腾冲重楼根茎的化学成分及抗菌活性研究
杨莹莉 ,白 雪,杨 珺,罗吉凤,段晓燕,王跃虎
1.中国科学院昆明植物研究所 资源植物与生物技术重点实验室和植物化学与西部植物资源持续利用国家重点实验室,云南 昆明 650201
2.中国科学院大学,北京 100049
重楼属ParisL.植物为藜芦科(Melanthiaceae)多年生草本植物,生长在常绿阔叶林、落叶阔叶林、针叶林、竹丛和灌木丛中[1-2];包括33 个种和15 个变种[2],主要分布于欧亚大陆的热带和温带地区,中国大部分地区均有分布。西南地区居多[2-3]。重楼属植物有很高的药用价值,在民间,有十几个种或变种作为药用植物被利用。据《中国药典》2020 年版记载,重楼药材的基原植物是云南重楼(滇重楼)ParipolyphyllaSmith var.yunnanensis(Franch.)Hand.-Mazz.和七叶一枝花P.polyphyllaSmith var.chinensis(Franch.) Hara。重楼具有清热解毒、消肿止痛、凉肝定惊等功效,用于治疗疔疮痈肿、咽喉肿痛、蛇虫咬伤、跌扑伤痛、惊风抽搐等病症[4]。云南重楼提取物[5]和球药隔重楼P.fargesiiFranch.根茎提取物[6]已被批准为化妆品原料。
腾冲重楼P.tengchongensisY.H.Ji, C.J.Yang &Y.L.Huang 仅发现于云南,生长于海拔2 750~3 200 m 常绿阔叶林、竹林和灌丛中[7],腾冲重楼被当地居民当作重楼药材使用,一般用于治疗毒蛇咬伤和跌打伤痛[8]。目前,关于腾冲重楼化学成分研究的文献较少,仅报道了从腾冲重楼中分离得到的20 个甾体皂苷类化合物,其中,化合物paristengoside A对人红白细胞白血病HEL 细胞和人乳腺癌MDAMB-231 细胞的生长有一定的抑制作用[9]。为了更全面地认识和合理利用腾冲重楼,本研究对腾冲重楼进行了化学成分研究和抗菌活性评价。从其根茎提取物中共分离出16 个化合物(图1),分别鉴定为parisvanioside A(1)、kingianoside K(2)、7-氧薯蓣皂苷(7-oxodioscin,3)、pariposide A(4)、parisvanioside B(5)、parisrugoside H(6)、(25R)-17α-羟基螺甾-5-烯-3β-基O-β-D-吡喃葡萄糖基-(1→4)-O-[α-L-吡喃鼠李糖基-(1→2)]-β-D-吡喃葡萄糖苷((25R)-17α-hydroxyspirost-5-en-3β-ylO-β-D-glucopyranosyl-(1→4)-O-[α-L-rhamnopyranosyl-(1→2)]-β-Dglucopyranoside,7)、麦冬皂苷Cʹ(ophiopogonin Cʹ,8)、纤细薯蓣皂苷(gracillin,9)、重楼皂苷Ⅰ(paris saponin Ⅰ,10)、重楼皂苷II(paris saponin II,11)、重楼皂苷Ⅵ(paris saponin Ⅵ,12)、pennogenin 3-O-β-chacotrioside(13)、重楼皂苷H(paris saponin H,14)、重楼皂苷Ⅶ(paris saponin Ⅶ,15)和β-蜕皮激素(β-ecdysone,16)。其中化合物1~7 首次从腾冲重楼中分离得到。
1 仪器与材料
HPD-100 型大孔树脂(上海吉至生化科技有限公司),柱色谱用硅胶(80~100、200~300、300~400 目,青岛美高集团有限公司),反相(RP)C18硅胶(40~75 μm,Fuji Silysia Chemical 有限公司),Sephadex LH-20 凝胶色谱(GE Healthcare Bio-Sciences AB),薄层色谱(TLC)板(100 mm×50 mm,青岛美高化工有限公司),SK3300LH 型超声波清洗器(上海科导超声仪器有限公司),旋转蒸发仪(瑞士BUCHI 公司),AL204 型电子分析天平(梅特勒托利多仪器有限公司),循环水真空泵(巩义市予华仪器有限责任公司),SB-1300 型水浴锅(上海爱朗仪器有限公司),500 MHz/800 MHz 核磁共振波谱仪(德国Brucker 公司),DFS 型快原子轰击离子源质谱仪(Thermo Fisher Scientific),Agilent 1200 Series 高效液相色谱仪半制备。
