Proteomic study of vitreous in proliferative diabetic retinopathy patients after treatment with aflibercept: a quantitative analysis based on 4D label-free technique
Ting-Ting Feng, Xiang Gao, An-Ran Liang, Bo-Wen Zhao, Guang-Hui He, Song Chen
1Clinical College of Ophthalmology, Tianjin Medical University, Tianjin 300070, China
2Tianjin Key Laboratory of Ophthalmology and Visual Science, Tianjin Eye Institute, Tianjin Eye Hospital, Tianjin 300020, China
3Key Laboratory of Ophthalmology, Anyang Eye Hospital,Anyang 455000, Henan Province, China
4Department of Ophthalmology, Jining No.1 People’s Hospital,Jining 272011, Shandong Province, China
Abstract
· KEYWORDS: vitreous; proliferative diabetic retinopathy;proteome; 4D label-free
INTRODUCTION
With the development of today’s society and changes in lifestyle, diabetes mellitus (DM) and its complication,diabetic retinopathy (DR), have become one of the leading causes of vision loss worldwide[1].The number of people with diabetes will increase to 552 million by 2030, compared to 366 million in 2011, and more than one-third of them have symptoms of DR.
Currently there are two main types of DR: 1) early-stage nonproliferative diabetic retinopathy (NPDR), characterized by microaneurysms and possible intraretinal haemorrhage,sclerotic exudates and cotton-spots; 2) late-stage proliferative DR (PDR), in which retinal and iris neovascularization occurs and results in preretinal fibrosis and detachment of the pulling retina[2].DR also produces diabetic macular edema(DME), which severely affects the patient’s vision and is caused by disruption of the outer retinal blood barrier and fluid accumulation[3].The main pathological mechanism of DR is that, in the presence of hyperglycemia, leukocyte adhesion to retinal capillaries leads to endothelial damage and an increase in vascular permeability, resulting in macular oedema and capillary obstruction.Subsequent retinal ischemia stimulates the production of vascular endothelial growth factor (VEGF),ultimately leading to retinal neovascularization and PDR.
VEGF, a cytokine and a key driver of intraocular neovascularization and associated pathological changes, is a promising therapeutic target.A series of pharmacological antibodies against VEGF have been designed to date for the prevention of neovascularization in PDR, notably bevacizumab(Avastin; Novartis), ranibizumab (Lucentis; Novartis), and the soluble fusion protein aflibercept (Eylea; Regeneron Pharmaceuticals).The emergence of intraocular drugs such as the aforementioned anti-VEGF agents has revolutionized the treatment of PDR, and aflibercept in particular has been designed to prolong the intravitreal half-life of the drug by altering its molecular structure, allowing the interval between injections to be extended to 2mo in patients with DME[4].However, after the widespread use of anti-VEGF drugs, it was found that also 15%-20% of patients with DR do not respond adequately or completely to anti-VEGF therapy and need to be co-administered with other therapies[5].Other therapies include steroids, topical medications, laser therapy, and an emerging array of other medications.The reasons for these results are currently unknown to us.
In response to the above problems, the emergence of proteomic analysis technology provides a good platform for extensive scanning of differentially expressed proteins (DEPs).The vast majority of proteins expressed in the genome can be accurately quantified and characterized by this technology[6].Isotope labelling for absolute quantification (iTRAQ),tandem mass tagging (TMT) and label-free quantification techniques are now widely used.In recent years, 4D labelfree techniques have been applied to proteomic analyses due to their high sensitivity and bioinformatics advantages,especially for vitreous fluids, which are rare samples and in which proteins are expressed in low abundance[7].In addition, label-free proteomics has significant advantages in the comprehensiveness of biomarker identification, allowing the identification of multiple relevant proteins differentially altered in the sample[8].
A series of current proteomics-based studies are attempting to elucidate intraocular factors associated with the pathogenesis of DR, and proteomics has the potential to identify new diagnostic and/or prognostic biomarkers associated with underlying pathophysiological processes in PDR patients undergoing anti-VEGF therapy, and can also further explain the reasons for the generation of anti-VEGF drug failures, we used the latest four-dimensional data-independent acquisition(4D-DIA) technology to analyze the vitreous humour of PDR patients using aflibercept and PDR patients without aflibercept using the latest 4D-DIA technology to perform vitreous humour analysis in order to better understand the mechanisms by which aflibercept affects the activity of the relevant signal pathways, thus guiding individualized anti-VEGF therapy and analyzing the intrinsic mechanisms of anti-VEGF drugs.
