CD3ε of a pan T cell marker involved in mouse Aspergillus fumigatus keratitis
Wen-Hao Shi, Li-Mei Wang, Hai-Jing Yan, Shi-Long Liu, Xian Yang, Xue-Jiao Yang, Cheng-Ye Che
1Department of Ophthalmology, the Affiliated Hospital of Qingdao University, Qingdao 266000, Shandong Province,China
2Weifang Medical University, Weifang 261053, Shandong Province, China
3Eye Hospital of Wenzhou Medical University, Wenzhou 325027, Zhejiang Province, China
4Department of Medical Engineering, the Affiliated Hospital of Qingdao University, Qingdao 266000, Shandong Province,China
Abstract
· KEYWORDS: CD3ε; adaptive immunity; Aspergillus fumigatus keratitis; interleukin-10
INTRODUCTION
Fungal keratitis (FK) is currently one of the main blinding factors[1].It usually occurs after corneal epithelial defects caused by vegetable trauma or other factors[2].Pathogenic fungi invade the corneal stroma from the damaged site to grow and multiply, and finally cause necrosis and inflammation[3].
The occurrence of FK depends on the interaction of fungal pathogenicity and body immunity.The immune mechanism which body fights against pathogenic microorganism mainly includes innate immunity and adaptive immunity[4].Studies have shown that when fungi invade cornea, they are recognized by pattern recognition receptors, initiating innate immunity, inducing local phagocytes (neutrophils,macrophagesetc.) to release cytokines and chemokines, which can enhance fungal clearance[4-6].Activated macrophages, as important antigen presenting cells, engulf apoptotic neutrophils and enhance T cell activation to initiate adaptive immunity.Once the adaptive immunity is activated, it will enhance the migration of activated macrophages and neutrophils, promote the outbreak of pro-inflammatory cytokines, trigger a strong hypersensitivity response, and even exacerbate corneal damage.Therefore, precise regulatory mechanisms are needed to regulate the innate and adaptive immunity of FK in order to develop new target therapies[7-9].
There is an important molecule in adaptive immunity—CD3 composed of γ, δ, ε, and ζ chains[10].The TCR/CD3 complex formed by CD3 and T cell antigen receptor (TCR),which participates in antigen recognition and immune signal transduction, is a sign of initiating adaptive immunity[11-12].CD3ε plays a core and multiple roles in the development and function of T cells[13-14].However, it has not been reported whether CD3ε plays a role in the immune process of FK.Therefore, our experiment further analyzed the expression of CD3ε in FK to understand the T cell immune function in mice with FK.
MATERIALS AND METHODS
Ethical ApprovalThe treatment of mice complies with the Use of Animals in Ophthalmic and Vision Research published by the Association for Research in Vision and Ophthalmology(ARVO) and the regulations of the Chinese Ministry of Science and Technology Guidelines on the Humane Treatment of Laboratory Animals (vGKFCZ-2006-398).
Experimental AnimalsFemale 8-week-old C57BL/6 mice were purchased from the Changzhou Cavens Laboratory(Jiangsu Province, China).
Fungal SpeciesAspergillus fumigatus(A.fumigatus) strain 3.0772 purchased from the China General Microbiological Culture Collection Center (Beijing, China) was cultured on sabouraud agar for 2-4d, and conidia were scraped with bacterial L-ring into phosphate buffered saline (PBS).It was then filtered with sterile cotton gauze to obtain a pure conidial suspension, and the concentration was adjusted to 5×107cfu/mL to be reserved.
Murine Models of Fungal Corneal InflammationMice were anesthetized by intraperitoneal injection of 8% chloral hydrate.Corneal intra-stromal injection method: a 33-gauge Hamilton syringe was used to inject 2.5 μL of conidia(5×107cfu/mL) into the corneal intra-stromal to establish mouse FK models.Corneal epithelial scratch method:completely scrape the epithelial layer in the center of the cornea with a diameter of 4 mm.Spread the fungal colony evenly on the corneal surface where the epithelium has been scraped,then cover the homemade contact lens and suture the eyelids.TreatmentNatamycin eye drops (Natacyn, Alcon Laboratories) and wedelolactone (S9042, Selleck Chem)were used to treat keratitis[15].Totally 5 μL of laminarin(SCH772984, Selleck Chem) or poly I (SM528906, Sigma)were injected under the conjunctiva.Before infection, mice were intraperitoneally injected with anti mouse CD3ε (1 mg/kg;A2104, Selleck Chem).The cornea of mice was observed daily under a slit lamp and photographed.According to the scoring criteria proposed by Wuet al[16], ocular disease was scored using clinical scores.The corneas of the mice collected were subjected to Western blot tests.
