Effectiveness of intravitreal ranibizumab for diabetic macular edema in vitrectomized versus non-vitrectomized eyes: a Meta-analysis
Yi-Heng Wang, Qian Xu, Jie Luan
1Department of Ophthalmology, Zhongda Hospital, Southeast University, Nanjing 210009, Jiangsu Province, China
2School of Medicine, Southeast University, Nanjing 210009,Jiangsu Province, China
Abstract
· KEYWORDS: diabetic macular edema; ranibizumab;vitrectomized eye
INTRODUCTION
Diabetic macular edema (DME), which is characterized by exudative fluid accumulation at the macula caused by long-term hyperglycemia, is a leading cause of blindness in the working-age population of most developed countries[1-3].The estimated prevalence of diabetic retinopathy and DME in the global diabetes mellitus population was 22.27% [95%confidence interval (CI), 19.73%-25.03%] and 6.81% (95%CI,6.74%-6.89%) respectively[2-3].The mechanism of DME is complex and mainly involves the disruption of the blood-retina barrier with an increase in vascular leakage[4].
Several clinical trials have confirmed that intravitreal injections of drugs targeting vascular endothelial growth factor (VEGF) can result in better visual outcomes than laser photocoagulation[5-6].Anti-VEGF drugs including aflibercept, ranibizumab, and bevacizumab have now become the first-line treatment for DME[4].Notably, studies such as RESOLVE[5]and RISE/RIDE[7]have proved that ranibizumab is an effective treatment for DME.Vitrectomy, also as a treatment for DME, can not only improve retinal oxygenation but also reduce the risk of retinal neovascularization[8].Studies affirm its effectiveness for both tractional and non-tractional DME cases[9-10].Currently, the effects of vitrectomy on the diffusion and clearance of anti-VEGF drugs remain controversial.The majority of anti-VEGF pharmacokinetics in vitreous, especially in vitrectomized eyes, are derived from animal models.Research evidence on the effect of vitrectomy on the pharmacokinetic properties of intravitreal drugs is scarce, and there is no clinical trial on anti-VEGF drugs.Considering that there is no guideline or consensus on anti-VEGF therapy for DME eyes after vitrectomy, the aim of this Meta-analysis is to evaluate the effectiveness of intravitreal ranibizumab (IVR) on DME between eyes with and without previous vitrectomy of different follow-up duration.
MATERIALS AND METHODS
Search StrategyPubMed, EMBASE, Web of Science,Cochrane, EBSCO were searched, up to May 2022, for articles published in English.The search keywords including “diabetic macular edema” and “anti-vascular endothelial growth factor or anti-VEGF or ranibizumab” and “vitrectomy or vitrectomized” were used to maximize the search accuracy.
This Meta study was approved by the Ethical Committee of Zhongda Hospital, Affiliated with Southeast University, in May.Ethics Approval number is 2022ZDSYLL112-P01.
Inclusion and Exclusion CriteriaAll studies included in this research followed the inclusion: 1) clinical comparative studies; 2) patients with DME who receive IVR therapy; 3)two groups according to the vitreous status: non-vitrectomized group and vitrectomized group; 4) the primary outcomes recorded postoperative best-corrected visual acuity (BCVA),central macular thickness (CMT) and the mean number of intravitreal injections.Additional outcomes collected included the rate of complications.
Exclusion criteria includes the following: 1) reviews, case reports, and non-comparative studies; 2) patients treated with dexamethasone (DEX) implant (Ozurdex) or other intravitreal injections of drugs; 3) duplicate literatures.
Study SelectionTwo independent researchers extracted the data fulfilling the inclusion and exclusion criteria, and evaluated the quality.If there are discrepancies, a third reviewer analyzed the data and quality.
Data Extraction and Quality AssessmentTwo researchers independently extracted the following data from the included articles: first author, publication year, region, study design,sample size, average age, intervention indicators, outcomes,and follow-up periods.Data was shown in the form of mean±standard deviation (SD).Afterwards, we used the Get Data software to estimate the mean and SD from the charts in the articles.
All studies were assessed by Methodological Index for Nonrandomized Studies (MINORS)[11], which contains 12 items with the highest scores of 24.Each criterion was scored 0, 1, or 2 (where 0 showed not reported; 1 showed reported but inadequate; 2 showed reported and adequate), and high quality was regarded if research gains a score of ≥18.As is concluded in Table 2, researches included were generally good.

