Neuroprotective and anti-inflammatory effects of eicosane on glutamate and NMDA-induced retinal ganglion cell injury
Zhen-Kai Wu, Huan-Yu Li, You-Lin Zhu, Meng-Qin Xiong, Jing-Xiang Zhong
1Department of Ophthalmology, the First Affiliated Hospital of Jinan University, Guangzhou 510632, Guangdong Province,China
2Changde Hospital, Xiangya School of Medicine, Central South University (the First People’s Hospital of Changde City), Changde 415000, Hunan Province, China
Abstract
· KEYWORDS: glaucoma; metabolites; eicosane
INTRODUCTION
Glaucoma, a disease characterized by the progressive loss of retinal ganglion cells (RGCs) and subsequent visual impairment, is a major cause of blindness worldwide.The pathogenesis of glaucoma is thought to involve oxidative stress and inflammation, which contribute significantly to RGC death.Plant extracts may protect against oxidative stress and inflammation in glaucoma, with many plant extracts demonstrating neuroprotective effects in animal models of glaucoma and other neurodegenerative diseases.These neuroprotective effects are thought to involve several mechanisms of action, including the reduction of oxidative stress, the inhibition of inflammation, and the promotion of cell survival[1-4].
The potential neuroprotective effects of specific active ingredients derived from plant extracts have been analyzed.For example, the neuroprotective properties of grape seed extract have been tested in various neurological conditions, including Alzheimer’s disease and stroke.The active components of grape seed extract, particularly flavonoids, have exhibited antioxidant and anti-inflammatory properties[5-6].Despite these advances, comprehensive analysis of all plant extract-derived ingredients with proven neuroprotective properties and their associated mechanisms remains challenging.The complexity of these interactions underscores the research required to determine the full spectrum of neuroprotective properties inherent in plant extracts.
A previous study showed that ethanol extracts ofEchium amoenumL (E.amoenumL.) have robust neuroprotective efficacy in models of glutamate-induced and optic nerve crush(ONC)-induced cell death.These effects were thought to be due to the inherent antioxidant and anti-inflammatory properties of these plant extracts.A comprehensive analysis of an ethanol extract ofE.amoenumL, using gas chromatographymass spectrometry (GC-MS), showed the presence of various compounds, including palmitic acid, hexadecanoic acid,octadecadienoic acid, docosane, campesterol, tetracosane,linolenic acid, eicosane, docosane, and nonacosane[7].However, the specific compound(s) responsible for mediating the neuroprotective effect of this extract remain unclear.Identifying these neuroprotective compounds is necessary for a comprehensive understanding of the therapeutic potential inherent in the ethanol extract ofE.amoenumL.
N-methyl-D-aspartate (NMDA) receptors are expressed on RGCs and play a pivotal role in the physiological function of these cells.Under pathological conditions such as glaucoma,however, these receptors may undergo overactivation,precipitating excitotoxicity and consequent damage to RGCs.The current study was designed to evaluate the potential neuroprotective effects of individual components derived fromE.amoenumL.extract.The underlying mechanisms of action of these compounds were assessed using anin vitromodel of glutamate-induced cell damage and anin vivomodel of NMDA-induced RGC injury in mouse glaucoma.Eicosane was found to significantly attenuate glutamateinduced damage to R28 cellsin vitroand to protect RGCs against NMDA-induced injury in a mouse glaucoma modelin vivo.The neuroprotective effects of eicosane were likely due to its inherent antioxidant and anti-inflammatory properties.These findings may provide clues to a potential treatment for mitigating glutamate-induced cellular damage and RGC injury in patients with glaucoma.
Alterations in the comprehensive metabolite profile of an organism under specific conditions can be assessed by metabolomics using liquid chromatography mass spectrometry (LC-MS) technology.This analytical method can facilitate investigation of overall biological function and changes in endogenous substances, including within the retinal microenvironment.Despite the pivotal role of RGCs in glaucoma pathology, few studies have explored the metabolic regulatory mechanisms within the retinal microenvironment in mouse glaucoma models.Analyzing the effects of neuroprotective agents on metabolic regulatory pathways in RGCs may provide insight into potential targets for RGC neuroprotection.The present study therefore included untargeted metabolomics analyses to investigate eicosane-associated changes in the metabolome of the retina in mice with NMDA-induced RGC damage.Eicosane treatment was found to induce increases in several metabolites, notably L-arginine and L-carnitine.Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis demonstrated that eicosane treatment modulated various metabolic pathways, including autophagy, the mTOR pathway, and the L-arginine metabolic pathway.These findings substantiate the protective effects of eicosane on RGC damage in glaucoma, effects likely due to the antioxidative stress properties of eicosane, its regulation of L-arginine and L-carnitine metabolism, and its modulation of autophagy and mTOR pathways.This study evaluated the potential neuroprotective properties of eicosane, a single compound derived fromE.amoenumL.extract, on RGCs in glaucoma models at the cellular and molecular levels, and provided clues to the treatment of patients with glaucoma.Taken together, these findings may contribute to elucidating the neuroprotective mechanism of eicosane, thereby establishing a foundation for subsequent clinical intervention.