实验用腾冲重楼根茎样品于2021 年10 月采自云南省兰坪县重楼种植基地,经中国科学院昆明植物研究所杨珺实验师鉴定为藜芦科重楼属植物腾冲重楼P.tengchongensisY.H.Ji, C.J.Yang & Y.L.Huang,凭证标本(YJ2021050)保存于中国科学院昆明植物研究所植物标本室。
2 提取与分离
腾冲重楼干燥根茎(3 kg)粉碎,加70%乙醇(3L)60 ℃超声提取6 次(每次1 h),滤过,取滤液,60 ℃减压回收溶剂,得粗提物浸膏(660.0 g),加水(1 L)混悬,分别用等体积石油醚、醋酸乙酯和正丁醇分别萃取5 次,60 ℃减压浓缩,得石油醚部分(118.1 mg)、醋酸乙酯部分(3.1 g)和正丁醇部分(182.0 g)。将正丁醇部分(170.2 g)用4 L 蒸馏水溶解,过 HPD100 大孔树脂柱,乙醇-水(30%→50%→70%→95%)梯度洗脱后得4 部分:T1(1.7 g)、T2(5.4 g)、T3(22.7 g)和T4(50.0 g)。T1(1.7 g)经RP C18硅胶柱色谱及Sephadex LH-20 凝胶柱(甲醇)分离纯化后,得到化合物16(78.1 mg)。T2(5.4 g)经RP C18硅胶柱色谱分段及HPLC(乙腈-水40∶60)分离纯化后得到化合物15(4.9 mg,tR=21.4 min)和14(11.7 mg,tR=27.0 min)。T3(22.7 g)经RP C18硅胶柱色谱得到T3-1(2.3 g)和T3-2(12.3 g)。T3-1 经硅胶柱色谱(二氯甲烷-甲醇15∶1→1∶1)得到T3-1-1(57.9 mg)、T3-1-2(567.8 mg)和T3-1-3(34.7 mg)3 个组分。T3-1-1 用Sephadex LH-20 凝胶柱色谱(甲醇)及HPLC(甲醇-水77∶23)分离纯化得到化合物1(1.1 mg,tR=25.5 min)、4(5.7 mg,tR=32.4 min)、7(2.8 mg,tR=22.3 min)和12(19.1 mg,tR=23.7 min)。T3-1-2用Sephadex LH-20凝胶柱色谱(甲醇)及HPLC(甲醇-水83∶17)分离纯化得到化合物5(1.1 mg,tR=32.5 min)、6(1.1 mg,tR=23.0 min)、3(0.9 mg,tR=29.3 min)和13(26.9 mg,tR=25.7 min)。T3-1-3 经HPLC(甲醇-水68∶32)分离纯化后得到化合物2(0.5 mg,tR=34.2 min)。T3-2 经硅胶柱色谱(二氯甲烷-甲醇15∶1→1∶1)得到T3-2-1(88.5 mg)、T3-2-2(1.0 g)和T3-2-3(10.1 mg)3 个组分。T3-2-1 用Sephadex LH-20 凝胶柱色谱(MeOH)及HPLC(甲醇-水85∶15)分离纯化得到化合物8(3.5 mg,tR=25.0 min)。T3-2-2 经RP C18硅胶柱色谱(80.7 mg)及HPLC(乙腈-水45∶55)分离纯化得到化合物11(6.7 mg,tR=17.9 min)和9(5.2 mg,tR=21.6 min)。T3-2-3 经反复重结晶(甲醇)得化合物10(9.1 mg)。
3 结构鉴定