Western BlotThe corneas were collected and placed in RIPA buffer (Solarbio), PMSF (Solarbio), phosphatase inhibitor cocktail I (MedChemExpress) at a ratio of 98∶1∶1 and lysed by ultrasound.A BCA kit (Solarbio) was used to determine the protein concentration.Then the amount of the sample required was calculated and added to the MeilunGel protein precast gels(8%-16%, 10 wells, HEPES-Tris, 1.5 mmol/L; Meilunbio)to separate the protein sample.Proteins were transferred to a polyvinylidene difluoride membrane (PVDF; Solarbio).The PVDF membrane was blocked in a Western blocking buffer(Beyotime) at 37℃ for 2h.The primary antibody was then added and incubated at 4℃ overnight (<16h).After washing 3 times with PBST (PBS+0.05% Tween 20; Bio-Rad), the membrane and the corresponding secondary antibody were incubated for 1h at 37℃.Western ECL blotting substrate(Bio-Rad) was added to the PVDF membrane to develop blots.Digital images were analyzed using a Vilber Solo 4S chemiluminescence imaging system.
Primary antibodies against the following proteins were used:anti-CD3ε (Abcam), anti-IL-10 (Cell Signaling Technology)and anti-β-actin (Cell Signaling Technology).HRP-tagged secondary antibodies were purchased from Cell Signaling Technology.
Statistical AnalysisThe statistical significance of experimental data were determined by an unpaired, two-tailed Student’st-test.P<0.05 indicated statistically significant differences.All experimental results are represented as mean±standard deviation (SD).
RESULTS
CD3ε Increased Earlier in Mice Corneas ofA.fumigatusKeratitis Developed by Intra-stromal Injection than Corneal Epithelium ScratchAt present, there were two main methods for establishing models of keratitis, namely intra-stromal injection method and corneal epithelial scratch method.We established mouse models ofA.fumigatuskeratitis using two different methods to observe the expression of CD3ε at different stages of the disease.On 12h, 1, 2, and 3d after infection, the slit lamp examination revealed that the mouse cornea gradually became opaque and the ulcer area gradually increased (Figure 1A, 1C).CD3ε expression in mouse cornea with intra-stromal injection was obviously increased on the next day (Figure 1B).The difference was that in the corneal epithelial scratch group, CD3ε increased from the third day(Figure 1D).Thus, intra-stromal injection method were used for subsequent research.
CD3ε was Down-regulated in Natamycin TreatedA.fumigatusKeratitis MiceCompared with the control group, the natamycin treatment group had more transparent corneas(Figure 2A) and lower clinical scores (Figure 2B) on the third day.Western blotting showed the protein of CD3ε was downregulated after natamycin treatment (Figure 2C).
CD3ε was Down-regulated in Wedelolactone TreatedA.fumigatusKeratitis MiceOn the second day of wedelolactone treatment, there was no significant decrease in corneal condition and clinical score in the treatment group.On the contrary, on the third day after wedelolactone treatment,corneal ulcers (Figure 3A) worsened and clinical scores(Figure 3B) increased.Compared with the control group,the protein of CD3ε was significantly down-regulated after wedelolactone treatment on the second and third day(Figure 3C).
