiquimod-induced psoriasis-like skin inflammation in BALB/c mice via down regulating NF-κB and activation of Nrf2 signaling pathways[J]. Int Immunopharmacol,2021,96:107754. doi:10.1016/j.intimp.2021.107754.
[23] GRAN F,KERSTAN A,SERFLING E,et al. Current developments in the immunology of psoriasis[J]. Yale J Biol Med,2020,93(1):97-110.
[24] SHEN K,JIA Y,WANG X,et al. Exosomes from adipose-derived stem cells alleviate the inflammation and oxidative stress via regulating Nrf2/HO-1 axis in macrophages[J]. Free Radic Biol Med,2021,165:54-66. doi:10.1016/j.freeradbiomed.2021.01.023.
[25] RAJENDIRAN A,SUBRAMANYAM S H,KLEMM P,et al. NRF2/itaconate axis regulates metabolism and inflammatory properties of T cells in children with JIA[J]. Antioxidants(Basel),2022,11(12):2426. doi:10.3390/antiox11122426.
[26] VAN NGUYEN T,PIAO C H,FAN Y J,et al. Anti-allergic rhinitis activity of alpha-lipoic acid via balancing Th17/Treg expression and enhancing Nrf2/HO-1 pathway signaling[J]. Sci Rep,2020,10(1):12528. doi:10.1038/s41598-020-69234-1.
[27] ANNUNZIATO F,ROMAGNANI C,ROMAGNANI S. The 3 major types of innate and adaptive cell-mediated effector immunity[J]. J Allergy Clin Immunol,2015,135(3):626-635. doi:10.1016/j.jaci.2014.11.001.
[28] OGAWA T,ISHITSUKA Y. The role of KEAP1-NRF2 system in atopic dermatitis and psoriasis[J]. Antioxidants(Basel),2022,11(7):1397. doi:10.3390/antiox11071397.
[29] GUO Z,LIN J,SUN K,et al. Deferoxamine alleviates osteoarthritis by inhibiting chondrocyte ferroptosis and activating the Nrf2 pathway[J]. Front Pharmacol,2022,13:791376. doi:10.3389/fphar.2022.791376.
[30] CHEN X,SU W,WAN T,et al. Sodium butyrate regulates Th17/Treg cell balance to ameliorate uveitis via the Nrf2/HO-1 pathway[J]. Biochem Pharmacol,2017,142:111-119. doi:10.1016/j.bcp.2017.06.136.
[31] TOKUYAMA M,MABUCHI T. New treatment addressing the pathogenesis of psoriasis[J]. Int J Mol Sci,2020,21(20):7488. doi:10.3390/ijms21207488.
[32] 朱玉婷,晏文,應理晟,等. 川芎嗪对银屑病小鼠皮损内TNF-α、IL-17、VEGF表达的影响[J]. 天津医药,2023,51(6):590-595. ZHU Y T,YAN W,YING L S,et al. Effects of tetramethylpyrazine on expression levels of TNF-α,IL-17 and VEGF in skin lesions of psoriatic mice[J]. Tianjin Med J,2023,51(6):590-595. doi:10.11958/20221433.
[33] HUANG Z,NG T K,CHEN W,et al. Nattokinase attenuates retinal neovascularization via modulation of Nrf2/HO-1 and glial activation[J]. Invest Ophthalmol Vis Sci,2021,62(6):25. doi:10.1167/iovs.62.6.25.
[34] ZHU Z,CHEN J,LIN Y,et al. Aryl hydrocarbon receptor in cutaneous vascular endothelial cells restricts psoriasis development by negatively regulating neutrophil recruitment[J]. J Invest Dermatol,2020,140(6):1233-1243. doi:10.1016/j.jid.2019.11.022.
[35] FURUE M,HASHIMOTO-HACHIYA A,TSUJI G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis[J]. Int J Mol Sci,2019,20(21):5424. doi:10.3390/ijms20215424.
