Bone morphogenetic protein-6 suppresses TGF-β2-induced epithelial-mesenchymal transition in retinal pigment epithelium
Xuan Liu, Ming Liu, Meng Ji, Bo Ma, Yu-Cen Hou, Xin-Yue Yao, Qiao-Chu Cheng, Li Chen
1Department of Ophthalmology, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, Shaanxi Province,China
2Department of Ophthalmology, Xi’an No.1 Hospital, Xi’an 710000, Shaanxi Province, China
3Shaanxi Institute of Ophthalmology, Xi’an 710000, Shaanxi Province, China
4Department of Ophthalmology, Ankang Hospital of Traditional Chinese Medicine, Ankang 725000, Shaanxi Province, China
Abstract
· KEYWORDS: bone morphogenetic protein-6; epithelialmesenchymal transition; transforming growth factor-β2;retinal pigment epithelial cells; cell migration
INTRODUCTION
Proliferative vitreoretinopathy (PVR) is a complex ophthalmic disease in which various cells proliferate and spread on the anterior and posterior surfaces of the retina and form fibrous cell membranes in the vitreous[1].It can further cause retinal contraction, traction, and recurrent retinal detachment.Surgical treatment could remove the membranes;however, it cannot stop cell proliferation and membrane reformation.Due to the poor prognosis of PVR, prevention and early intervention are the key strategies for PVR treatment[2].
Epithelial-mesenchymal transition (EMT) in retinal pigment epithelium (RPE) cells triggered by various growth factors and cytokines is recognized as the key mechanism of PVR pathogenesis[3-4].EMT in RPE cells results in the loss of cell-to-cell junctions, mobility and proliferation, and morphological transition to mesenchymal cells.Phenotypic changes in EMT include a decrease in epithelial markers such as zonule atresia-1 (ZO-1) and E-cadherin and an increasein mesenchymal markers such as α-smooth muscle actin(α-SMA), vimentin and fibronectin[3].

Table 1 Genetic sequences of bone morphogenetic protein-6 small interfering RNA
Transforming growth factor-β2(TGF-β2), a typical inducer of EMT, has been widely used in models of RPE cell fibrosis[5].TGF-β2was reported to be aberrantly expressed in retinal membranes from PVR patients[6].
Bone morphogenetic protein 6 (BMP-6), a member of the TGF-β superfamily, was found to be significantly reduced in patients with diabetic retinopathy.Hence, it is speculated that BMP-6 is involved in the pathogenesis of diabetic retinopathy[7].In the kidney, fibrosis decreases with age,which is related to increased BMP signalling, most likely due to higher BMP-6[8].Furthermore, overexpression of BMP-6 significantly decreased the phosphorylation level of extracellular regulated protein kinases (ERK) to inhibit proliferationin vitro[9].However, it has not been reported that the downregulation of BMP-6 could lead to fibrosis in RPE.
Our previous research showed that BMP-6 can alleviate oxidative stress injury[10], which could damage RPE cells and aggravate the fibrosis of RPE cells induced by TGF-β[11].Therefore, we further explored whether BMP-6 also plays an important role in preventing the fibrosis of RPE cells.Due to the poor prognosis of PVR, prevention and early intervention are the key strategies for PVR treatment.Our study might provide an important potential theory for future options for the prevention and treatment of PVR.
MATERIALS AND METHODS
Cell Culture and TreatmentAdult retinal pigment epithelial cell line-19 (ARPE-19) cells are a commercialized RPE cell line, mentioned below as RPE cells, purchased (American Type Culture Collection, USA) and culturedin vitroas previously described[10].ARPE-19 cells were cultured in conventional DMEM/F12 medium supplemented with 10% foetal bovine serum and penicillin and streptomycin and incubated at 37℃with saturated humidity and 5% CO2.Cells were cultured in serum-free medium for 24h before the treatments.