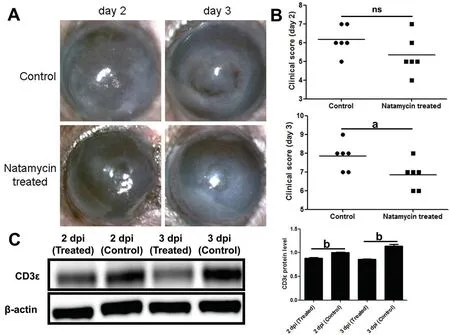
Figure 2 CD3ε was down-regulated in natamycin treated A. fumigatus keratitis mice A, B: Subconjunctival injection of natamycin was used to treat fungal keratitis in mice for 2d and 3d.The mouse corneal slit lamp pictures (A) and clinical scores (B) were compared.C: After euthanasia,corneas (six per group) were taken for Western blotting to detect the protein of CD3ε.dpi: Day post infection.aP≤0.05, bP≤0.01, ns: P>0.05.

Figure 3 CD3ε was down-regulated in wedelolactone treated A. fumigatus keratitis mice A, B: Subconjunctival injection of wedelolactone was used to treat fungal keratitis in mice for 2d and 3d.The mouse corneal slit lamp pictures (A) and clinical scores (B) were compared.C: After euthanasia,corneas (six per group) were taken for Western blotting to detect the protein of CD3ε.Dpi: Day post infection.aP≤0.05, cP≤0.001, ns: P>0.05.
CD3ε Up-regulated byA.fumigatusInfection was Independent of Dectin-1 and LOX-1Our previous research demonstrated that Dectin-1 and LOX-1 were involved in the inflammatory response ofA.fumigatuskeratitis.We wanted to know whether Dectin-1 and LOX-1 regulated the expression of CD3ε.So subconjunctival injection of laminarin and poly I inhibited the production of LOX-1 (Figure 4A) and Dectin-1(Figure 4B), but found that CD3ε did not decrease.
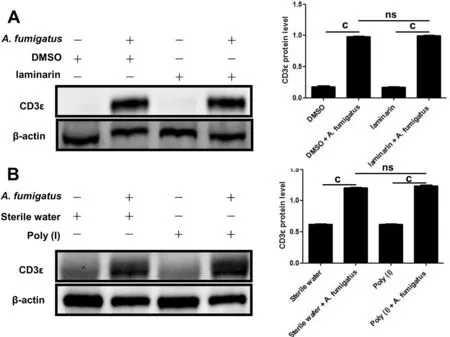
Figure 4 CD3ε up-regulation in response to A. fumigatus infection was independent of Dectin-1 and LOX-1 The LOX-1 inhibitor (poly I;A) and Dectin-1 inhibitor (laminarin; B) were pretreated with mice.Two days later, mouse corneas (six per group) were taken for Western blotting to detect CD3ε (cP≤0.001, ns: P>0.05).
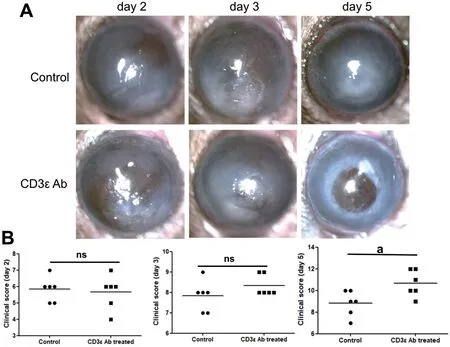
Figure 5 Disease response after CD3ε antibody treatment Intraperitoneal injection of anti-mouse CD3ε (145-2C11) was used to pretreat fungal keratitis in mice.The mouse corneal slit lamp pictures (A) and clinical scores (B) were compared on day 2, 3 and 5 (aP≤0.05, ns: P>0.05).
Disease Response after CD3ε AntibodyTo further investigate the role of CD3ε in the progression of keratitis,we injected anti-mouse CD3ε (145-2C11) intraperitoneally.Compared with the control group, there were no significant changes in the cornea and clinical scores on the first three days of the disease course, but there was a significant deterioration on the fifth day (Figure 5A, 5B).
Blockade of CD3ε Decreased Expression of IL-10 in MouseA.fumigatusKeratitisIn order to explore the mechanism of CD3ε chain in the adaptive immunity, Western blotting was performed on the corneas pretreated by intraperitoneal injection of CD3ε on the 5thday, and it was found that the expression of IL-10, an anti-inflammatory factor, was significantly downregulated (Figure 6A).
