瑞马唑仑调节EPAC1/RAP1信号通路对急性心肌梗死大鼠心肌损伤的影响
肖锦亮 汪威廉 但家朋

基金項目:荆州市2020年医疗卫生科技计划项目(2020HC05)
作者单位:荆州市中心医院(长江大学附属荆州医院)麻醉科(邮编434000)
作者简介:肖锦亮(1983),男,主治医师,主要从事心脏大血管麻醉方面研究。E-mail:ijpr9v@163.com
△通信作者 E-mail:cunganiv51@163.com
摘要:目的 探讨瑞马唑仑调节环腺苷酸激活的交换蛋白1(EPAC1)/RAS相关蛋白1(RAP1)信号通路对急性心肌梗死(AMI)大鼠心肌损伤的影响。方法 将大鼠按照随机数字表法分为假手术组、模型组、瑞马唑仑组、瑞马唑仑+8-CPT(EPAC1的激动剂)组,每组20只。除假手术组外,其余各组大鼠通过左前降支结扎法构建AMI大鼠模型;小动物超声仪检测心功能指标;HE染色检测心肌组织病理情况;化学比色法检测大鼠心肌组织超氧化物歧化酶(SOD)和丙二醛(MDA)水平;JC-1染色法检测大鼠心肌细胞线粒体膜电位;TUNEL染色检测心肌细胞TUNEL阳性率;Western blot检测心肌组织EPAC1、RAP1、胱天蛋白酶3(Caspase-3)蛋白表达水平。结果 与假手术组相比,模型组大鼠心肌组织结构被严重破坏且浸润大量炎性细胞;心功能指标左心室舒张末期内径(LVEDD)、左心室收缩末期内径(LVESD),心肌组织MDA水平,心肌细胞TUNEL阳性率,心肌组织EPAC1、RAP1、Caspase-3蛋白表达水平均明显升高;左心室射血分数(LVEF)、左心室短轴缩短率(LVFS),心肌组织SOD水平,心肌细胞线粒体膜电位明显降低(P<0.05)。与模型组相比,瑞马唑仑组大鼠心肌损伤缓解,炎性细胞浸润减轻,心功能指标LVEDD、LVESD,心肌组织MDA水平,心肌细胞TUNEL阳性率,心肌组织EPAC1、RAP1、Caspase-3蛋白表达水平均明显降低;LVEF、LVFS,心肌组织SOD水平,心肌细胞线粒体膜电位明显升高(P<0.05)。EPAC1的激动剂减弱了瑞马唑仑对AMI大鼠心肌损伤的缓解作用。结论 瑞马唑仑可能通过抑制EPAC1/RAP1信号通路抑制心肌细胞凋亡,减轻AMI大鼠心肌损伤。
关键词:心肌梗死;心肌损伤;瑞马唑仑;环腺苷酸激活的交换蛋白1;RAS相关蛋白1
中图分类号:R542.22文献标志码:ADOI:10.11958/20230890
Effect of remimazolam on myocardial injury in rats with acute myocardial infarction by regulating the EPAC1/RAP1 signaling pathway
XIAO Jinliang, WANG Weilian, DAN Jiapeng△
Department of Anesthesiology, Jingzhou Central Hospital (Jingzhou Hospital Affiliated to Changjiang University),
Jingzhou 434000, China
△Corresponding Author E-mail: cunganiv51@163.com
Abstract: Objective To investigate the effect of remimazolam on myocardial injury in rats with acute myocardial infarction (AMI) by regulating exchange proteins directly activated by cAMP (EPAC1)/RAS-related protein 1 (RAP1) signaling pathway. Methods Rats were divided into the sham operation group, the model group, the remazolam group and the remazolam+8-CPT (EPAC1 agonist) group according to random number table method, with 20 rats in each group. Except for