SUBJECTS AND METHODS
Ethical ApprovalThe study protocol was approved by the Medical Ethics Committee of Anyang Eye Hospital (No.AYLS-2022-01) and after being informed of the purpose of the study, all patients agreed to participate in this study.
ParticipantsPatients who meet the following criteria may be included in the PDR group: 1) patients with a diagnosis of active PDR requiring vitrectomy; 2) patients who cannot be treated with pan-retinal photocoagulation (PRP) because of nonabsorbable vitreous haemorrhage or neovascularized retinal fibrovascular membrane detachment, or for whom other treatments are not indicated; 3) patients who have not received intravitreous steroids or anti-VEGF injections in the past 6mo.Excluded from this condition: 1) other retinal diseases; 2) the presence of glaucoma or other ocular diseases; 3) the presence of intraocular infections or inflammation; 4) a history of penetrating ocular trauma; 5) those who have had intraocular surgery in the last 6mo; 6) inability to undergo pars plana vitrectomy (PPV) due to uncontrolled systemic diseases.
Liquid Chromatography-Mass Spectrometry AnalysesEach sample was separated using the HPLC Liquid Phase System Easy nLC at nanolitre flow rates.Buffer A was 0.1% formic acid aqueous solution and B was 0.1% formic acid acetonitrile aqueous solution (acetonitrile 84%).The chromatographic column was equilibrated with 95% liquid A.The samples were upsampled from an autosampler onto a supercolumn (Thermo Scientific Acclaim PepMap100,100 μm×2 cm, nanoViper C18) and passed through an analytical column (Thermo Scientific EASY column, 10 cm),ID 75 μm, 3 μm, C18-A2) at a flow rate of 300 nL/min.
After chromatographic (MS) separation, the samples were analyzed by mass spectrometry using a timsTOF Pro mass spectrometer.The detection method was positive ion, the ion source voltage was set to 1.5 kV, and both MS and MS/MS(Tandem mass spectrometry) were analyzed by time-of-flight(TOF) detection.The scanning range of the mass spectrometer was set to 100-1700 m/z.The data acquisition method was in parallel accumulated serial fragmentation (PASEF) mode, with 10 PASEF mode acquisitions of parent ions after the first level of MS acquisition, with a cyclic window time of 1.17s.For secondary spectra with charge numbers in the range of 0-5, the dynamic exclusion time of the tandem mass spectrometry scan was set to 24s to avoid repeated scans of the parent ions.
Bioinformatics and Functional Enrichment AnalysisUse MaxQuant software (version 1.6.14) to identify and quantitatively analyze the obtained Liquid Chromatography-Mass Spectrometry (LC-MS/MS) data.First, normalize the quantitative information of the target protein set to the (-1, 1) interval.Then, use the Complexheatmap R package (R Version 3.4) to classify both the sample and protein expression dimensions simultaneously, generating a hierarchical clustering heatmap.Use the InterProScan software package to run the scanning algorithm InterPro database in an integrated manner to characterize the sequence’s functionality and obtain domain annotation information of the target protein sequence in the Pfam database.Use Blast2GO and KEGG Automatic Annotation Server (KAAS) software to perform Gene Ontology (GO) annotation and KEGG pathway annotation on the target protein set, respectively.Perform GO annotation or (or KEGG pathway, or Domain) annotation enrichment analysis on the target protein set using Fisher’s exact test.From STRING (http://string-db.org/), the information in the database is used to search for direct and indirect interactions between target proteins.Generate interactive networks and analyze them using Cytoscape software (version 3.2.1).
RESULTS
Characteristics of Study PopulationTable 1 summarizes the patient baseline and demographic data.