[36] KELEL M,YANG R B,TSAI T F,et al. FUT8 remodeling of EGFR regulates epidermal keratinocyte proliferation during psoriasis development[J]. J Invest Dermatol,2021,141(3):512-522. doi:10.1016/j.jid.2020.07.030.
[37] GAUTAM P,MAENNER S,CAILOTTO F,et al. Emerging role of IκBζ in inflammation: emphasis on psoriasis[J]. Clin Transl Med,2022,12(10):e1032. doi:10.1002/ctm2.1032.
[38] ZHANG Y,TANG J,ZHOU Y,et al. Short-term exposure to dimethyl fumarate (DMF)inhibits LPS-induced IκBζ expression in macrophages[J]. Front Pharmacol,2023,14:1114897. doi:10.3389/fphar.2023.1114897.
[39] DAI X,MURAKAMI M,SHIRAISHI K,et al. EGFR ligands synergistically increase IL-17A-induced expression of psoriasis signature genes in human keratinocytes via IκBζ and Bcl3[J]. Eur J Immunol,2022,52(6):994-1005. doi:10.1002/eji.202149706.
[40] KIM E N,KAYGUSUZ O,LEE H S,et al. Simultaneous quantitative analysis of ginsenosides isolated from the fruit of panax ginseng C.A. meyer and regulation of HO-1 expression through EGFR signaling has anti-inflammatory and osteogenic induction effects in HPDL cells[J]. Molecules,2021,26(7):2092. doi:10.3390/molecules26072092.
[41] DUAN X,LIU X,LIU N,et al. Inhibition of keratinocyte necroptosis mediated by RIPK1/RIPK3/MLKL provides a protective effect against psoriatic inflammation[J]. Cell Death Dis,2020,11(2):134. doi:10.1038/s41419-020-2328-0.
[42] ZHAO P,WEI Y,SUN G,et al. Fetuin-A alleviates neuroinflammation against traumatic brain injury-induced microglial necroptosis by regulating Nrf-2/HO-1 pathway[J]. J Neuroinflammation,2022,19(1):269. doi:10.1186/s12974-022-02633-5.
[43] DENG G,CHEN W,WANG P,et al. Inhibition of NLRP3 inflammasome-mediated pyroptosis in macrophage by cycloastragenol contributes to amelioration of imiquimod-induced psoriasis-like skin inflammation in mice[J]. Int Immunopharmacol,2019,74:105682. doi:10.1016/j.intimp.2019.105682.
[44] SHOU Y,YANG L,YANG Y,et al. Inhibition of keratinocyte ferroptosis suppresses psoriatic inflammation[J]. Cell Death Dis,2021,12(11):1009. doi:10.1038/s41419-021-04284-5.
[45] DONG H,QIANG Z,CHAI D,et al. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1[J]. Aging,2020,12(13):12943-12959. doi:10.18632/aging.103378.
[46] 穆利萍,王吉,邱成省,等. 高良姜中具有神經保护作用的化学成分研究[J]. 云南农业大学学报(自然科学),2023,38(4):627-635. MU L P,WANG J,QIU J C,et al. Study on chemical constituents with neuroprotective effects from alpinia officinarum hance[J]. Journal of Yunnan Agricultural University(Natural Science),2023,38(4):627-635. doi:10.12101/j.issn.1004-390X(n).202211027.
[47]PARK J Y,PARK S H,OH S W,et al. Yellow chaste weed and its components,apigenin and galangin,affect proliferation and oxidative stress in blue light-irradiated HaCaT cells[J]. Nutrients,2022,14(6):1217. doi:10.3390/nu14061217.
[48] LEE J J,NG S C,HSU J Y,et al. Galangin reverses H2O2-induced dermal fibroblast senescence via SIRT1-PGC-1α/Nrf2 signaling[J]. Int J Mol Sci,2022,23(3):1387. doi:10.3390/ijms23031387.