Figure 1 Flowchart of database search and study identification.
Statistical AnalysisReview Manager 5.3 was used to analyze the extracted statistics.Continuous variable outcomes and dichotomous outcomes were estimated by mean difference(MD) and risk ratio (RR) with 95%CI.Statistical heterogeneity was assessed by calculatingI2.I2ranges from 0 to 100%.The 25%, 50%, and 75% express low, moderate, and high heterogeneity respectively[12].IfI2results were between 50%and 100%, the random effects model analysis was used,otherwise, the fixed effects model was employed.Publication bias was evaluated by funnel plots and Egger’s test.P< 0.05 were considered significant statistically.
RESULTS
Search ResultsA total of 96 records were found through database searching, and 94 studies remained after removing duplicates.After screening titles and abstracts 54 studies were excluded and 40 full-text articles were left for assessment.Finally, six non-randomized studies[13-18]in English were included in this Meta-analysis.The literature selection process is indicated in Figure 1.
Characteristics of the Included StudiesCharacteristics of the included studies are shown in Table 1[13-18].A total of 641 eyes were included, with 112 eyes having vitrectomy before as the vitrectomized group and 529 eyes without previous vitrectomy as the non-vitrectomized group.Four studies were prospective and the remaining two were retrospective.The follow-up time was at least 6mo (6-36mo).The quality assessment with the MINORS ranging from 18 to 20 points was shown in Table 2.
Meta-Analysis
Mean BCVA at 6 and 12moAt 6mo, data from four studies[13-14,16-17]assessing 247 eyes (77 vitrectomized eyes,170 non-vitrectomized eyes) reported the BCVA.Significantlybetter final BCVA was discovered in the non-vitrectomized group than in the vitrectomized group (MD=0.18, 95%CI: 0.10 to 0.26,P<0.00001; Figure 2A).A total of 3 literatures[15-17]of 410 eyes compared the BCVA between vitrectomized group(45 eyes) and non-vitrectomized group (365 eyes) at 12mo,and showed statistically significant difference between two groups, in favor of the non-vitrectomized group (MD=0.13,95%CI: 0.05 to 0.21,P=0.002; Figure 2A).The fixedmodel was applied for the evaluation of mean BCVA, and no heterogeneity was observed (I2=0).
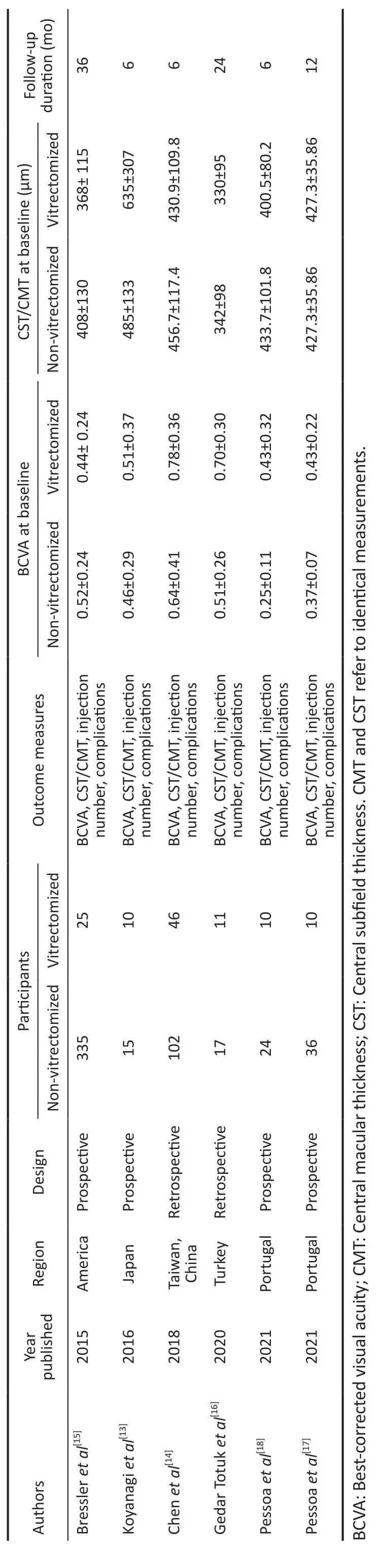
Table 1 Characteristics of the included studies
Mean Improvement in BCVA at 6 and 12moData from four studies[13-14,16-17]of 247 eyes reported the mean change of BCVA between the two groups.The mean change of BCVA from baseline to 6mo was not significant (MD=0.05, 95%CI:-0.02 to 0.13,P=0.14; Figure 2B).Moreover, data of 12mo came to the same conclusion.Three studies[15-17]of 410 eyes showed the mean change of BCVA from baseline to 12mo, the non-vitrectomized group represented a similar improvement with the vitrectomized group (MD=0.03, 95%CI: -0.04 to 0.09,P=0.43; Figure 2B).There was no statistical heterogeneity and the fixed effects model was used.