Analysis of Significantly DEPsThe extracted vitreous humour was examined through strict quality control and 9069 identified peptides and 1481 identified proteins were obtained(Figure 1A).A total of 1481 proteins were found to be coexpressed in both groups (Figure 1B).Upon further analysis,a total of 58 proteins were identified as DEPs (Figure 1C, 1D,Table 2), of which 47 DEPs were up-regulated and 11 DEPs were down-regulated.Subcellular organelles (Organelle) are micro-organisms (such as mitochondria and endoplasmic reticulum) of the cytoplasm with certain morphology and function, which are the sites where proteins perform their functions, and analyzing the subcellular localization of the identified proteins can help to further explore the function of DEPs.In this experiment, we used the subcellular structure prediction software CELLO to analyze the proteins, which was shown as a pie chart, and found that the highest number of 37 proteins were distributed in the extracellular, followed by 15 proteins in the nucleus, and 7 proteins in the plasma membrane.
Gene Ontology Functional AnalysisGO functional enrichment analysis showed that DEPs were classified into 43 GO terms (Figure 2A), including 22 biological processes, 14 cellular components, and 7 molecular functions.In biological processes, these differentially expressed proteins were mainly involved in cellular process (51 DEPs), response to stimulus(44 DEPs), metabolic process (43 DEPs), organic substance metabolic process (43 DEPs), primary metabolic process (42 DEPs), biological regulation (41 DEPs).
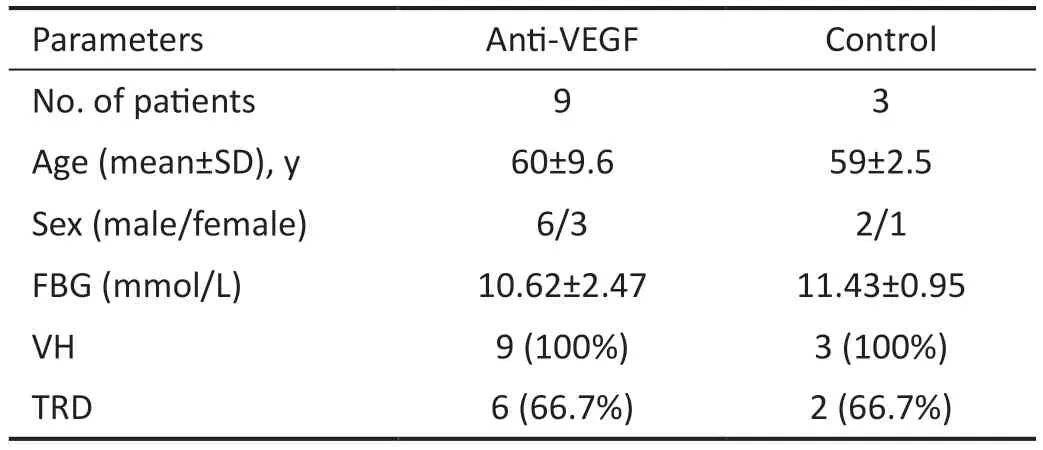
Table 1 Patient baseline and demographic data
In cellular component, extracellular region contains 53 proteins, followed by DEPs mainly involved in cell (53 DEPs),cell part (52 DEPs), extracellular region part (50 DEPs),extracellular space (47 DEPs).In addition, organelle (45 DEPs),membrane-bounded organelle (45 DEPs), intracellular (41 DEPs), intracellular part (41 DEPs) and cytoplasm (41 DEPs)were also among the terms with high enrichment in cellular component.
The most enriched terms in Molecular Function are binding,which contains 48 proteins.The next most enriched molecular functions are protein binding (32 DEPs), ion binding (27 DEPs), catalytic activity (19 DEPs), signaling receptor binding (18 DEPs) and cation binding (18 DEPs).The above results suggested that some overexpressed DEPs were mainly concentrated in inflammatory, endothelial cell proliferation-related terms, such as “regulation of epithelial cell differentiation”, “leukocyte migration”, “regulation of reproductive process” and “cell migration” (Figure 2B).
In addition, DEP was enriched in cellular components for platelet alpha granule lumen, platelet alpha granule, cell surface, extracellular region and extracellular matrix (Figure 2C).Among the molecular functions, “receptor ligand activity”,“receptor regulator activity”, “cytokine activity”, and“cytokines” were the most important.Among the molecular functions, receptor ligand activity, receptor regulator activity,cytokine activity and growth factor activity were significantly enriched (Figure 2D).During the follow-up process we constructed GO Directed Acyclic Graph (DAG) structures(Figure 2E-2G).The results of significant enrichment under the three classifications are consistent with the bubble diagram.