化合物 1:白色无定型粉末,分子式为C39H58O14。ESI-MSm/z: 774 [M+Na]+,。1H-NMR (500 MHz, pyridine-d5)δ: 6.64(1H, d,J= 8.6 Hz, H-7), 6.37 (1H, brs, H-1ʺ), 6.29 (1H,d,J= 8.6 Hz, H-6), 5.47 (1H, m, H-11), 4.88 (1H, d,J= 7.3 Hz, H-1ʹ), 4.26 (2H, m, H2-6ʹ), 1.74 (3H, d,J=6.2 Hz, H3-6ʺ), 1.20 (3H, s, H3-19), 1.04 (3H, d,J= 6.7 Hz, H3-21), 0.87 (3H, s, H3-18), 0.65 (3H, d,J= 4.7 Hz,H3-27);13C-NMR (125 MHz, pyridine-d5)δ: 143.7 (C-9), 136.4 (C-6), 130.5 (C-7), 119.1 (C-11), 109.2 (C-22), 101.7 (C-1ʺ), 100.7 (C-1ʹ), 82.7 (C-5), 80.5 (C-16),79.3 (C-3ʹ), 77.9 (C-5ʹ), 77.5 (C-8), 77.2 (C-2ʹ), 74.0(C-4ʺ), 73.5 (C-3), 72.6 (C-3ʺ), 72.2 (C-2ʺ), 71.3 (C-4ʹ),69.2 (C-5ʺ), 66.7 (C-26), 62.1 (C-6ʹ), 61.3 (C-17), 48.3(C-14), 42.1 (C-20), 41.3 (C-13), 41.2 (C-12), 38.4 (C-10), 33.3 (C-4), 32.8 (C-1), 31.5 (C-23), 30.3 (C-25),29.6 (C-15), 29.2 (C-2), 28.9 (C-24), 25.3 (C-19), 18.5(C-6ʺ), 17.0 (C-27), 16.9 (C-18), 14.3 (C-21)。以上数据与文献报道数据基本一致[10],故鉴定化合物1 为parisvanioside A。
化合物 2:白色无定型粉末,分子式为C44H68O17。ESI-MSm/z: 891 [M+Na]+,。1H-NMR (500 MHz, pyridine-d5)δ: 5.74(1H, brs, H-6), 6.30 (1H, brs, H-1ʺ), 5.92 (1H, brs, H-1ʹʹʹ), 4.93 (1H, m, H-1ʹ), 4.32, 4.23 (2H, m, H2-6ʹ), 4.25,4.16 (2H, m, H2-5ʹʹʹ), 1.75 (3H, d,J= 5.9 Hz, H3-6ʺ),1.13 (3H, d,J= 6.8 Hz, H3-21), 1.10 (3H, s, H3-19),0.83 (3H, s, H3-18), 0.66 (3H, d,J= 4.3 Hz, H3-27);13C-NMR (125 MHz, pyridine-d5)δ: 200.7 (C-7), 164.9(C-5), 126.1 (C-6), 109.4 (C-1ʹʹʹ), 109.1 (C-22), 101.6(C-1ʺ), 100.2 (C-1ʹ), 86.5 (C-4ʹʹʹ), 82.5 (C-2ʹʹʹ), 80.8 (C-16), 77.7 (C-2ʹ), 77.5 (C-3ʹʹʹ), 76.8 (C-3), 76.8 (C-3ʹ),76.8 (C-5ʹ), 76.7 (C-4ʹ), 73.9 (C-4ʺ), 72.6 (C-3ʺ), 72.4(C-2ʺ), 69.3 (C-5ʺ), 66.6 (C-26), 62.3 (C-6ʹ), 61.7 (C-17), 61.2 (C-5ʹʹʹ), 49.8 (C-14), 49.6 (C-9), 44.8 (C-8),41.7 (C-20), 41.0 (C-13), 38.6 (C-4), 38.5 (C-10), 38.5(C-12), 36.2 (C-1), 34.1 (C-15), 31.6 (C-23), 30.4 (C-25), 29.5 (C-2), 29.0 (C-24), 20.9 (C-11), 18.4 (C-6ʺ),17.1 (C-27), 16.8 (C-19), 16.2 (C-18), 14.9 (C-21)。以上数据与文献报道数据基本一致[11],故鉴定化合物2 为kingianoside K。