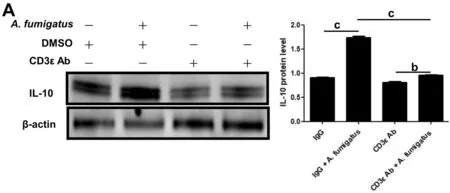
Figure 6 Blockade of CD3ε decreased expression of IL-10 in mouse A. fumigatus keratitis After euthanasia, corneas (six per group) were taken for Western blotting to detect the protein of IL-10 (bP≤0.01, cP≤0.001).
DISCUSSION
In agriculture-based developing countries, plant-induced corneal damage is the main risk factor for FK[17].Fungal infections of the eye can cause corneal edema, opacity, ulcers and even perforations, which can lead to decreased vision and even blindness[18-19].
The CD3 present on the surface of T cells is a characteristic sign of mature T cells, transducing the activation signal generated by TCR recognition antigen[20].The combination of CD3 and TCR leads to the differentiation and activation of T cells and changes in the expression of cytokines and effectors[21-22].The ε, γ, δ, and ζ chains of CD3 each contained one (ε, γ, and δ) or three (ζ) tyrosine-based immunoreceptor activation motifs (ITAM) are phosphorylated by the Src kinase Lck, which initiates the downstream T cell signaling cascade[14,23].The studies we had performed found that the expression of CD3ε chain, an important signal molecule in TCR signal, was significantly increased in FK.Elevated CD3ε chain expression late in the disease course seemed to indicate the differentiation and activation of T cells, which indicated the adaptive immune stage.Compared with the scraping epithelial method to establish models, by the intra-stromal injection, the CD3ε expression in the corneal increased relatively earlier and entered the adaptive immune stage faster.This may be due to the fact that inherent phagocytes residing in the corneal stroma layer can quickly recognize fungi injected into the stroma,thereby presenting antigens to activate T cells and trigger applicable immunity more quickly.
Natamycin is currently the first-line drug for the treatment of FK[24-25].As reported by others, natamycin binded to the ergosterol on the fungal cell membrane to change the permeability, which inhibited the growth of the fungus[26-27].Consistent with our observation, natamycin enhanced the cornea transparency with FK.Our experiment also found that the CD3ε expression was decreased after natamycin treatment.The direct bactericidal effect of natamycin only activated an appropriate amount of phagocytes, these phagocytes cleared the residue, and avoided excessive adaptive immune activation,which significantly improved FK.This explained CD3ε as a activation marker for T cell was decreased after treatment with natamycin.
Our laboratory previous studies found that wedelolactone exerted a protective effect on the first day after the remedy of FK, while cornea began to worsen significantly on the third day after consecutive treatment.It was related to the reduction of inflammatory cell infiltration during the early immune response stage, inhibiting caspase-1 and thus inhibiting IL-1β release[15].Wedelolactone had a certain anti-inflammatory effect in the innate immune stage, avoiding immune storms and leading to good corneal transparency in the early stage of treatment.This explained the decrease of CD3ε in the early stages of treatment.However, the corneal transparency was significantly reduced from the third day of the wedelolactone treatment, while CD3ε was still expressed at a low level.We suspected that this result was related to the corresponding lag of adaptive immunity after medication.That was to say, whether it was an inhibitor of caspase-11 on the non-classical pyroptosis pathway that inhibited pyroptosis[28]or exerted anti-inflammatory effects by inhibiting the activation of inflammasomes[15], wedelolactone would delay T cell activation mediated by CD3ε, thereby affecting subsequent adaptive immunity.A small amount of T cell activation was not sufficient to limit fungal proliferation and tissue repair, thereby exacerbating the symptoms of keratitis and gradually leading to perforation.Previous studies have also shown that the combination of wedelolactone and natamycin treatment significantly reduced the severity of the entire course ofA.fumigatuskeratitis[15].Therefore, maintaining the balance between innate and adaptive immunity is the key to curing FK.In recent years, it has been the focus of our laboratory to determine the signals and effectors of the innate immune response in the mouse FK model.However, the impact of innate and adaptive immunity on FK is still of great concern.Much information about the role of their links in the pathogenesis remains to be elucidated.