the sham operation group, AMI rat model was constructed by ligation of left anterior descending branch in the other groups. Ultrasonic apparatus for small animals was applied to detect cardiac function indicators. HE staining was applied to detect the pathological condition of myocardial tissue. Chemical colorimetry was applied to detect levels of superoxide dismutase (SOD) and malondialdehyde (MDA) in myocardial tissue of rats. JC-1 staining method was applied to detect the mitochondrial membrane potential of rat cardiomyocytes. TUNEL staining was used to detect the TUNEL positive rate of myocardial cells. Western blot assay was applied to detect expression levels of EPAC1, RAP1 and Caspase-3 proteins in myocardial tissue. Results Compared with the sham operation group, the myocardial tissue structure of rats in the model group was severely damaged and infiltrated with a large number of inflammatory cells. Cardiac function indicators left ventricular end diastolic diameter (LVEDD), left ventricular end systolic diameter (LVESD), myocardial tissue MDA level, myocardial cell TUNEL positive rate, and myocardial tissue EPAC1, RAP1 and Caspase-3 protein expression levels were obviously increased, and the left ventricular ejection fraction (LVEF), left ventricular fraction shortening (LVFS), myocardial tissue SOD level, cardiomyocytes mitochondrial membrane potential were obviously decreased (P<0.05). Compared with the model group, the myocardial tissue structure of rats in the ramazolam group was obviously restored, and inflammatory cell infiltration was reduced. The cardiac function indicators LVEDD, LVESD, myocardial tissue MDA level, myocardial cell TUNEL positive rate, and myocardial tissue EPAC1, RAP1 and Caspase-3 protein expression levels were obviously decreased, and LVEF, LVFS, myocardial tissue SOD level, cardiomyocytes mitochondrial