KEGG Pathway Enrichment AnalysisTo further explore the physiological characteristics of vitreous samples from both groups of patients, we performed KEGG pathway enrichment analysis to identify the major biochemical metabolic pathways involved in differential metabolites.The results showed 270 differential metabolites enriched in 20 metabolic pathways (Figure 3A).The “Proteoglycans in cancer” metabolic pathway was associated with the highest number of differential metabolites,containing a total of 7 DEPs.This was followed by the“Complement and coagulation cascades” with a total of 6 DEPs.KEGG pathway analysis showed that the significantly enriched pathway associated with it after anti-VEGF treatment was proteoglycans in cancer, followed by mitogen-activated protein kinase (MAPK) signaling pathway and Ras signaling pathway,both of which were associated with inflammatory response(Figures 3B, 4).Among them, upregulated DEPs were mainly enriched in VEGF signaling pathway, EGFR tyrosine kinase inhibitor resistance, rheumatoid arthritis, glycosphingolipid biosynthesis-globo and isoglobo series (Figure 3C).Inaddition, the downregulated DEPs were mainly associated with Chagas disease, endocrine resistance, longevity regulating pathway and Hedgehog signaling pathway.
PPI Network Highlights Anti-VEGF Treatment Response ProteinsFinally, we generated a PPI network for the identified DEPs using the STRING database (http://string-db.org/).One of the important ways for proteins to function is to interact with other proteins to exert biological regulation through interprotein-mediated pathways, or to form complexes, in which proteins with high connectivity may be the key sites for the whole signal transduction pathway.Among them, we found that the core top five protein targets with high connectivity are tissue inhibitor matrix metalloproteinase 1 (TIMP1), VEGF-A,thrombospondin 1, serine protease inhibitor clade E member 1(SERPINE1) and matrix metalloproteinase (MMP)-9 (Figure 4).
DISCUSSION
Currently, patients with PDR are facing the problem of multiple use and partial ineffectiveness of anti-VEGF therapy,which undoubtedly increases the cost of treatment for patients and makes it more difficult to promote the use of anti-VEGF drugs.The purpose of this experiment is to address the problem of drug resistance after the widespread use of anti-VEGF therapeutic drugs.In our previous experiments, we have analyzed the vitreous fluid samples of patients with foramen ovale retinal detachment by 4D-DIA, and found that DEPs were mainly enriched in the cell adhesion pathway compared with the control group, and that DEPs were associated with the heat shock protein content, glycolysis, and acute inflammatory processes, which provided a reference for finding biomarkers for the pathogenesis of rhegmatogenous retinal detachment[9].DDA technique, which is capable of capturing information on all acquired ions, reduces randomness in the detection process, has higher reproducibility and stability, and increased accuracy.Anti-VEGF drugs may affect these underlying pathophysiological processes differently, and more studies on drug-specific phenotypic changes after drug use in PDR patients are necessary.
VEGF in PDR controls angiogenesis through the VEGFR1 and VEGFR2 signal pathways[10], the latter being the main receptor for VEGF-induced neoangiogenesis and permeability-enhancing effects[11].The aflibercept selected in this experiment is a chimeric receptor consisting of VEGF receptor 1 (VEGFR1) and VEGFR2 binding domains, which binds with high affinity to VEGF-A, VEGF-B, and placental growth factor.Differences in the effects of different anti-VEGF therapies on VEGFR1/2 dynamics may be due to differences in the types of these drugs, and an in-depth analysis of the mechanisms has the potential to identify key targets in which anti-VEGF therapy is ineffective and resistant.In this experiment, related receptor tyrosine kinase 1 (FLT1)and kinase insert domain receptor (KDR) were found to be highly expressed after anti-VEGF, with FLT1 associated with VEGFR1 and KDR associated with VEGFR2.This is different from previous results of VEGFR1 and VEGFR2 changes in atrial fluid samples obtained by aflibercept after vitreous injection in patients with PDR[12].However, in the same way,the VEGF signal pathway will be activated in PDR patients after receiving anti-VEGF therapy[13].