Knockdown with siRNA Against BMP-6RPE cells in logarithmic growth phase were used to prepare a single-cell suspension in culture medium, which was uniformly inoculated into a 6-well plate at 5×105cells/mL cells per well, and the cells were cultured to 60% density at 37℃ and 5% CO2saturation under humid conditions.After starvation for 2h with serum-free medium, RPE cells were transfected with siRNA BMP-6-homo1087, BMP-6-homo1634 and BMP-6-homo885(Table 1 for the gene sequences) or with scrambled control siRNA.Lipofectamine RNAiMAX (200 μL) was used as the transfection reagent (13778075, Thermo Fisher, USA) in one well of a six-well plate under 80% confluent cells, according to the vendor’s instructions.Protein was extracted after 48h of transfection, and BMP-6 protein expression was determined by reverse transcription-polymerase chain reaction (RT-PCR).RNA was extracted from all transfected cells and control cells by the TRIzol method and reverse transcribed into cDNA with the following reaction conditions: 50℃ for 2min, 95℃ for 10min, and 40 cycles of 95℃ for 30s and 60℃ for 30s.The final data were analysed by the 2-△△CTmethod.The primer sequences were as follows: the upstream primer for BMP-6 was 5’-CTTACGACAAGCAGCCCTTC-3’, and the downstream primer was 5’-ATCTGAAGCACTGGAGACCC-3’.The upstream primer for GAPDH was 5’-TCAAAGGTG GTGAGCGG-3’, and the downstream primer was 5’-TCAAAGGTGGAGGGGT-3’.The segment with the best inhibitory effect was selected for subsequent experiments.
BMP-6 Expression Plasmid and Overexpression of BMP-6 in RPEPIRES2-ZsGreen1 and the target fragment homo BMP-6 were digested with BamHI and Xho1, and after running the gel, the band in the gel was recovered and purified.The recovered and purified target fragment homo BMP-6(BamHI/Xho1) was ligated with the recovered and purified vector pIRES2-ZsGreen1 (BamHI/Xho1).Eventually, pIRES2-ZsGreen1-homo BMP-6 was constructed.The primers used for sequencing were universal primers.The upstream primer was complementary to the CMV region, 74 bp from the MCS region, and the downstream primer was complementary to the IRES region, 101 bp from the MCS region.The target sequence was 1542 bp in length, and the PCR product size was approximately 1700 bp.The expression level of BMP-6 mRNA was detected by RT-PCR.After the product was verified, the plasmid pIRES2-ZsGreen1-homo BMP-6 was extracted on a large scale for subsequent experiments.
Transwell Migration TestCells were suspended in serumfree medium, counted with a cell counting plate, and diluted with serum-free medium to 5×105cells/mL for later use.Then,600 μL of complete culture medium was added to the lower chamber, and 200 μL of the serum-free cell suspension was added to the upper chamber of a Transwell chamber.With different treatment interventions, the cells were cultured for 48h in an incubator containing 5% CO2at 37℃.The Transwell chamber was removed, the chamber was carefully cleaned with phosphate buffer saline (PBS), and the cells were fixed with a 70% iced ethanol solution for 1h.The cells were dyed with 5 g/L crystal violet dye solution, maintained at room temperature for 20min, and washed with PBS, after which the non-migrated cells on one side of the upper chamber were wiped away with a clean cotton ball, and the remaining cells were observed and photographed under a microscope.
Western BlottingWestern blot analysis was performed and semi-quantitatively analysed using the following antibodies:rabbit anti-BMP-6 (Affinity, AF5196, China), rabbit anti-Ecadherin (Abcam, Ab40772, USA), mouse anti-vimentin(Boster, Bm0135, USA), mouse anti-α-SMA (Boster, Bm0002,USA), rabbit anti-GAPDH (Hangzhou Xianzhi, AB-P-R001,China), rabbit anti-fibronectin (Wuhan Sanying, 15613-1-AP, China), rabbit anti-ERK (CST, 9102S, USA), rabbit antip-ERK (EST, 9101S, USA), HRP-labelled goat anti-rabbit secondary antibody (Wuhan Boshide, BA1054, China), and HRP-labelled sheep anti-mouse secondary antibody (Wuhan Boshide, BA1051, China).