化合物 3:白色无定型粉末,分子式为C45H70O17,,ESI-MSm/z:907 [M+Na]+。1H-NMR (500 MHz, pyridine-d5)δ:6.40 (1H, brs, H-1ʺ), 5.84 (1H, brs, H-1ʹʹʹ), 5.76 (1H, s,H-6), 1.74 (3H, d,J= 6.2 Hz, H3-6ʹʹʹ), 1.62 (3H, d,J=6.0 Hz, H3-6ʺ), 1.12 (3H, d,J= 6.9 Hz, H3-21), 1.10(3H, s, H3-19), 0.82 (3H, s, H3-18), 0.66 (3H, d,J= 4.8 Hz, H3-27);13C-NMR (125 MHz, pyridine-d5)δ: 200.8(C-7), 165.0 (C-5), 126.0 (C-6), 109.0 (C-22), 102.6 (C-1ʹʹʹ), 101.6 (C-1ʺ), 100.2 (C-1ʹ), 81.1 (C-16), 78.3 (C-4ʹ), 77.6 (C-5ʹ), 77.2 (C-2ʹ), 76.8 (C-3ʹ), 76.5 (C-3),73.8 (C-4ʹʹʹ), 73.6 (C-4ʺ), 72.5 (C-3ʹʹʹ), 72.4 (C-3ʺ), 72.3(C-2ʹʹʹ), 72.2 (C-2ʺ), 70.2 (C-5ʹʹʹ), 69.2 (C-5ʺ), 66.5 (C-26), 61.6 (C-17), 61.0 (C-6ʹ), 49.7 (C-14), 49.5 (C-9),44.7 (C-8), 41.7 (C-20), 40.9 (C-13), 38.6 (C-4), 38.5(C-10), 38.4 (C-12), 36.2 (C-1), 34.1 (C-15), 31.5 (C-23), 30.3 (C-25), 29.5 (C-2), 28.9 (C-24), 20.8 (C-11),18.4 (C-6ʹʹʹ), 18.2 (C-6ʺ), 17.0 (C-19), 16.8 (C-27), 16.2(C-18), 14.9 (C-21)。以上数据与文献报道数据基本一致[12],故鉴定化合物3 为7-氧薯蓣皂苷。
化合物 4:白色无定型粉末,分子式为C39H60O14,,ESI-MSm/z:775 [M+Na]+。1H-NMR (500 MHz, pyridine-d5)δ:6.49 (1H, d,J= 8.5 Hz, H-7), 6.37 (1H, d,J= 1.2 Hz,H-1ʺ), 6.22 (1H, d,J= 8.5 Hz, H-6), 4.88 (1H, d,J=7.3 Hz, H-1ʹ), 1.74 (3H, d,J= 6.2 Hz, H3-6ʺ), 1.08 (3H,d,J= 6.9 Hz, H3-21), 0.93 (3H, s, H3-19), 0.88 (3H, s,H3-18), 0.66 (3H, d,J= 5.8 Hz, H3-27);13C-NMR (125 MHz, pyridine-d5)δ: 136.1 (C-6), 130.7 (C-7), 109.3(C-22), 101.9 (C-1ʺ), 101.1 (C-1ʹ), 82.2 (C-5), 80.9 (C-16), 79.6 (C-3ʹ), 78.7 (C-8), 78.1 (C-5ʹ), 77.5 (C-2ʹ),74.2 (C-4ʺ), 74.0 (C-3), 72.8 (C-3ʺ), 72.5 (C-2ʺ), 71.6(C-4ʹ), 69.4 (C-5ʺ), 66.9 (C-26), 62.8 (C-17), 62.4 (C-6ʹ), 51.8 (C-9), 51.5 (C-14), 42.3 (C-13), 41.5 (C-20),39.6 (C-12), 37.6 (C-10), 35.0 (C-1), 34.5 (C-4), 31.8(C-23), 30.6 (C-25), 29.2 (C-2), 29.2 (C-24), 29.1 (C-15), 23.2 (C-11), 18.7 (C-19), 18.2 (C-6ʺ), 17.3 (C-27),17.0 (C-18), 14.9 (C-21)。以上数据与文献报道数据基本一致[13],故鉴定化合物4 为pariposide A。
化合物 5:白色无定型粉末,分子式为C45H70O18,,ESI-MSm/z:921 [M+Na]+。1H-NMR (800 MHz, pyridine-d5)δ:6.50 (1H, d,J= 8.5 Hz, H-7), 6.35 (1H, d,J= 8.5 Hz,H-6), 6.32 (1H, brs, H-1ʺ), 5.81 (1H, brs, H-1ʹʹʹ), 4.78(1H, d,J= 7.1 Hz, H-1ʹ), 1.74 (3H, d,J= 6.1 Hz, H3-6ʹʹʹ), 1.61 (3H, d,J= 6.2 Hz, H3-6ʺ), 1.10 (3H, d,J=6.9 Hz, H3-21), 0.94 (3H, s, H3-19), 0.89 (3H, s, H3-18),0.67 (3H, d,J= 5.0 Hz, H3-27);13C-NMR (200 MHz,pyridine-d5)δ: 135.2 (C-6), 130.2 (C-7), 109.0 (C-22),102.3 (C-1ʹʹʹ), 101.5 (C-1ʺ), 100.3 (C-1ʹ), 81.9 (C-5),80.4 (C-16), 78.3 (C-8), 77.9 (C-4ʹ), 77.2 (C-2ʹ), 76.2(C-5ʹ), 73.5 (C-4ʹʹʹ), 73.5 (C-4ʺ), 73.3 (C-3), 72.2 (C-3ʺ), 72.1 (C-3ʹʹʹ), 72.0 (C-2ʹʹʹ), 71.9 (C-2ʺ), 69.9 (C-5ʺ),69.0 (C-5ʹʹʹ), 66.4 (C-26), 62.2 (C-17),60.6 (C-6ʹ), 51.3(C-14), 51.0 (C-9), 41.9 (C-13), 41.0 (C-20), 39.1 (C-12), 37.0 (C-10), 34.5 ( C-1), 33.9 (C-4), 31.3 (C-2),30.0 (C-25), 29.5 (C-23), 28.7 (C-15), 28.5 (C-24), 22.8(C-11), 18.1 (C-6ʹʹʹ), 18.0 (C-19), 17.8 (C-27), 16.8 (C-18), 16.5 (C-6ʺ), 14.4 (C-21)。以上数据与文献报道数据基本一致[10],故鉴定化合物5 为parisvanioside B。
化合物 6:白色无定型粉末,分子式为C44H66O17,,ESI-MSm/z:889 [M+Na]+。