membrane potential were obviously increased (P<0.05). Agonists of EPAC1 attenuated the mitigating effect of remazolam on myocardial injury in AMI rats. Conclusion Remimazolam may inhibit the EPAC1/RAP1 signaling pathway, inhibit myocardial cell apoptosis and alleviate myocardial injury in AMI rats.
Key words: myocardial infarction; myocardial damage; remimazolam; exchange proteins directly activated by cAMP; RAS-related protein 1
急性心肌梗死(acute myocardial infarction,AMI)是由冠状动脉闭塞引起的一种心血管疾病,会导致不可逆的心肌损伤[1-2]。研究表明,AMI发作时氧化应激和炎症反应会通过激活不同的信号通路诱导心肌细胞的凋亡[3]。研究显示线粒体功能障碍会导致心肌细胞能量供应不足,进而诱导心肌细胞异常凋亡[4-5]。因此,抑制线粒体功能障碍将减轻心肌损伤。瑞马唑仑是一种在咪达唑仑的基础上改良的新型超短效苯二氮类麻醉药,其镇静效果优于咪达唑仑,且患者恢复较快[6]。咪达唑仑可抑制心肌缺血/再灌注(ischemia-reperfusion,I/R)损伤诱导的心肌细胞凋亡[7],而关于咪达唑仑类似物瑞马唑仑对心肌损伤影响的相关研究较少。环磷酸腺苷激活的交换蛋白(exchange proteins directly activated by cAMP,EPAC)是近年发现的一种环磷酸腺苷(cyclic adenosine monophosphate,cAMP)效应分子,RAS相关蛋白1(RAS-related protein 1,RAP1)是EPAC1下游效应分子[8]。有研究表明EPAC1/RAP1信号通路在心肌I/R中被激活,导致心肌细胞凋亡[9]。本研究旨在探究瑞马唑仑对AMI大鼠心肌损伤的影响及其可能的机制,进一步豐富瑞马唑仑的药用价值。
1 材料与方法
1.1 实验动物 SPF级雄性SD大鼠80只,6~7周龄,体质量180~200 g,购自湖北贝恩特生物科技有限公司,动物生产许可证号:SCXK(鄂)2021-0027。本研究经本院动物伦理委员会批准。
1.2 主要试剂与仪器 瑞马唑仑(江苏恒瑞医药股份有限公司);超氧化物歧化酶(SOD)、丙二醛(MDA)试剂盒(南京建成生物工程研究所);EPAC1激动剂8-对氯苯硫基(8-p-Chlorophenylthio,8-CPT,美国MCE公司);JC-1染色试剂盒(Sigma公司);苏木精-伊红(HE)染色试剂盒、TUNEL凋亡试剂盒(碧云天公司);兔源EPAC1、RAP1、胱天蛋白酶-3(Caspase-3)、甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GAPDH)一抗以及羊抗兔二抗(CST公司)。MYLAB? X5 VET小动物超声仪(上海玉研科学仪器有限公司);DM2000光学显微镜(徕卡显微系统上海贸易有限公司);Multiskan SkyHigh酶标仪(美国赛默飞公司)。
1.3 给药及分组 将大鼠按照随机数字表法分为假手术组、模型组、瑞马唑仑组、瑞马唑仑+8-CPT(EPAC1的激动剂)组,每组20只。除假手术组外,其余各组大鼠分别通过左前降支结扎法构建AMI模型[10],即先将大鼠用戊巴比妥钠进行麻醉,然后于左侧第3、4肋骨间开胸,挤压胸腔充分暴露心脏,缝线结扎冠状动脉左前降支,逐层缝合,心电图显示ST段持续抬高则建模成功;假手术组仅穿线,不结扎。造模后瑞马唑仑组大鼠静脉注射瑞马唑仑(1.89 mg·kg-1·d-1)[11];瑞马唑仑+8-CPT组静脉注射瑞马唑仑(1.89 mg·kg-1·d-1)和8-CPT(30 μmol·kg-1·d-1)[9];假手术组、模型组静脉注射等量的生理盐水,各组大鼠连续给药7 d。