[49] 张婷,姜海慧,冯石卜,等. 山茱萸果核不同产地间的抗氧化活性研究[J]. 陕西中医药大学学报,2023,46(2):23-26. ZHANG T,JIANG H H,FENG S B,et al. Study on antioxidant activity of fructus corni fruit core from different places of origin[J]. Journal of Shaanxi University of Chinese Medicine,2023,46(2):23-26. doi:10.13424/j.cnki.jsctcm.2023.02.004.
[50] 朱君,张鑫悦,崔敏,等. 4种中草药提取液的工艺优化及抗菌效果对比研究[J]. 常熟理工学院学报(自然科学),2023,37(2):65-71. ZHU J,ZHANG X Y,CUI M,et al. Comparative study on process optimization and antibacterial effects of 4 kinds of Chinese herbal medicine extracts[J]. Journal of Changshu Institute of Technology(Natural Sciences),2023,37(2):65-71. doi:10.16101/j.cnki.cn32-1749/z.2023.02.011.
[51] 张金卫. 没食子酸通过Nrf2调控角蛋白6、16、17治疗银屑病的研究[D]. 广州:广州中医药大学,2019. ZHANG J W. Gallic acid inhibites the expressioin of keratin 6,16,17 throught Nrf2 in psoriasis-like disease[D]. Guangzhou:Guangzhou University of Chinese Medicine,2019.
[52] CHEN S,DONG L,QUAN H,et al. A review of the ethnobotanical value,phytochemistry,pharmacology,toxicity and quality control of tussilago farfara L.(coltsfoot)[J]. J Ethnopharmacol,2021,267:113478. doi:10.1016/j.jep.2020.113478.
[53] LEE J,SONG K,HIEBERT P,et al. Tussilagonone ameliorates psoriatic features in keratinocytes and imiquimod-induced psoriasis-like lesions in mice via NRF2 activation[J]. J Invest Dermatol,2020,140(6):1223-1232. doi:10.1016/j.jid.2019.12.008.
[54] IBRAHIM A,ABDEL G S,FAWZI K M,et al. Baicalin lipid nanocapsules for treatment of glioma:characterization,mechanistic cytotoxicity,and pharmacokinetic evaluation[J]. Expert Opin Drug Deliv,2022,19(11):1549-1560. doi:10.1080/17425247.2022.2139370.
[55] WANG P W,LIN T Y,YANG P M,et al. Therapeutic efficacy of scutellaria baicalensis georgi against psoriasis-like lesions via regulating the responses of keratinocyte and macrophage[J]. Biomed Pharmacothe,2022,155:113798. doi:10.1016/j.biopha.2022.113798.
[56] 榮光莉. AZT、芍药苷和靛玉红对小鼠银屑病模型的作用及机制比较[D]. 广州:广州中医药大学,2019. RONG G L. Comparison of the effects and mechanisms of AZT, paeoniflorin and indirubin on psoriasis mouse model[D]. Guangzhou:Guangzhou University of Chinese Medicine,2019.
[57] CHHABRA G,SINGH C K,AMIRI D,et al. Recent advancements on immunomodulatory mechanisms of resveratrol in tumor microenvironment[J]. Molecules,2021,26(5):1343. doi:10.3390/molecules26051343.
[58] 高进涛,何荣安,莫文飞,等. 白藜芦醇对H2O2诱导的HaCaT细胞氧化应激损伤的保护作用[J]. 长江大学学报(自然科学版),2019,16(5):88-92. GAO J T,HE R A,MO W F,et al. Resveratrol on H2O2-induced oxidative stress in HaCaT cells damage protection[J]. Journal of Yangtze University(Natural Science Edition),2019,16(5):88-92. doi:10.16772/j.cnki.1673-1409.2019.05.018.
(2023-10-27收稿 2023-11-16修回)
(本文编辑 李鹏)