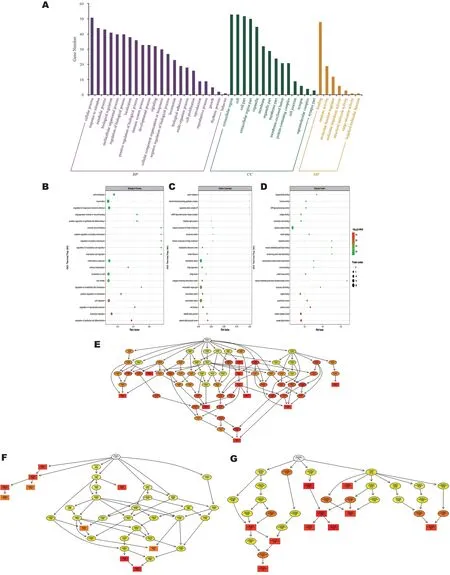
Figure 2 GO classification analysis A: GO classification analysis (anti-VEGF vs control); Each bar represents the number of DEPs.B, C, D: GO function enrichment bubble chart (BP)/(CC)/(MF).Top 20 significantly enriched GO terms; Dot size represents DEP counts.E, F, G: Directed acyclic graphs of the three main classifications (BP)/(CC)/(MF).The main node of the DAG represents the top 10 with the highest enrichment degree, with a darker color indicating a higher enrichment degree.GO: Gene Ontology; VEGF: Vascular endothelial growth factor; DEP:Differentially expressed proteins; BP: Biological process; CC: Cellular component; MF: Molecular function; DAG: Directed Acyclic Graph.
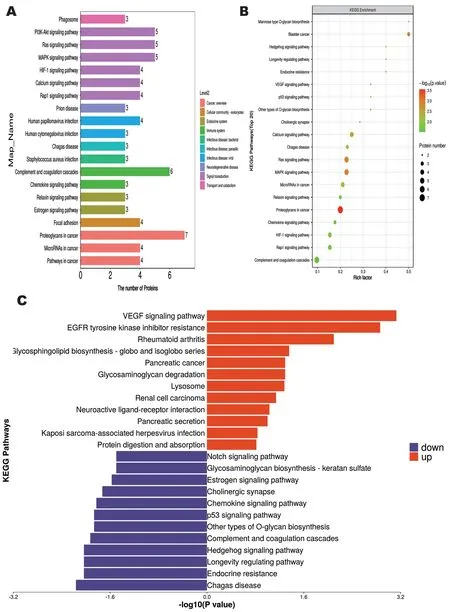
Figure 3 KEGG pathway enrichment analysis A: KEGG pathway annotation and attribute histogram of DEPs; Different colors represent the 7 branches of the KEGG metabolic pathway.B: KEGG pathway enrichment bubble plot; C: Butterfly diagram of up- and down-regulated DEPs.KEGG: Kyoto Encyclopedia of Genes and Genomes; DEP: Differentially expressed proteins.
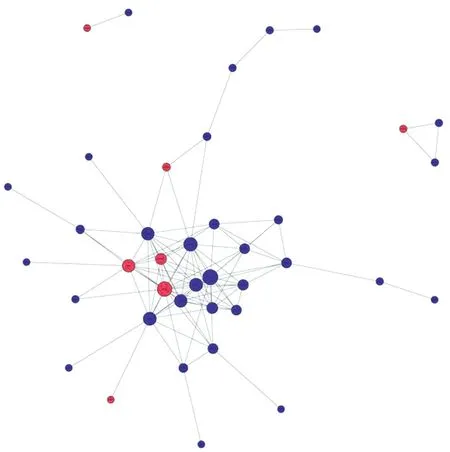
Figure 4 Protein-protein interaction networks Circles represent proteins, horizontal lines represent links, blue represents downregulation, red represents up-regulation.