MATERIALS AND METHODS
Ethical ApprovalAll animal experiments were conducted in accordance with the Animal Ethics Guidelines of Xiangya Hospital, Central South University (permit number: 202108022).AnimalsMice fed a normal diet were orally administered 20 mg/kg eicosane once daily for 10d.Subsequently, 1 μL 50 mmol/L NMDA was injected into the vitreous cavity of both eyes using a 33-gauge needle.Control mice were fed a normal diet and were intravitreally injected with the same volume of phosphate buffered saline (PBS).
Cell Viability, Cell Death Assays and Reactive Oxygen Species ProductionThe individual constituents ofE.amoenumL.extract, including palmitic acid, hexadecanoic acid, ocadecadienoic acid, docosane, campesterol, tetracosane,linolenic acid, docosane, eicosane and nonacosane, were obtained from Targetmol (Boston, MA, USA).R28 cells were treated with 10 μmol/L of each compound and 10 mmol/L glutamate for 12h, and cell viability, cell death and reactive oxygen species (ROS) production were assayed as described previously[7].Briefly, cell viability was determined using Cell Counting Kit-8 (CCK-8; Servicebio, China) assays,and cell death was determined using Apoptosis and Necrosis Assay Kits (Beyotime, Shanghai, China), according to the manufacturers’ instructions.ROS production was determined using the fluorescent probe 2’-7’-dichlorofluorescin diacetate(Sigma-Aldrich, St.Louis, MO, USA).All experiments were repeated three times.
Immunostaining of Frozen SectionsMouse eyes were dissected; fixed in 4% paraformaldehyde at room temperature for 2h; dehydrated in 10%, 20%, and 30% sucrose; and embedded in optimal cutting temperature compound (O.C.T.)mounting medium.Frozen sections were cut using a cryostat microtome apparatus and immunostained with anti-Tuj1(1:500, Millipore, MAB1637), anti-RBPMS (1:500, Abcam,ab152101) and rabbit anti-Iba1 (1:300, Abcam, ab178847)antibodies.The nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI).Slides were examined using an Axio Imager.M2 (Carl Zeiss, Germany).Cells positive for Iba1 and RBPMS were quantitatively analyzed using Image J software.Quantitative Real-Time Polymerase Chain ReactionTotal RNA was extracted from mouse retinas using TRIzol Reagent.Following reverse transcription, reverse transcription polymerase chain reaction (RT-PCR) was performed using the following primers, nanufactured by Sangon Biotech (Shanghai, China): tumor mecrosis factor(TNF)-α (forward, 5’-ACGGCATGGATCTCAAAGAC-3’,and reverse, 5’-AGATAGCAAATCGGCTGACG-3’); interleukin(IL)-1β (forward, 5’-GCAACGGGAAGATTCTGAAG-3’,and reverse, 5’-TGACAAACTTCTGCCTGACG-3’);inducible nitric oxide synthase (iNOS) (forward,5’-ACGAGACGGATAGGCAGAGA-3’, and reverse,5’-CACATGCAAGGAAGGGAACT-3’); IL-4 forward,5’-TCAACCCCCAGCTAGTTGTC-3’, and reverse,5’-TGTTCTTCGTTGCTGTGAGG-3’).β-actin (forward,5’-CACGATGGAGGGGCCGGACTCATC-3’, and reverse,5’-TAAAGACCTCTATGCCAACACAGT-3’).
Liquid Chromatography-Mass Spectroscopy and Untargeted Metabolomics AnalysesTwo days after NMDAinduced injury, retinas were collected from the eicosane and control mouse groups (n=6 each).The samples were homogenized in methanol by ultrasonication for 2min, placed in an ice-water bath for 10min, and centrifuged at 12000×g for 10min at 4°C.The supernatants were collected, and metabolites were analyzed using a Dionex Ultimate 3000 RS UHPLC fitted with a Q-Exactive plus quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific) in both ESI-positive and ESI-negative ion modes.Compounds were identified based on their precise mass-to-charge ratios (M/z) and isotopic distribution using The Human Metabolome (HMDB, http://hmdb.ca)and EMDB (http://ebi.ac.uk/emdb/) databases.Principle component analysis (PCA) was performed using a data matrix containing both the positive and negative ion data.Significant differences in metabolite concentrations between the two groups were determined using two-tailed Student’st-tests.