Statistical AnalysisAll experiments were repeated three times, and the data and pictures were statistically analysed by GraphPad Prism 8.0 software.All measurement data are presented as mean±standard deviation.An independent samplet-test was used for comparisons between two groups, singlefactor analysis of variance was used for comparisons of three groups or more, and Tukey’s HSD test was used in the comparisons among multiple groups.P<0.05 was considered statistically significant.
RESULTS
TGF-β2 Induces EMT in RPE cells and BMP-6 ExpressionRPE cells were treated with TGF-β2or vehicle for 48h.The cell morphology showed a typical spindle-like shape in the TGF-β2treatment group (Figure 1A, 1B).The number of cells in the TGF-β2group that migrated through the Transwell was significantly higher than that in the control group (122±5.57vs63.67±4.04,P<0.05; Figure 1C, 1D, 1G).
Under TGF-β2treatment, the α-SMA, fibronectin, and vimentin were increased in protein expression, while the E-cadherin was significantly reduced (P<0.05; Figure 1E, 1F).BMP-6 was significantly reduced in the TGF-β2group compared with the control group (0.41±0.13vs0.70±0.08,P<0.05; Figure 1E, 1F).
BMP-6 mRNA after BMP-6 siRNA Transfection in RPE CellsRT-PCR was used to detect the expression of BMP-6 mRNA in RPE cells transfected with different small fragments of BMP-6 siRNA.The expression level of BMP-6 mRNA in the RPE cells in the control group was 1.00.After the transfection of BMP-6-homo1087, BMP-6-homo1634 and BMP-6-homo885, the relative expression level of BMP-6 mRNA in RPE cells decreased significantly to 0.29±0.03, 0.73±0.13 and 0.59±0.06, respectively (allP<0.05).Transfection of BMP-6-homo1087 had the greatest inhibitory effect on BMP-6 mRNA expression, so BMP-6-homo1087 was chosen for follow-up experiments.
Knockdown of BMP-6 Promoted EMTWith the transfection of BMP-6 siRNA, knockdown of BMP-6 in RPE cells was efficiently displayed at lower protein levels.With the transfection of BMP-6 siRNA, the morphology of RPE cells changed from an oval shape to a spindle-like shape, similar to typical mesenchymal cells (Figure 2A, 2B).The cell migration assay showed that the number of cells migrating through the transparent wells in the BMP-6 siRNA group was significantly greater than that in the control group (115.67±1.53vs57±7,P=0.0001; Figure 2C, 2D, 2G).Morphology change and promoted cell migration both indicated EMT occurring in RPE cells under the silencing of BMP-6.The α-SMA,fibronectin, and vimentin were significantly increased under the transfection of BMP-6 siRNA compared to the control treatment.In contrast, E-cadherin, an epithelial marker, was significantly reduced, as expected in the EMT process (P<0.05;Figure 2E, 2F).
BMP-6 mRNA Levels After BMP-6 OverexpressionRT-PCR was used to detect the expression of BMP-6 mRNA in RPE cells transfected with BMP-6 overexpression.The expression level of BMP-6 mRNA in the RPE cells in the control group was 1.00.After the transfection of empty plasmid or BMP-6 overexpression, the relative expression level of BMP-6 mRNA in empty plamid was 1.140±0.034 (P>0.05vscontrol group),the relative expression level of BMP-6 mRNA in BMP-6 overexpression increased significantly to 2.599±0.06 (P<0.05vscontrol group).