1.4 小动物超声仪评估心功能指标末次给药结束后,戊巴比妥钠麻醉大鼠,通过小动物超声仪检测各组大鼠心功能指标,包括左心室射血分数(left ventricular ejection fraction,LVEF)、左心室短轴缩短率(left ventricular fractional shortening,LVFS)、左心室舒张末期内径(left ventricular end-diastolic diameter,LVEDD)、左心室收缩末期内径(left ventricular end-systolic diameter,LVESD)。
1.5 样品制备 各组大鼠在心功能检测后予以处死,分离心脏组织,每组中取5只大鼠心脏组织固定于多聚甲醛中用于HE染色和TUNEL染色;5只大鼠心脏组织用于检测心肌组织SOD和MDA水平;5只大鼠心脏组织用于检测心肌细胞线粒体膜电位;5只大鼠心脏组织用于Western blot检测。
1.6 HE染色检测心肌组织病理情况 将固定于多聚甲醛中的心脏组织通过石蜡包埋,制成5 μm病理切片,HE染色,并通过光镜观察各组大鼠心肌组织病理损伤。
1.7 化学比色法检测心肌组织SOD和MDA水平 取各组大鼠适量梗死心肌组织(假手术组取同一位置的心肌组织),称质量后加入9倍生理盐水进行混合并制成匀浆,离心后取上清液,按试剂盒说明书测定SOD和MDA,分别在450、532 nm波长处检测SOD、MDA的吸光度(A)值,计算表达水平。
1.8 JC-1染色法检测心肌细胞线粒体膜电位 将梗死心肌组织碎块提取出线粒体后,按照JC-1线粒体膜电位检测试剂盒说明书进行操作,上镜检测。红绿荧光比值代表线粒体膜电位水平。
1.9 TUNEL染色观察心肌细胞凋亡 将固定于多聚甲醛中心脏组织进行石蜡包埋制备病理切片,按照说明书进行TUNEL染色,计算TUNEL阳性率。
1.10 Western blot检测心肌组织EPAC1、RAP1、Caspase-3蛋白表达水平 将心肌组织剪碎后加入RIPA裂解液,充分匀浆裂解后,离心获得上清液即蛋白,BCA法进行定量后,进行Western blot检测。具体步骤为电泳,转膜,脱脂奶粉封闭,4 ℃孵育EPAC1、RAP1、Caspase-3、GAPDH一抗(稀释比均为1∶1 000)过夜,室温孵育二抗(稀释比均为1∶3 000)1 h,将膜用ECL显色,凝胶成像系统拍照,通过Image J软件分析蛋白相对表达。
1.11 统计学方法 采用SPSS 25.0软件进行数据分析,计量数据以均数±标准差([x] ±s)表示。多组间比较用单因素方差分析,组间多重比较采用SNK-q检验,P<0.05为差异有统计学意义。
2 结果
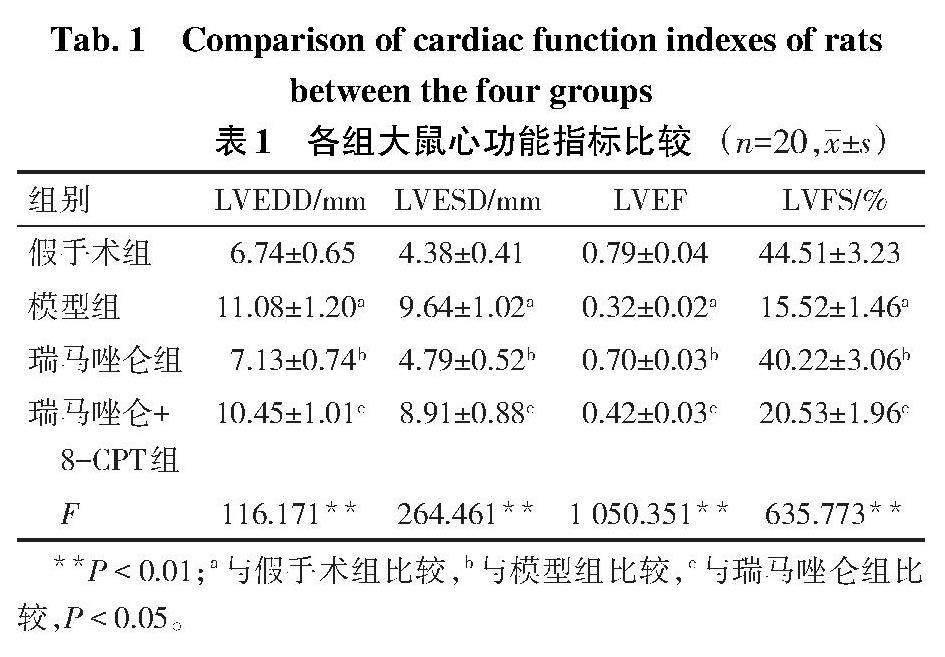
2.1 各组大鼠心功能相关指标比较 与假手术组相比,模型组大鼠LVEDD、LVESD均升高,LVEF、LVFS降低(P<0.05);与模型组相比,瑞马唑仑组大鼠LVEDD、LVESD均降低,LVEF、LVFS升高(P<0.05);与瑞马唑仑组相比,瑞马唑仑+8-CPT组大鼠LVEDD、LVESD升高,LVEF、LVFS降低(P<0.05),见表1。
2.2 各组大鼠心肌组织病理损伤 假手术组大鼠心肌组织结构完整,细胞排列有序;与假手术组相比,模型组大鼠心肌组织结构受损严重,大量炎性细胞浸润;与模型组相比,瑞马唑仑组大鼠心肌组织结构恢复,少量炎性细胞浸润;与瑞马唑仑组相比,瑞马唑仑+8-CPT组大鼠心肌组织结构受损严重,大量炎性细胞浸润,见图1。
2.3 各组大鼠心肌组织SOD和MDA水平比较 与假手术组相比,模型组大鼠心肌组织SOD水平降低,MDA水平升高(P<0.05);与模型组相比,瑞马唑仑组大鼠心肌组织SOD水平升高,MDA水平降低(P<0.05);与瑞马唑仑组相比,瑞马唑仑+8-CPT组大鼠心肌组织SOD水平降低,MDA水平升高(P<0.05),见表2。
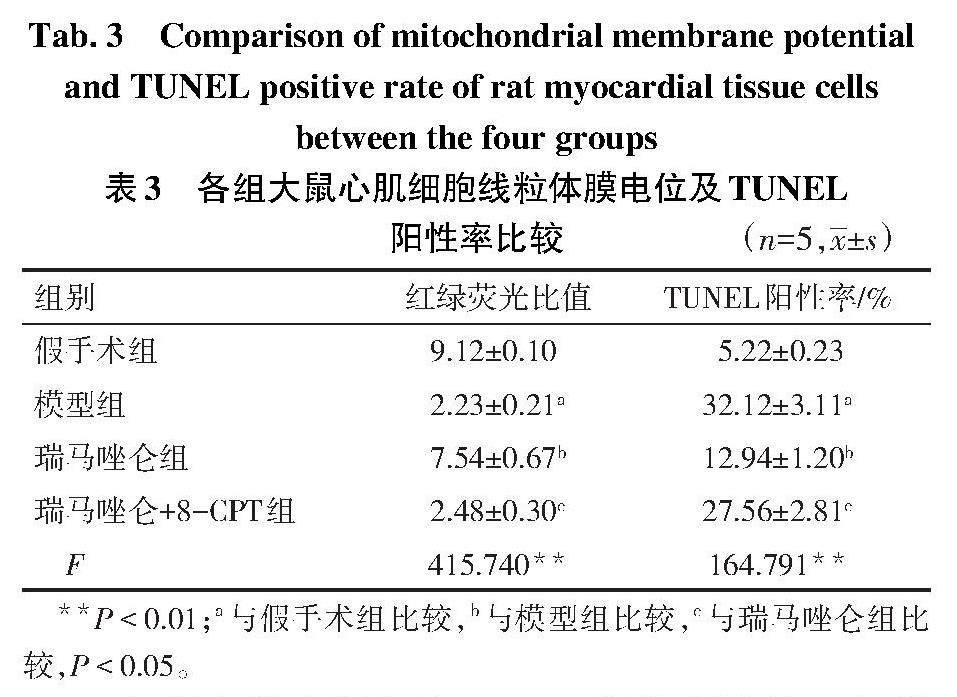
2.4 各组大鼠心肌细胞线粒体膜电位比较 与假手术组相比,模型組大鼠心肌细胞红绿荧光比值降低,线粒体膜电位下降(P<0.05);与模型组相比,瑞马唑仑组大鼠心肌细胞线粒体膜电位上升(P<0.05);与瑞马唑仑组相比,瑞马唑仑+8-CPT组大鼠心肌细胞线粒体膜电位下降(P<0.05),见表3,图2。