Statistical AnalysisData are presented as the mean percentage of control ±standard error of mean (SEM) and compared by one-way analysis of variance with Tukey’s multiple comparison tests.All statistical analyses were performed using GraphPad Prism 8, with statistical significance set atP<0.05.
RESULTS
Protective Effects of Eicosane on Glutamate-Induced R28 Cell DeathThe protective effects of individual components inE.amoenumL.ethanol extract on retinal neurons were assessed in R28 cells with glutamate-induced damage.The R28 retinal cell line, an adherent retinal precursor cell line derived from rat retina, is widely used forin vitrostudies of retinal diseases.CCK-8 assays showed that tricosane and eicosane significantly augmented cell viability, with eicosane exhibiting the most pronounced protective effect (Figure 1A).Subsequent cell death assays showed that 10 μmol/L eicosane notably attenuated glutamate-induced damage to R28 cells(Figure 1B, 1C), as well as significantly reducing glutamateinduced ROS production (Figure 1D).These findings collectively indicated that eicosane can protect retinal cells against glutamate-induced death, thereby providing empirical support for its neuroprotective efficacy.
Neuroprotective and Anti-inflammatory Effects of Eicosane on NMDA-induced RGC Injury in a Mouse Glaucoma ModelTo determine whether eicosane has a protective effect on NMDA-induced RGC injury in mice, mice were orally administered 20 mg/kg eicosane once daily for 10d, followed by intravitreal injection of NMDA.Immunofluorescence staining of retinal frozen sections showed that NMDA injection significantly reduced the number of RBPMS positive cells in the RGC layer, and that eicosane treatment effectively rescued NMDA-induced RGC loss (Figure 2A, 2B).TUNEL staining showed that eicosane treatment markedly attenuated NMDA-induced RGC cell death (Figure 2C, 2D).Microglial activation has been reported to damage RGCs in the retina,and inhibiting this activation may have a protective effect on RGCs in glaucoma.Iba1 immunofluorescence staining to detect microglia showed that the number of activated microglia was lower in the eicosane group than in the control group(Figure 3A, 3B).Moreover, the glutamate-induced elevation of mRNAs encoding proinflammatory cytokines such as TNF-α,iNOS and IL-1β was significantly decreased by eicosane treatment, whereas the glutamate-induced reduction of mRNA encoding the anti-inflammatory cytokine IL-4 was significantly increased upon eicosane treatment (Figure 3C).Taken together,these findings demonstrate that eicosane has neuroprotective and anti-inflammatory effects in thisin vivomodel of NMDAinduced glaucoma.

Figure 1 Protective effects of eicosane on glutamate-induced R28 cell death A: CCK-8 assays showing the effects of different components of E. amoenum ethanol extract on the viability of R28 cells treated with 10 mmol/L glutamate for 24h.B: Effects of 10 μmol/L eicosane on the death of R28 cells treated with 10 mmol/L glutamate for 24h.C: Quantification of the percentage of dead cells.D: ROS production in glutamatetreated R28 cells, as determined by fluorescence-activated cell sorting.Scale bar=20 μm.Data are mean±SEM; aP<0.05, bP<0.01, cP<0.001.ROS:Reactive oxygen species; PI: Propidium iodide; SEM: Standard error of mean.

Figure 2 Neuroprotective effects of eicosane on NMDA-induced RGC injury in a mouse glaucoma model A: Retinas from different groups were harvested and frozen sections were immunostained with antibodies to RBPMS and Tuj1.B: Quantification of the number of RGCs in retinal sections.C: TUNEL staining of retinas from different groups.D: Quantification of the number of death cells in retinal sections.Scale bar=50 μm.Data are mean±SEM (n=6); aP<0.05, cP<0.001.RBPMS: RNA-binding protein with multiple splicing; Tuj1: Class III β-tubulin; TUNEL: (TdT) dUTP nickend labeling.NMDA: N-methyl-D-aspartate; RGC: Retinal ganglion cell; SEM: Standard error of mean.