BMP-6 Overexpression Impeded TGF-β2-induced EMTRPE cells were transfected with BMP-6 overexpression plasmid or empty plasmid as a control group, followed by treatment with TGF-β2for 48h or corresponding vehicle.In the empty plasmid group treated with TGF-β2, the cell morphology changed into a typical spindle-like shape (Figure 3A, 3B).The overexpression of BMP-6+impeded the morphological changes induced by treatment with TGF-β2(Figure 3C, 3D).Meanwhile, TGF-β2promoted significantly less cell infiltration in the BMP-6 overexpression group than in the control group(P=0.076; Figure 3E-3I).
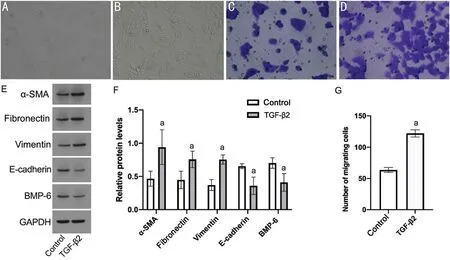
Figure 1 The effect of TGF-β2 on RPE cell morphology, cell migration, EMT-related biomarkers and BMP-6 A: Morphology of RPE cells in the control group under a microscope (×100); B: Morphology of RPE cells in the TGF-β2 group under a microscope (×100); C: Cell migration in the control group, as determined by the Transwell method (×200); D: Cell migration in the TGF-β2 group, as determined by the Transwell method(×200); E: Western blotting was used to detect the effect of TGF-β2 on related proteins; F: Analysis of the influence of TGF-β2 on related proteins by Western blotting; G: Analysis of the cell migration number after TGF-β2 treatment.aP<0.05 vs control.TGF-β2: Transforming growth factor β2;RPE: Retinal pigment epithelium; EMT: Epithelial-mesenchymal transition; α-SMA: α-smooth muscle actin; BMP-6: Bone morphogenetic protein 6.

Figure 2 Effect of BMP-6 siRNA on RPE cell morphology, cell migration, BMP-6, and EMT-related biomarkers A: Morphology of RPE cells in the control group under a microscope (×100); B: Morphology of RPE cells in the BMP-6 siRNA group under a microscope (×100); C: Cell migration in the control group, as determined by the Transwell method (×200); D: Cell migration in the BMP-6 siRNA group, as determined by the Transwell method (×200); E: Western blotting was used to detect the effect of BMP-6 siRNA on related proteins; F: Analysis of the effect of BMP-6 siRNA on related proteins by Western blotting; G: Analysis of the cell migration number after BMP-6 siRNA treatment.aP<0.05 vs control.BMP-6: Bone morphogenetic protein-6; siRNA: Small interfering RNA; RPE: Retinal pigment epithelium; EMT: Epithelial-mesenchymal transition;α-SMA: α-smooth muscle actin.
Overexpression of BMP-6 Inhibited the Effects of TGF-β2 on EMT and the ERK Signalling Pathway in RPE CellsWe further determined the abrogation of the effects of TGF-β2on RPE cell fibrosis by overexpression of BMP-6 and its potential underlying signalling pathway.The overexpression of BMP-6 repressed the effects of TGF-β2on EMT markers.Treatment of RPE cells with BMP-6+overexpression with TGF-β2resulted in significantly lower protein expression of α-SMA, fibronectin, and vimentin and significantly higher protein expression of E-cadherin than treatment with TGF-β2without BMP-6 overexpression (P<0.05; Figure 4).Our data showed that BMP-6+overexpression can impede TGF-β2-induced EMT in REP cells.
The ratio of p-ERK/ERK with TGF-β2treatment was significantly higher than that of the control group (P<0.05).With the overexpression of BMP-6, the ratio of p-ERK/ERK was significantly lower than that in the group without BMP-6 overexpression after TGF-β2treatment.Therefore,ERK is an important signalling pathway through which BMP-6 overexpression can prevent TGF-β2-induced EMT by activating the ERK signalling pathway (Figure 4).