2.5 各组大鼠心肌细胞TUNEL阳性率比较 与假手术组相比,模型组大鼠心肌细胞TUNEL阳性率升高(P<0.05);与模型组相比,瑞马唑仑组大鼠心肌细胞TUNEL阳性率降低(P<0.05);与瑞马唑仑组相比,瑞马唑仑+8-CPT组大鼠心肌细胞TUNEL阳性率升高(P<0.05),见表3、图3。
2.6 各组大鼠心肌组织EPAC1、RAP1、Caspase-3蛋白表达水平比较 与假手术组相比,模型组大鼠心肌组织EPAC1、RAP1、Caspase-3蛋白表达水平升高(P<0.05);与模型组相比,瑞马唑仑组大鼠心肌组织EPAC1、RAP1、Caspase-3蛋白表达水平降低(P<0.05);与瑞马唑仑组相比,瑞马唑仑+8-CPT组大鼠心肌组织上述蛋白表达水平升高(P<0.05),见表4、图4。
3 讨论
AMI是临床常见心血管疾病,近年来我国心肌梗死发病率逐渐升高,尽管该病的诊疗技术取得了进展,但心肌梗死时出现心肌坏死,心肌细胞凋亡导致心脏出现不可逆损伤,AMI病死率仍然很高[12-13]。因此,寻求可以抑制心肌细胞凋亡,从而减轻AMI后心肌损伤的方法具有重要意义。
瑞马唑仑是一种新型镇静药物,广泛应用于成人全身麻醉和重症监护病房(intensive care unit,ICU)镇静[14]。有研究表明咪达唑仑对心肌具有保护作用,其可减少心肌I/R损伤大鼠心肌梗死面积,缓解心脏组织损伤并抑制心肌细胞凋亡[7]。而瑞马唑仑是咪达唑仑类似物,且具有比咪达唑仑更好的临床效果,因此推测瑞马唑仑可能对心肌具有保护作用,减少心肌梗死面积,减轻心肌损伤。本研究表明瑞马唑仑可明显缓解心肌损伤,提高AMI大鼠心脏收缩和舒张能力,与Zhou等[7]研究结果基本一致。有研究显示心肌缺血时出现大量氧自由基堆积,从而导致氧化应激损伤[15]。抗氧化酶SOD和氧化酶MDA常用来反映氧化应激情况。本文结果显示,经瑞马唑仑干预后,AMI大鼠MDA水平降低,SOD水平升高,提示瑞马唑仑可减轻大鼠心肌的氧化应激损伤。线粒体是细胞凋亡的一个关键因素,几乎所有导致细胞凋亡的刺激都会诱发线粒体结构破坏和功能障碍,且线粒体膜电位变化也是心肌细胞早期凋亡的指标[16]。有研究显示芪苈强心胶囊可通过缓解AMI大鼠氧化损伤减轻线粒体功能障碍,并抑制心肌细胞异常凋亡,保护大鼠心脏功能[17]。本研究结果显示,AMI大鼠心肌细胞线粒体膜电位降低,心肌细胞凋亡率升高,瑞马唑仑干预后大鼠心肌细胞线粒体膜电位升高,且心肌细胞凋亡率下降,与Zhao等[17]研究结果相似,提示瑞马唑仑处理可促进AMI大鼠线粒体功能障碍恢复,抑制心肌细胞凋亡,从而缓解AMI大鼠心肌氧化损伤。
cAMP是一种关键的二级信使,可直接诱导EPAC激活,发挥多种生物学效应。EPAC包含EPAC1和EPAC2两种主要亚型,其中EPAC1在心脏、子宫、卵巢等组织中大量表达[18],EPAC的效应器为RAP,RAP是小G蛋白的RAS超家族成员之一[19]。多个研究表明心肌I/R损伤通过激活EPAC1-RAP1信号诱导心肌细胞凋亡[20-21]。也有研究表明激活EPAC1-RAP1信号可引起线粒体功能障碍,从而导致心肌I/R损伤[9]。本研究结果表明AMI大鼠心肌组织中EPAC1、RAP1蛋白水平明显升高,EPAC1-RAP1信号通路被激活,而瑞马唑仑干预后,大鼠心肌组织中EPAC1、RAP1蛋白水平明显降低,与Yang[9]等研究结果相似,提示瑞马唑仑可能通过抑制EPAC1/RAP1信号通路减轻AMI大鼠心肌损伤。进一步使用8-CPT干预AMI大鼠后,发现8-CPT逆转了瑞马唑仑对AMI大鼠心肌损伤的保护作用,进一步证实瑞马唑仑可通过抑制EPAC1/RAP1信号通路减轻AMI大鼠心肌损伤。
综上所述,瑞马唑仑可通过抑制EPAC1/RAP1信号通路抑制心肌细胞凋亡,从而减轻AMI大鼠心肌损伤,但是否存在其他通路调控此过程,仍需进一步研究。
参考文献
[1] CHI X,SHAN L,HU Y,et al. Bromodomain-containing protein 7 contributes to myocardial infarction-induced myocardial injury through activating Wnt/β-catenin signaling[J]. Ann Palliat Med,2021,10(10):10756-10767. doi:10.21037/apm-21-2433.