Mean CMT at 6 and 12moA total of 4 literatures[13-14,16-17]of 247 eyes demonstrated data on CMT at 6mo.The summary MD in patients was statistically significant (MD=34.51,95%CI: 11.22 to 57.80,P=0.004; Figure 2C) in favor of nonvitrectomized group over vitrectomized group.However,the subgroup analysis suggested that the advantage of nonvitrectomized group over vitrectomized group was not evident at 12mo (MD=20.71, 95%CI: -3.28 to 44.71,P=0.09; Figure 2C).Meta-analysis did not show significant heterogeneity at any of these follow-up periods (6mo,I2=0; 12mo,I2=0).
Mean Reduction in CMT at 6 and 12moFour studies[13-14,16-17]with 247 eyes compared the mean change from baseline to 6mo in terms of the reduction of CMT between the two groups.The difference between non-vitrectomized group and vitrectomized group showed statistically significant(MD=53.37, 95%CI: 28.03 to 78.72,P<0.0001; Figure 2D),in favor of non-vitrectomized group, and without statistically significant heterogeneity (I2=0).Additionally, this superiority was still observed at 12mo.Significant reduction in CMT was shown in favor of non-vitrectomized group (MD=49.65,95%CI: 19.58 to 79.72,P=0.01; Figure 2D) with no significant heterogeneity (I2=0).
Visual Improvement in Vitrectomized GroupIn four studies[13-14,16-17], the BCVA significantly improved after IVR at 6mo in vitrectomized group (MD= -0.18, 95%CI: -0.28 to-0.08,P=0.0006; Figure 2E), this superiority was still observed at 12mo.BCVA data from three studies[15-17]at 12mo, with MD of -0.13, also showed the improvement was statistically significant difference (P=0.02; Figure 2E).No significantheterogeneity was detected among the studies at 6mo (I2=39%)and 12mo (I2=0).

Table 2 Quality assessment using methodological index for nonrandomized studies
Anatomic Improvement in Vitrectomized GroupThe pooled assessment of the CMT from baseline to 6 and 12mo of follow-up in vitrectomized group was displayed in Figure 2F.Four studies[13-14,16-17](n=77 eyes) reported variations of CMT from baseline to 6mo after IVR treatment, which reduced with an average of 82.55 μm (95%CI: -113.71 to -51.39,P<0.00001;Figure 2F).The assessment was performed in three studies[15-17](n=44 eyes) at 12mo and demonstrated a significant reduction from baseline with an average of 66.83 μm (95%CI: -101.43 to-32.24,P=0.0002; Figure 2F).
Mean Number of Intravitreal Injection at 6 and 12moFour studies[13-15,18]assessing 549 eyes indicated that the mean number of ranibizumab injection in vitrectomized eyes was significantly more than that in non-vitrectomized eyes during 6-month period (MD=0.60, 95%CI: 0.16 to 1.04,P=0.008;Figure 2G).There was a large amount of heterogeneity and the random effects model was used.When the study by Bressleret al[15]was excluded from the Meta-analysis, the remaining 3 literatures showed no statistical heterogeneity (I2=0,P<0.00001) and the difference was still statistically significant between two groups (MD=0.88, 95%CI: 0.67 to 1.10,P<0.00001;Figure 2H).
The study by Pessoaet al[17]only showed the mean number of IVR injection in non-vitrectomized eyes (7.72; 95%CI:6.71-8.74) was not significantly different from that in the vitrectomized eyes (7.86; 95%CI: 5.39-10.33) during 12mo of follow-up (P>0.05).Similarly, there was no statistically significant difference between the two groups in the mean number of IVR injection according to Gedar Totuket al[16].Safety OutcomesMeta-analysis was limited cause lacking adequate data about complications.Significant adverse effects reported by Bressleret al[15]which included 29 eyes (9%,99%CI: 5% to 13%) in non-vitrectomized group including vitreous hemorrhage, traction retinal detachment, or venous occlusive disease, compared to no eye (0, 99%CI, 0 to 19%)in the vitrectomized group.One case of retinal detachment and one case of iatrogenic cataract were reported in the study by Pessoaet al[17].