Figure 3 Overexpression of BMP-6 reversed the effects of TGF-β2 on the morphology and migration of RPE cells A: Morphology of RPE cells in the control group under a microscope (×100); B: Morphology of RPE cells in the BMP-6+ group under a microscope (×100); C: Morphology of RPE cells in the TGF-β2 group under a microscope (×100); D: Morphology of RPE cells in the TGF-β2+BMP-6+ group under a microscope (×100); E:Cell migration in the control group, as determined by the Transwell method (×200); F: Cell migration in the TGF-β2 group, as determined by the Transwell method (×200); G: Cell migration in the BMP-6+ group, as determined by the Transwell method (×200); H: Cell migration in the TGFβ2+BMP-6+ group, as determined by the Transwell method (×200); I: Analysis of the cell migration results.aP<0.05 vs control group, cP<0.05 vs empty plamid+TGF-β2 group.TGF-β2: Transforming growth factor β2; RPE: Retinal pigment epithelium; BMP-6+: Bone morphogenetic protein 6 overexpression.

Figure 4 Overexpression of BMP-6 antagonized the effects of TGF-β2 on EMT and the ERK signalling pathway A: Western blot showing related bands; B-G: Analysis of EMT-associated biomarkers and ERK.aP<0.05 vs control group; cP<0.05 vs empty plamid+TGF-β2 group.TGF-β2:Transforming growth factor β2; RPE: Retinal pigment epithelium; EMT: Epithelial-mesenchymal transition; α-SMA: α-smooth muscle actin; ERK:Extracellular regulated protein kinases; BMP-6+: Bone morphogenetic protein 6 overexpression.
DISCUSSION
This study shows for the first time that BMP-6 repress EMT of RPE cell by blocking the ERK signalling pathway.The overexpression of BMP-6 inhibited the migration of RPE cells and mesenchymal morphology changes induced by TGF-β2.In contrast, knockdown of BMP-6 could upregulate EMT,showing morphological changes, promoted cell migration and alteration of EMT markers.
EMT is not only the pathogenic mechanism of PVR but also an important factor that alters the therapeutic effect of intraocular surgery.Therefore, the repression of EMT could be a potential therapy to impede the progression of PVR.TGF-β plays a role in inducing the phenotypic transformation of RPE cells into myofibroblasts, which is related to fibrosis complications such as PVR[12].TGF-β has been used to induce EMT in various experimentsin vitro, such as in kidney, breast, lung, and RPE cells[13].Our study applied TGF-β-induced EMT to evaluate the relevant roles of BMP, which is a useful modelin vitro.We observed that RPE cells displayed a morphological change to mesenchymal formation, upregulated cell migration, elevated mesenchymal markers and decreased epithelial markers by TGF-β2treatment.
The BMP signalling pathway is not only involved in the growth and development of normal tissues but is also related to the development of various tumour types.Nevertheless,the BMP signalling pathway also plays an important role in the proliferation and fibrosis of tumours and the kidney[14].However, the role of the BMP signalling pathway in the pathogenesis of PVR remains unclear.Our previous research showed that BMP-6 could protect RPE cells from apoptosis induced by oxidative stress through the MAPK and SMAD signalling pathwaysin vitro[15].
Many previous studies have shown that BMP can inhibit cellular EMT induced by TGF.Songet al[16]recently found that overexpression of BMP-7 could reverse EMT caused by TGF-β through the Wnt3/β-catenin signalling pathway.The BMP-6 protein is part of the superfamily of TGF-β ligands, participates in iron homeostasis, inhibits invasion by increasing adhesions and cell-cell type interactions and induces angiogenesis directly on vascular endothelial cells[17].Yaoet al[18]found that BMP-7 with primary human RPE cells could inhibit the EMT process induced by TGF-β.Another study reported that the BMP inhibitor gremlin could promote RPE proliferation and neovascularization[19].Reports also confirmed that the BMP-4 signalling pathway plays a very important role in the differentiation of RPE cells.The role of BMP in maintaining the physiological function of RPE cells was confirmed by the fact that inhibition of the BMP signalling pathway affected the late differentiation of RPE cells[20].However, BMP-6 has never been reported to impact EMT in RPE cells.