Dectin-1 and LOX-1, both belonging to pattern recognition receptors and expressed in multiple types of cells in keratitis,including corneal epithelial cells, macrophages, neutrophils,etc., mediate the activation of innate immunity to eliminate fungi[29-30].Previous studies in our laboratory have found that the corneal epithelium expressed and up-regulated LOX-1 afterA.fumigatusinfection, and inhibiting LOX-1 reduced the infiltration of polymorphonuclear neutrophils (PMN)and monocytes, as well as IL-1β activation[31-33].Dectin-1 which could recognize the β-glucan in fungal cell walls, was necessary for immunity against fungi, triggering phagocyte activation and activating cytokines including IL-1β, IL-6,CXCL 1, CCL 2 and others[34-36].So far, it was known that the connection of fungal components with Dectin-1 triggered Th1 and Th17-mediated immune responses against fungi[6,36-37].Dectin-1 deficiency leaded to impaired T cell-mediated immune function and loss of control of fungal infections[38].
However, our experiments found that LOX-1 and Dectin-1 did not participate in the regulation of CD3ε expression inA.fumigatuskeratitis.This may be due to the following reasons:In FK, the transmission of immune signals mediated by LOX-1 and Dectin-1 may be conducted by CD3γ, δ and ζ instead of CD3ε,which further activates the adaptive immunity of downstream T cells.These also need our follow-up experiments to verify.
IL-10 is a major anti-inflammatory cytokine produced by different types of immune cells, including neutrophils,macrophages, dendritic cell, B cells, and a variety of T cell subset[39].Due to the importance of IL-10 in the immune response and its deficiency association with certain autoimmune,inflammatory diseases, and cancer[40-44], understanding the molecular and cellular mechanisms regulating this cytokine in FK is crucial.In this study, CD3ε was associated with the regulation of IL-10 in FK.Preprocessing suppressed CD3ε expression, thus inhibiting the activation of some T cells in the early stages.Due to the fact that the body primarily activated innate immunity in the early stage, the keratitis did not seem to have significantly worsened.However, as the course of keratitis entered its later stages, adaptive immunity began to play a greater role.T cells that mainly secrete IL-10 were repressed due to the suppression of CD3ε.The expression of IL-10 was reduced, unable to exert anti-inflammatory and tissue repair functions, leading to worsening of corneal ulcers in the later stage, swelling of the posterior elastic layer, and even corneal perforation.However, which types of immune T cells, activated by CD3ε, regulating IL-10 secretion still require our subsequent experiments to verify.In summary, our study revealed that CD3ε, as an important marker of T cell activation, playing an anti-inflammatory and repair role by regulating IL-10 secretion, was involved in the adaptive immune stage ofA.fumigatuskeratitis in mice.However, LOX-1 and Dectin-1 did not participate in regulation of CD3ε expression inA.fumigatuskeratitis.Maintaining the balance between innate immunity and adaptive immunity was the key to treating FK.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.82171019); the Natural Science Foundation of Shandong Province (No.ZR2021MH368);Traditional Chinese Medicine Research Project of Qingdao(No.2020-zyy055); Shandong Qingdao Outstanding Health Professional Development Fund.
Conflicts of Interest: Shi WH,None;Wang LM,None;Yan HJ,None;Liu SL,None;Yang X,None;Yang XJ,None;Che CY,None.
 International Journal of Ophthalmology2024年4期
International Journal of Ophthalmology2024年4期
- International Journal of Ophthalmology的其它文章
- Algorithm of automatic identification of diabetic retinopathy foci based on ultra-widefield scanning laser ophthalmoscopy
- Neuroprotective effects of acteoside in a glaucoma mouse model by targeting Serta domain-containing protein 4
- Neuroprotective and anti-inflammatory effects of eicosane on glutamate and NMDA-induced retinal ganglion cell injury
- Bone morphogenetic protein-6 suppresses TGF-β2-induced epithelial-mesenchymal transition in retinal pigment epithelium
- Dry eye rate and its relationship with disease stage in patients with primary hypertension: a cross-sectional study in Vietnam
- Assessment of tear film parameters post-treatment with commercial eyelid cleaning wipes: a pilot study