Figure 3 Anti-inflammatory effects of eicosane on NMDA-induced RGC injury in a mouse glaucoma model A: Retinas from different groups were harvested and immunostained with antibody to Iba1.B: Quantification of the number of microglia in retinal sections.Data are shown as the mean±SEM (n=6).C: Relative levels of TNF-α, iNOS and IL-1β mRNAs, as determined by real-time PCR analysis.Scale bar=50 μm.Data are shown as the mean±SEM (n=3); aP<0.05, bP<0.01, cP<0.001 versus the control group.RGCs: Retinal ganglion cells; Iba1: Lonized calcium binding adaptor molecule 1; iNOS: Inducible nitric oxide synthase; NMDA: N-methyl-D-aspartate; SEM: Standard error of mean.
Metabolomics Analyses of Metabolites and Pathways Involved in Responses to Eicosane TreatmentThe effects of eicosane on metabolite changes in mouse retinas with NMDA-induced RGC damage were assessed by metabolomics analyses.Heat map clustering of the differentially expressed metabolites showed a distinctive metabolites expression pattern in NMDA after eicosane treatment when compared to NMDA treated group.Eicosane treatment was found to increase the levels of multiple metabolites in the retina,including L-arginine and L-carnitine (Figure 4A), with the most changed metabolites shown in Figure 4B.The degree of linear correlation between pairs of metabolites was assessed by correlation analysis, with red indicating positive and blue indicating negative correlation.KEGG pathway analysis showed that pathways enriched by eicosane treatment included those associated with the process of autophagy, the mTOR pathway and the L-arginine metabolic pathway (Figure 4C).Highly positive correlations were observed between propionylcarnitine and L-carnitine, and between L-arginine and acetyl-L-carnitine (Figure 4D).These results indicated that these pathways may be putative downstream effectors induced by NMDA after eicosane treatment.
Neuroprotective Effects of Eicosane may be due to its Modulation of Metabolites ProductionAnalysis of individual metabolites showed that eicosane treatment of mice with NMDA-induced RGC injury increased L-arginine, acetyl-L-carnitine, butanoyl-carnitine and L-carnitine contents, but reduced docosahexaenoic acid (DHA) content (Figure 5A).To determine whether eicosane-induced L-arginine and L-carnitine have protective effects on retinal neurons, L-arginine and L-carnitine were added individually to R28 cells with glutamate-induced damage.CCK-8 results showed that both of these metabolities significantly increased cell viability (Figure 5B), and L-arginine and L-carnitine treatment also reduced glutamate-induced cell death (Figure 5C).Overall, these results suggest that eicosane may exert its neuroprotective effects by increasing the contents of these metabolites.
DISCUSSION
Eicosane is a naturally occurring hydrocarbon compound found in several plants, includingDrosera indicaL.andBarringtonia asiaticaL[8-9].Eicosane exhibits notable antiinflammatory and antimicrobial properties[10].For example,in a diabetic rat wound model, the administration of eicosane and octadecane accelerated wound healing.Both compounds were found to possess robust free radical scavenging activity and interacted with matrix metalloproteinases (MMPs)[11].Eicosane functions as a ligand, binding to the taste receptor BminGR59b ofBactrocera minax, a significant citrus pest.This interaction mediates precise host plant selection for oviposition through the insect’s taste system[12].A study utilizing thermal desorption-gas chromatography-mass spectrometry to analyze sebum volatiles collected from Parkinson’s patients and healthy individuals identified a cluster of volatile metabolites associated with Parkinson’s disease,including perillic aldehyde, hippuric acid, eicosane, and octadecanal[13].Interestingly, eicosane levels were significantly higher, while perillic aldehyde levels were lower, in the sebum of Parkinson’s patients than in healthy individuals[13].Based on these findings, the current study assessed the ability of eicosane to protect RGCs from glutamate-induced injury, bothin vitroand in anin vivoglaucoma model.
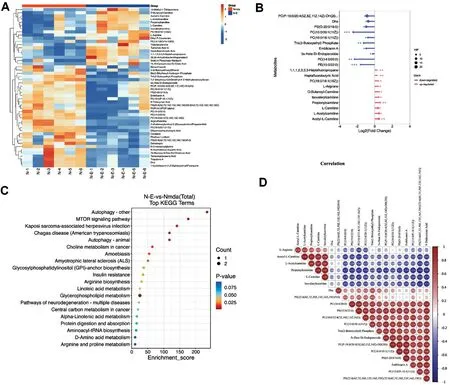
Figure 4 Metabolomics analyses of metabolites and pathways in response to eicosane treatment A: Heat map clustering of the NMDA group and the NMDA+eicosane group.Yellow blocks represent upregulated metabolites and blue blocks represent downregulated metabolites.B:Lollipop map of the most changed metabolites.C: KEGG analysis of the differentially expressed metabolites.D: Degree of linear correlation between pairs of metabolites, as determined by Pearson correlation analysis.Red indicates a positive correlation, and blue indicates a negative correlation.KEGG: Kyoto Encyclopedia of Genes and Genomes; NMDA: N-methyl-D-aspartate.