Publication Bias and Sensitivity AnalysisFunnel plot(Figure 3) of Meta-analysis indicated no obvious publication bias.Because of the limited studies, a subgroup analysis couldn’t be performed to interpret the source of heterogeneity.After the exclusion of the study[15]in the Meta-analysis of mean number of intravitreal injection, the remaining 3 literatures showed no statistical heterogeneity (I2=0,P<0.00001).We suspect that the study may be the source of heterogeneity, because of the imbalance of baseline such as poorer levels of initial visual acuity and thinner central subfield thicknesses.
DISCUSSION
To the best of our knowledge, this study is the first Metaanalysis to evaluate the efficacy of IVR on DME between eyes with and without previous vitrectomy.Previous clinical trials have consistently demonstrated the efficacy of ranibizumab in treating DME without prior vitrectomy[5,7,19].However, there remains a dearth of research investigating whether vitrectomy alters the effects of ranibizumab on DME.Therefore, clinical assisted evidence should be provided when ophthalmologists treat DME in vitrectomized patients.In the Meta-analysis,by pooling 6 comparative studies involving a total of 641 eyes and our results indicated that: 1) Both vitrectomized and non-vitrectomized group could achieve functional and anatomical improvement at 6 and 12mo after ranibizumab injections; 2) Although the mean reduction in CMT among non-vitrectomized eyes was significantly greater than in vitrectomized group at the month 6 and 12mo visits, there was a similar trend in the mean visual gain in two groups; 3)In contrast to non-vitrectomized group, more ranibizumab treatment burden was found in vitrectomized group at 6mo.However, no significant difference in the number of IVR injections was found between two groups at long-term (12mo)follow-up.

Figure 2 Forest plot A: Forest plot comparing mean BCVA after IVR at 6 and 12mo follow-up (vitrectomized group vs non-vitrectomized group);B: Forest plot comparing the mean improvement in BCVA after IVR at 6 and 12mo follow-up (vitrectomized group vs non-vitrectomized group); C:Forest plot comparing mean CMT after IVR at 6 and 12mo follow-up (vitrectomized group vs non-vitrectomized group); D: Forest plot comparing the mean reduction in CMT after IVR at 6 and 12mo follow-up (vitrectomized group vs non-vitrectomized group); E: Forest plot comparing mean BCVA at baseline with after IVR in vitrectomized group; F: Forest plot comparing mean CMT at baseline with after IVR in vitrectomized group;G: Forest plot comparing mean number of intravitreal injection at 6mo between vitrectomized group and non-vitrectomized group; H: Forest plot comparing mean number of intravitreal injection at 6mo between vitrectomized group and non-vitrectomized group after the exclusion of Bressler et al.IVR: Intravitreal ranibizumab; BCVA: Best-corrected visual acuity; CMT: Central macular thickness.

Figure 3 Funnel plots for the Meta-analysis A: Mean BCVA at 6 and 12mo; B: Mean improvement in BCVA at 6 and 12mo.BCVA: Bestcorrected visual acuity.
Vitrectomy for DME was first reported by Lewiset al[20]in 1992.Although the mechanism of vitrectomy on DME remains to be indistinct, many researchers reported that vitrectomy was effective for both visual and anatomic improvement on DME[10,21], for vitrectomy could decrease the amount of VEGF and proinflammatory cytokines[22].In addition, some studies reported that vitrectomy could increase vitreous oxygenation, and increased oxygen tension is likely to reduce the concentration of VEGF[8,23-24].In this sense, vitrectomy can hinder the development of neovascularization and macular edema.Currently, vitrectomy for DME has gained rapid and widespread acceptance[10].But DME still exists in some patients after vitrectomy and need intravitreal anti-VEGF drug treatment.Although literatures included in our Meta-analysis demonstrated that the recovery is slower in vitrectomized eyes compared with non-vitrectomized eyes[13-16], significant anatomical and functional improvements were shown in eyes with previous vitrectomy of the Meta-analysis.