[2] ECKNER D,PAUSCHINGER M,ADEMAJ F,et al. Clinical implications of the fourth universal definition of myocardial infarction[J]. Herz,2020,45(6):520-527. doi:10.1007/s00059-020-04948-6.
[3] JINAWONG K,PIAMSIRI C,APAIJAI N,et al. Treatment with apoptosis inhibitor restores cognitive impairment in rats with myocardial infarction[J]. Biochim Biophys Acta Mol Basis Dis,2023,1869(7):166809. doi:10.1016/j.bbadis.2023.166809.
[4] RABINOVICH-NIKITIN I,RASOULI M,REITZ C J,et al. Mitochondrial autophagy and cell survival is regulated by the circadian clock gene in cardiac myocytes during ischemic stress[J]. Autophagy,2021,17(11):3794-3812. doi:10.1080/15548627.2021.1938913.
[5] JIANG W,ZHANG Y,ZHANG W,et al. Hirsutine ameliorates myocardial ischemia-reperfusion injury through improving mitochondrial function via CaMKII pathway[J]. Clin Exp Hypertens,2023,45(1):2192444. doi:10.1080/10641963.2023.2192444.
[6] LIU G,XIONG Y. Analysis of stress response and analgesic effect of remazolam combined with etomidate in painless gastroenteroscopy[J]. Contrast Media Mol Imaging,2022,2022:4863682. doi:10.1155/2022/4863682.
[7] ZHOU W,CAI D. Midazolam suppresses ischemia/reperfusion-induced cardiomyocyte apoptosis by inhibiting the JNK/p38 MAPK signaling pathway[J]. Can J Physiol Pharmacol,2022,100(2):117-124. doi:10.1139/cjpp-2021-0289.
[8] WANG X,ZHANG Y,YANG Y,et al. Curcumin pretreatment protects against hypoxia/reoxgenation injury via improvement of mitochondrial function, destabilization of HIF-1α and activation of Epac1-Akt pathway in rat bone marrow mesenchymal stem cells[J]. Biomed Pharmacother,2019,109:1268-1275. doi:10.1016/j.biopha.2018.11.005.
[9] YANG H,XUE W,DING C,et al. Vitexin mitigates myocardial ischemia/reperfusion injury in rats by regulating mitochondrial dysfunction via Epac1-Rap1 signaling[J]. Oxid Med Cell Longev,2021,2021:9921982. doi:10.1155/2021/9921982.