The precise mechanisms underlying glaucoma and the protective effects of plant extracts in the context of glaucoma remain incompletely understood[14].Green tea and its concentrated extracts might reduce intraocular pressure (IOP)in people who have risk factors for glaucoma[15].Current evidence suggests, however, that eicosane mitigates oxidative stress, suppresses inflammation, and enhances cell survival.Arginine, a pivotal amino acid, plays a fundamental role in various metabolic pathways, including the synthesis of nitric oxide (NO), a crucial mediator of vasodilation and neurotransmission.Within the retina, arginine metabolism is required to maintain normal blood flow and regulate the function of RGCs[16].Under pathological conditions such as diabetes and glaucoma, however, arginine metabolism can become dysregulated, leading to oxidative stress, inflammation,and neuronal damage.
Carnitine, a coenzyme involved in fatty acid metabolism, has exhibited neuroprotective effects across various pathological conditions.Arginine levels have been reported higher in the plasma or aqueous humor of individuals with primary openangle glaucoma (POAG) than in controls[17-18].Similarly, the concentrations of spermidine and various acyl-carnitines and lysophosphatidylcholines[19], as well as L-carnitine and L-acetylcarnitine[20], have been reported higher in the aqueous humor of patients with than without open angle glaucoma.The present study also found that the levels of L-arginine,L-carnitine and acetyl-L-carnitine were higher in the retinas of mice with NMDA-induced RGC injury glaucoma mice, and that the levels of these metabolites were increased by eicosane treatment.Furthermore, L-arginine or L-carnitine treatment was found to significantly increase the viability of R28 cells with glutamate-induced damage.Taken together, these findings suggest that the neuroprotective activity of eicosane may be due to its mediation of L-arginine or L-carnitine metabolism.Additional studies, however, are needed to better understand the roles of arginine and carnitine metabolism in retinal diseases and to develop potential therapeutic strategies to modulate these metabolic pathways in the treatment of glaucoma.
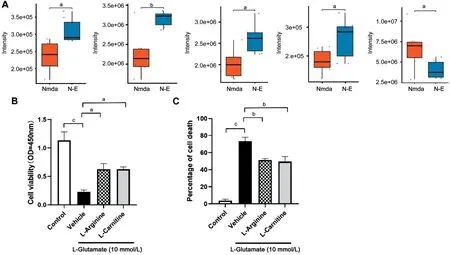
Figure 5 Eicosane-associated neuroprotection may be due to its modulation of metabolite production A: Effects of eicosane on L-arginine,acetyl-L-carnitine, butanoyl-carnitine, L-carnitine, and docosahexaenoic acid (DHA) contents on NMDA-induced RGC injury in a mouse model of glaucoma; B: Effects of L-arginine and L-carnitine on the viability of R28 cells treated with 10 mmol/L glutamate for 24h, as determined by CCK-8 assays; C: Effects of L-arginine and L-carnitine on cell death of R28 cells treated with 10 mmol/L glutamate for 24h, as determined by PI staining.Data are mean±SEM; aP<0.05, bP<0.01, cP<0.001.PI: Propidium iodide; NMDA: N-methyl-D-aspartate; RGCs: Retinal ganglion cells;SEM: Standard error of the mean.
ACKNOWLEDGEMENTS
Foundation:Supported by the Science and Technology Innovation Program of Hunan Province (No.2018SK50208).
Conflicts of Interest:Wu ZK,None;Li HY,None;Zhu YL,None;Xiong MQ,None;Zhong JX,None.
 International Journal of Ophthalmology2024年4期
International Journal of Ophthalmology2024年4期
- International Journal of Ophthalmology的其它文章
- Algorithm of automatic identification of diabetic retinopathy foci based on ultra-widefield scanning laser ophthalmoscopy
- CD3ε of a pan T cell marker involved in mouse Aspergillus fumigatus keratitis
- Neuroprotective effects of acteoside in a glaucoma mouse model by targeting Serta domain-containing protein 4
- Bone morphogenetic protein-6 suppresses TGF-β2-induced epithelial-mesenchymal transition in retinal pigment epithelium
- Dry eye rate and its relationship with disease stage in patients with primary hypertension: a cross-sectional study in Vietnam
- Assessment of tear film parameters post-treatment with commercial eyelid cleaning wipes: a pilot study