1H-NMR (800 MHz, pyridine-d5)δ:6.41 (1H, s, H-1ʺ), 6.29 (1H, s, H-6), 5.92 (1H, s, H-1ʹʹʹ), 4.95 (1H, d,J= 7.5 Hz, H-1ʹ), 1.79 (3H, d,J= 6.0 Hz, H3-6ʺ), 1.33 (1H, s, H-19), 1.13 (3H, d,J= 7.0 Hz,H3-21), 0.83 (3H, s, H3-18), 0.67 (3H, d,J= 5.2 Hz, H3-27);13C-NMR (200 MHz, pyridine-d5)δ: 185.3 (C-7),161.1 (C-5), 133.0 (C-9), 126.7 (C-6), 109.3 (C-1ʹʹʹ),109.1 (C-22), 102.6 (C-1ʺ), 101.5 (C-1ʹ) 86.4 (C-4ʹʹʹ),82.4 (C-2ʹʹʹ), 81.8 (C-16), 77.9 (C-4ʹ), 77.6 (C-3), 77.3(C-3ʹʹʹ), 76.7 (C-3ʹ), 76.7 (C-2ʹ), 76.7 (C-5ʹ), 73.8 (C-4ʺ), 72.4 (C-3ʺ), 72.3 (C-2ʺ), 69.2 (C-5ʺ), 66.5 (C-26),62.2 (C-5ʹʹʹ), 61.1 (C-6ʹ), 60.2 (C-17), 47.9 (C-14), 42.1(C-10), 42.0 (C-20), 40.4 (C-13), 38.4 (C-4), 35.5 (C-12), 34.4 (C-1), 32.9 (C-15), 31.6 (C-23), 31.5 (C-25),30.3 (C-2), 29.7 (C-24), 23.9 (C-11), 23.2 (C-19), 18.4(C-6ʺ), 18.2 (C-27), 17.0 (C-18), 15.8 (C-19)。以上数据与文献报道数据基本一致[14],故鉴定化合物6 为parisrugoside H。
化合物 7:白色无定型粉末,分子式为C45H72O18,,ESI-MSm/z:923 [M+Na]+。1H-NMR (800 MHz, pyridine-d5)δ:6.38 (1H, s, H-1ʺ), 5.28 (1H, brd,J= 4.1 Hz, H-6), 5.01(1H, d,J= 7.5 Hz, H-1ʹ), 1.76 (3H, d,J= 6.3 Hz, H3-6ʺ), 1.23 (3H, d,J= 7.2 Hz, H3-21), 1.05 (3H, s, H-19),0.95 (3H, s, H3-18), 0.67 (3H, d,J= 6.0 Hz, H3-27);13C-NMR (200 MHz, pyridine-d5)δ: 140.5 (C-5), 121.5(C-6), 109.6 (C-22), 104.2 (C-1ʹʹʹ), 101.5 (C-1ʺ), 101.0(C-1ʹ), 89.9 (C-17), 89.7 (C-16), 82.4 (C-4ʹ),79.4 (C-5ʹʹʹ), 78.0 (C-3ʹʹʹ), 77.6 (C-3), 77.6 (C-3ʹ), 77.4 (C-2ʹ),77.0 (C-5ʹ), 74.7 (C-2ʹʹʹ), 73.9 (C-4ʺ), 72.6 (C-3ʺ), 72.3(C-2ʺ), 71.5 (C-4ʹʹʹ), 69.2 (C-5ʺ), 66.6 (C-26), 62.4 (C-6ʹʹʹ) , 62.2 (C-6ʹ), 52.7 (C-14), 49.9 (C-9), 44.9 (C-20),44.5 (C-13), 38.7 (C-4), 37.3 (C-1), 36.9 (C-10), 32.1(C-7), 31.8 (C-15), 31.5 (C-12), 31.4 (C-8), 30.3 (C-25), 30.1 (C-2), 29.9 (C-23), 28.5 (C-24), 20.7 (C-11),19.2 (C-19), 18.4 (C-6ʺ), 17.0 (C-27), 16.9 (C-18), 9.5(C-21)。以上数据与文献报道数据基本一致[15],故鉴定化合物7 为 (25R)-17α-羟基螺甾-5-烯-3β-基O-β-D-吡喃葡萄糖基-(1→4)-O-[α-L-吡喃鼠李糖基-(1→2)]-β-D-吡喃葡萄糖苷。