Complement C1q tumor necrosis factor-related protein 5(C1QTNF5) was found to be an adipocytokine, which was found to be increased in vitreous fluid samples from patients after abciximab injection.Past studies on C1QTNF5 have focused on its role in the pathogenesis of late-onset retinal degeneration (L-ORD) pathogenesis, where the S163R mutation in C1QTNF5 in retinal pigment epithelium (RPE)leads to the autosomal dominant disease L-ORD.A recent study concluded that it may act as a biomarker for PDR by analysing PDR data in GEO[14].C1QTNF3, a member of the C1QTNF5 family, is also one of the biomarkers for DR, and supplementation with C1QTNF3 is now thought to prevent the development of diabetes in overweight individuals[15].In ophthalmology C1q/TNF-related protein 3 (CTRP3) activates the adenosine 5’-monophosphate-activated protein kinase signalling pathway andin vivoandin vitroenhances the expression of Occludin and Claudin-5 (tight junction proteins)and ultimately ameliorates human retinal endothelial cell damage induced by high glucose/high lipid[16].It has also been demonstratedin vitrothat overexpression of CTRP3 improves cell viability of ARPE-19 cells under hyperglycaemia (HG)by reducing reactive oxygen species and malondialdehyde levels and increasing superoxide dismutase activity through attenuating HG-induced oxidative stress in ARPE-19 cells.Apoptosis rate of CTRP3 overexpressing ARPE-19 cells was significantly decreased in CTRP3 overexpressing ARPE-19 cells[17].Another study concluded that CTRP3 expression was reduced in HG-stimulated human peripapillary retinal cells.In addition, CTRP3 overexpression enhanced the viability of HG-induced HRP while abrogating the effects of apoptosis and oxidative stress, and forkhead box O4 (FOXO4) was upregulated in HG-induced humen retinal pericytes (HRP) when FOXO4 binds to the CTRP3 promoter and represses CTRP3 transcription, thereby regulating the Nrf2/NF-κB signaling pathway.The up-regulation of FOXO4 reversed the effects of elevated CTRP3 on the proliferation, apoptosis, and cell death of HG-induced HRP.HG-induced HRP proliferation, apoptosis and oxidative stress.In conclusion, the protective effect of CTRP3 against HG-induced oxidative damage in DR by regulating Nrf2/NF-κB signalling could be inhibited by FOXO4[18].
Clusterin (CLU) is a multifunctional glycoprotein that not only protects the blood-retinal barrier in high-glucose environments,but also acts as a neuroretinoprotective agent[19], and even serves to alleviate ocular surface diseases caused by obesity and type 2 diabetes[20].In a high-glucose environment, CLU effectively inhibited VEGF-induced hyperpermeability of human retinal microvascular endothelial cells and retina.The anti-permeability activity of CLU was associated with the restoration of tight junction proteins as a way to reduce DR vascular leakage and restore tight junction protein expression.In conclusion, CLU may be associated with three main aspects[21]: 1) CLU binds to misfolded proteins in an ATPindependent manner, stabilizing them into a soluble form and preventing their aggregation; 2) cytoprotection, which is mainly reflected in the inhibition of optic rod cell death in rats with retinitis pigmentosa by CLU, which is likely to occur through the activation of Akt and STAT3 inhibitors,activation of Akt and STAT3 to inhibit cell death, followed by inhibition of Bax, thereby interfering with its contribution to optic rod cell death[22]; 3) anti-inflammatory effects, CLU binds to circulating lipoproteins, CLU binds to low density lipoproteins (LDL), which accumulates in human plasma, and exerts a protective effect against LDL aggregation, preventing endothelial cell activation and limiting pro-inflammatory responses.Second, CLU has the ability to inhibit complement activation, and in a mouse model of hyperoxic acute lung injury characterized by excessive lung inflammation, CLU knockout mice showed greater injury and mortality compared to wild-type mice.Recombinant CLU attenuated hyperoxiainduced apoptosis, and the inhibitory effect of CLU was similarly confirmed in retinal vascular development andex vivofree radical injury in mice[23].