Some doubts remain on the real effect of intravitreal anti-VEGF drug once the vitreous has been removed.This Metaanalysis showed that no significant difference was detected in improvement in BCVA, whilst the mean reduction in CMT among non-vitrectomized eyes was significantly greater than in vitrectomized eyes.Additionally, more ranibizumab treatment burden was found in vitrectomized group at 6mo than non-vitrectomized group in the Meta-analysis, while there was no statistically significant difference between the two groups during 12mo follow-up.Although we know vitrectomy is an effective treatment of DME, the rapid drug diffusion and clearance from the vitreous cavity after vitrectomy may reduce intravitreal anti-VEGF treatment success.Several animal studies[25-27]reported intraocular pharmacokinetics from the vitreous cavity in vitrectomized eyes.A study in rabbits has shown that VEGF clearance increased after vitrectomy[25].Another study using monkeys revealed that the half-life of bevacizumab was shorter in vitrectomized eyes than in non-vitrectomized eyes[26].Conversely, other animal study suggested that the clearance of bevacizumab in vitrectomized eyes may be comparable to those without vitrectomy before[27].There lacks clinical trial evidence about the effects of vitrectomy on the pharmacokinetic properties of intravitreal anti-VEGF drugs.In the Gedar Totuket al’s[16]study, there was no significant difference in number of IVR injections between vitrectomized and non-vitrectomized group at the long-term follow-up (24mo).This result is also in agreement with that of the Bressleret al’s[15]2015 study, which found that the cumulative number of IVR injections with prior vitrectomy were comparable to those eyes without vitrectomy before at 3y visit.Based on these findings, we can find that although the improvements were slower, ranibizumab was still an effective treatment for eyes with DME after vitrectomy.
The emerging popularity of anti-VEGF drugs has raised concerns about the safety of their use.Several studies have reported that the use of anti-VEGF drugs in oncology may lead to systemic events such as hemorrhagic and cardiovascular events[28-29].In the included studies of our Meta-analysis,Pessoaet al[17]reported one acute myocardial infarction and one stroke.Low incidence of serious ocular adverse events occurred on intravitreal anti-VEGF injections[30].Only one research in our Meta-analysis reported ocular adverse events, such as vitreous hemorrhage, traction retinal detachment or venous occlusive disease, and the complication rate of vitrectomized eye was not higher than that without vitrectomy before[15].Prior Meta-analyses have primarily focused on comparing the efficacy of different anti-VEGF therapies[31-32].Our current Meta-analysis stands as the first study to explore the effectiveness of ranibizumab specifically in DME patients with and without prior vitrectomy.Notably, considering the absence of established guidelines or consensus for managing DME in vitrectomized eyes using anti-VEGF treatment, our research aims to evaluate both the efficacy and safety of IVR to furnish clinical practice with evidence-based insights.
Our Meta-analysis has some limitations.The researches of the Meta-analysis included four prospective studies and the two retrospective studies.Further prospective researches of larger samples are needed to confirm the result.
In conclusion, results of this Meta-analysis suggested that both vitrectomized and non-vitrectomized group exhibit favorable visual and anatomical responses to anti-VEGF therapy.Compared with non-vitrectomized group, similar rate of vision improvement and less reduction in macular thickness in DME eyes was found in vitrectomized group.More ranibizumab treatment burden was found in vitrectomized group at 6mo than non-vitrectomized group, while no significant difference between the two groups was found during 12mo of follow-up.
ACKNOWLEDGEMENTS
Conflicts of Interest:Wang YH,None;Xu Q,None;Luan J,None.
 International Journal of Ophthalmology2024年4期
International Journal of Ophthalmology2024年4期
- International Journal of Ophthalmology的其它文章
- Algorithm of automatic identification of diabetic retinopathy foci based on ultra-widefield scanning laser ophthalmoscopy
- CD3ε of a pan T cell marker involved in mouse Aspergillus fumigatus keratitis
- Neuroprotective effects of acteoside in a glaucoma mouse model by targeting Serta domain-containing protein 4
- Neuroprotective and anti-inflammatory effects of eicosane on glutamate and NMDA-induced retinal ganglion cell injury
- Bone morphogenetic protein-6 suppresses TGF-β2-induced epithelial-mesenchymal transition in retinal pigment epithelium
- Dry eye rate and its relationship with disease stage in patients with primary hypertension: a cross-sectional study in Vietnam