[10] 姚書霞,史璇,韩松,等. 乔松素抑制TLR4/NF-κB/NLRP3信号通路对急性心肌梗死大鼠炎性损伤的影响[J/OL].中国免疫学杂志,2022[2023-06-12]. YAO S X,SHI X,HAN S,et al. Influences of Pinocembrin on inflammatory injury in rats with acute myocardial infarction by inhibiting TLR4/NF-κB/NLRP3 signaling pathway[J/OL]. Chinese Journal of Immunology,2022[2023-06-12]. https://kns.cnki.net/kcms/detail/22.1126.R.20221120.1735.002.html.
[11] 彭蕊,王倩,杨天爽,等. 不同剂量瑞马唑仑复合舒芬太尼在无痛胃镜检查术中的比较[J]. 临床麻醉学杂志,2023,39(4):389-392. PENG R,WANG Q,YANG T S,et al. Comparison of different doses of remimazolam combined with sufentanil in painless gastroscopy[J]. J Clin Anesthesiol,2023,39(4):389-392. doi:10.12089/jca.2023.04.010.
[12] 孙向华,杨菲. 基于Nrf2/HO-1信号通路探讨阿托伐他汀对急性心肌梗死大鼠模型心肌细胞的影响[J]. 中西医结合心脑血管病杂志,2023,21(6):1042-1046. SUN X H,YANG F. Exploring the effects of atorvastatin on cardiomyocytes in a rat model of acute myocardial infarction based on the Nrf2/HO-1 signaling pathway[J]. Journal of Integrative Medicine On Cardio-Cerebrovgascular Disease,2023,21(6):1042-1046. doi:10.12102/j.issn.1672-1349.2023.06.012.
[13] TRIPATHI H,DOMINGUES A,DONAHUE R,et al. Combined transplantation of human MSCs and ECFCs improves cardiac function and decrease cardiomyocyte apoptosis after acute myocardial infarction[J]. Stem Cell Rev Rep,2023,19(2):573-577. doi:10.1007/s12015-022-10468-z.
[14] KILPATRICK G J. Remimazolam:non-clinical and clinical profile of a new sedative/anesthetic agent[J]. Front Pharmacol,2021,12:690875. doi:10.3389/fphar.2021.690875.
[15] 钱厚霖,周述芝,毕小波,等. 右美托咪定调控Nrf2/HO-1通路对H2O2诱导心肌细胞氧化应激损伤的作用研究[J]. 中国现代医学杂志,2023,33(7):40-45. QIAN H L,ZHOU S Z,BI X B,et al. Effect of dexmedetomidine regulating Nrf2/HO-1 pathway on H2O2-induced oxidative stress injury in cardiomyocytes[J]. China Journal of Modern Medicine,2023,33(7):40-45. doi:10.3969/j.issn.1005-8982.2023.07.007.
[16] DAUBERT M A,ADAMS K,YOW E,et al. NT-proBNP goal achievement is associated with significant reverse remodeling and improved clinical outcomes in HFrEF[J]. JACC Heart Fail,2019,7(2):158-168. doi:10.1016/j.jchf.2018.10.014.
[17] ZHAO Q,LI H,CHANG L,et al. Qiliqiangxin attenuates oxidative stress-induced mitochondrion-dependent apoptosis in cardiomyocytes via PI3K/AKT/GSK3β signaling pathway[J]. Biol Pharm Bull,2019,42(8):1310-1321. doi:10.1248/bpb.b19-00050.
[18] LEE K. Epac:new emerging cAMP-binding protein[J]. BMB Rep,2021,54(3):149-156. doi:10.5483/BMBRep.2021.54.3.233.
[19] FAZAL L,LAUDETTE M,PAULA-GOMES S,et al. multifunctional mitochondrial Epac1 controls myocardial cell death[J]. Circ Res,2017,120(4):645-657. doi:10.1161/CIRCRESAHA.116.309859.
[20]WANG X,CHE X,JIANG Q,et al. Epac1/Rap1 signaling pathway is involved in the pathogenesis of myocardial ischemia/reperfusion injury in rats[J]. Chin J Pharmacol Toxicol,2018,32(4):309-310.
[21] CHE X,WANG X,ZHANG J,et al. Vitexin exerts cardioprotective effect on chronic myocardial ischemia/reperfusion injury in rats via inhibiting myocardial apoptosis and lipid peroxidation[J]. Am J Transl Res,2016,8(8):3319-3328.
(2023-06-12收稿 2023-10-19修回)
(本文編辑 李国琪)