Our study revealed a significant decrease in BMP-6 in RPE cells that underwent TGF-β-induced EMT.We further determined the role of BMP-6 in the development of EMT in RPE cells.The knockdown of BMP-6viasiRNA transfection demonstrated changes in cell morphology, cell migration and the expression of EMT markers, including α-SMA,fibronectin, vimentin, and E-cadherin.Our results indicated that the knockout of endogenous BMP-6 with specific siRNAs increased the expression of the mesenchymal markers α-SMA and fibronectin and decreased the expression of the epithelial marker E-cadherin.We confirmed that the knockdown of BMP-6 could upregulate EMT.
We further investigated whether BMP-6 overexpression could reverse TGF-β-induced EMT.The reversed mesenchymal morphology, downregulated cell migration ability and increased epithelial marker expression were detected, which showed that BMP-6 overexpression could reverse TGF-βinduced EMT.Notably, the overexpression of BMP-6 had no significant impact on cell morphology, migration or EMTrelated markers in RPE cells without TGF-β treatment.However, TGF-β treatment can significantly prevent EMT development in RPE cells.Our study results showed that overexpression of BMP-6 could impede EMT occurrence in RPE cells.BMP-6 could potentially prevent PVR.
Our study further indicated that the deactivation of ERK is the key pathway related to the repressive effect of BMP-6 on EMT.A study by Paoet al[21]suggested that the overexpression of miR-4516 suppresses TGF-β2-induced EMT in a PVR model, and its role in PVR depends on ERK signalling.Research has also suggested that inhibition of ERK signalling pathways suppresses proliferation in human RPE cells[22].Our study results were aligned with their findings.Thus, the effect of BMP-6 against TGF-induced fibrosis may be related to the ERK signalling pathway.
Our study did not dig deeper into the relationship between BMP and related mechanisms of ERK channels.Our findings were limited to the modelin vitro.In the future, we could further validate these results in patient samples and/or establish an animal model.
In conclusion, our study shows that BMP-6 can inhibit the migration of RPE cells and EMT induced by TGF-β.These results suggest a potential novel mechanism to prevent fibrosis in RRE cells, hence providing a new perspective for the prevention and treatment of PVR.
ACKNOWLEDGEMENTS
The authors thank American Journal Experts for language polishing.
Foundations:Supported by the Key Research & Development Program of Shaanxi Province (No.2022SF-311; No.2024SFYBXM-328; No.2024SF-YBXM-325); the Natural Science Basic Research Program of Shaanxi Province, China(No.2021JQ-385).
Conflicts of Interest: Liu X,None;Liu M,None;Ji M,None;Ma B,None;Hou YC,None;Yao XY,None;Cheng QC,None;Chen L,None.
 International Journal of Ophthalmology2024年4期
International Journal of Ophthalmology2024年4期
- International Journal of Ophthalmology的其它文章
- Algorithm of automatic identification of diabetic retinopathy foci based on ultra-widefield scanning laser ophthalmoscopy
- CD3ε of a pan T cell marker involved in mouse Aspergillus fumigatus keratitis
- Neuroprotective effects of acteoside in a glaucoma mouse model by targeting Serta domain-containing protein 4
- Neuroprotective and anti-inflammatory effects of eicosane on glutamate and NMDA-induced retinal ganglion cell injury
- Dry eye rate and its relationship with disease stage in patients with primary hypertension: a cross-sectional study in Vietnam
- Assessment of tear film parameters post-treatment with commercial eyelid cleaning wipes: a pilot study