As shown in the previous PPI analysis we obtained through the STRING database, TIMP1 is the core protein of the whole regulatory network, and its role as a novel inflammatory factor is associated with the progression of several inflammatory diseases[24], in ocular diseases, TIMP-1 has been shown to inhibit neovascularization in chick chorioallantoic membrane,but it can induce mice to have more neovascularization[25],while intravitreal injection of VEGF promotes TIMP1 expression[26].In addition to binding to VEGF, TIMP blocks the catalysis of MMP and has additional biological activities,including the regulation of cell growth and differentiation,apoptosis, angiogenesis, and tumorigenesis, as shown by the results of a vitreous sample assay in patients with proliferative vitreoretinopathy (PVR) and PDR, which demonstrated that TIMP-1 and TIMP-3 were expressed in the vascular endothelium of the preoptic membrane of PDR retinas as well as in the vascular endothelium of expressed in myofibroblasts and leukocytes of PDR and PVR retinal precursors, TIMP-1 may be a biomarker of disease activity[27].In contrast, another analysis of PDR and PVR fibrovascular membrane samples revealed that MMP involved in matrix degradation in fibrovascular tissue as well as TIMP-1 and TIMP-2 were found in most PDR membranes and their expression did not differ from that in proliferating membranes in PVR, suggesting the existence of a common pathway of extracellular matrix degradation in the pathological process leading to retinal neovascularization and fibrosis[28], suggesting that regardless of whether PDR or PVR leads to proliferating fibrous membranes, treatment against TIMP1 may be a therapeutic target for both of these diseases.Also during the course of this experiment, the differential SIRPα alterations in the vitreous samples of the two groups aroused our interest.Recently experimentalists have identified the involvement of lymphoid angiogenesis with aberrant endothelial differentiation and progenitor cells in the neovascularization process associated with PDR[29], which offers the potential to improve DR treatment.In particular,CD47/SIRPα is an innate negative immunoregulatory axis that transmits a “don’t eat me” signal to macrophages and causes tumor cells to respond to immunity signals to macrophages and makes tumor cells resistant to immune surveillance[30].CD47/SIRPα-based therapy has been shown to be an effective treatment for both solid tumors and haematological malignancies, and these findings highlight the tremendous power of mobilizing macrophages for anti-tumor activity in tumor immunotherapy.The unsustainable efficacy of antineovascular therapies such as aflibercept in tumors was found to be due to its ability to upregulate CD47 expression in the tumor microenvironment, and the combination of CD47-blocking fusion proteins promotes the anti-neovascular effects of aflibercept in tumors, which provides new means of inhibition of angiogenesis as well as a target of action[31],and whether this target of action could be further be used to inhibit retinal neovascularization will be further demonstrated experimentally.
In conclusion, the results of this study provide new insights into the proteomic changes in vitreous samples of PDR patients treated with aflibercept, in which the SIRPα target finds a new target for solving anti-VEGF resistance.Although further experiments are needed to verify this hypothesis.Only 12 eligible patients were recruited in the sample collection for this trial; such a small sample size may have led to biased analyses, and also the vitreous samples, while better reflecting retinal tissue conditions, did not directly observe changes in the associated DEPs before and after aflibercept injection.Considering the implications of our results, we are in the process of recruiting more patients and collecting more detailed clinical data to validate our metabolomics findings.
ACKNOWLEDGEMENTS
Foundations:Supported by Tianjin Key Medical Discipline Specialty Construction Project (No.TJYXZDXK-016A);Henan Provincial Department of Science and Technology (No.LHGJ20200802).
Conflicts of Interest: Feng TT,None;Gao X,None;Liang AR,None;Zhao BW,None;He GH,None;Chen S,None.
 International Journal of Ophthalmology2024年4期
International Journal of Ophthalmology2024年4期
- International Journal of Ophthalmology的其它文章
- Algorithm of automatic identification of diabetic retinopathy foci based on ultra-widefield scanning laser ophthalmoscopy
- CD3ε of a pan T cell marker involved in mouse Aspergillus fumigatus keratitis
- Neuroprotective effects of acteoside in a glaucoma mouse model by targeting Serta domain-containing protein 4
- Neuroprotective and anti-inflammatory effects of eicosane on glutamate and NMDA-induced retinal ganglion cell injury
- Bone morphogenetic protein-6 suppresses TGF-β2-induced epithelial-mesenchymal transition in retinal pigment epithelium
- Dry eye rate and its relationship with disease stage in patients with primary hypertension: a cross-sectional study in Vietnam