化合物 8:白色无定型粉末,分子式为C39H62O12,,ESI-MSm/z:745 [M+Na]+。1H-NMR (500 MHz, pyridine-d5)δ:6.36 (1H, d,J= 1.0 Hz, H-1ʺ), 5.29 (1H, brd,J= 5.5 Hz, H-6), 5.02 (1H, d,J= 7.2 Hz, H-1ʹ), 1.76 (3H, d,J= 6.2 Hz, H3-6ʺ), 1.12 (3H, d,J= 7.0 Hz, H3-21), 1.03(3H, s, H3-19), 0.80 (3H, s, H3-18), 0.67 (3H, d,J= 5.5 Hz, H3-27;13C-NMR (125 MHz, pyridine-d5)δ: 140.9(C-5), 121.8 (C-6), 109.3 (C-22), 102.1 (C-1ʺ), 100.4(C-1ʹ), 81.2 (C-16), 79.7 (C-2ʹ), 78.3 (C-3), 78.0 (C-3ʹ),77.9 (C-5ʹ), 74.2 (C-4ʺ), 72.9 (C-3ʺ), 72.6 (C-2ʺ), 71.8(C-4ʹ), 69.6 (C-5ʺ), 66.9 (C-26), 62.9 (C-17), 62.9 (C-6ʹ), 56.7 (C-14), 50.3 (C-9), 42.0 (C-20), 40.5 (C-13),39.9 (C-12), 39.0 (C-4), 37.5 (C-1), 37.2 (C-10), 32.3(C-7), 32.3 (C-15), 31.9 (C-23), 31.7 (C-8), 30.6 (C-25), 30.2 (C-2), 29.3 (C-24), 21.2 (C-11), 19.5 (C-19),18.7 (C-6ʺ), 17.4 (C-27), 16.4 (C-18), 15.1 (C-21)。以上数据与文献报道数据基本一致[16],故鉴定化合物8 为麦冬皂苷Cʹ。
化合物 9:白色无定型粉末,分子式为C45H72O17,,ESI-MSm/z:907 [M+Na]+。1H-NMR (500 MHz, pyridine-d5)δ:6.40 (1H, brs, H-1ʺ), 5.30 (1H, brd,J= 5.1 Hz, H-6),5.11 (1H, d,J= 7.8 Hz, H-1ʹʹʹ), 4.95 (1H, d,J= 7.2 Hz,H-1ʹ), 3.96 (1H, m, H-3), 1.77 (3H, d,J= 6.2 Hz, H3-6ʺ), 1.14 (3H, d,J= 7.0 Hz, H3-21), 1.10 (3H, s, H3-19), 0.82 (3H, s, H3-18), 0.69 (3H, d,J= 5.1 Hz, H3-27);13C-NMR (125 MHz, pyridine-d5)δ: 140.8 (C-5),121.9 (C-6), 109.3 (C-22), 104.6 (C-1ʹʹʹ), 102.3 (C-1ʺ),100.0 (C-1ʹ), 89.6 (C-3ʹ), 81.1 (C-16), 78.8 (C-5ʹʹʹ), 78.6(C-3ʹʹʹ), 77.9 (C-3), 77.7 (C-5ʹ), 77.0 (C-2ʹ), 75.0 (C-2ʹʹʹ), 74.2 (C-4ʺ), 72.8 (C-3ʺ), 72.5 (C-2ʺ), 71.5 (C-4ʹʹʹ),69.6 (C-4ʹ), 69.6 (C-5ʺ), 66.9 (C-26), 62.9 (C-17), 62.4(C-6ʹʹʹ), 62.4 (C-6ʹ), 56.7 (C-14), 50.3 (C-9), 42.0 (C-20), 40.5 (C-13), 39.9 (C-12), 38.7 (C-4), 37.5 (C-1),37.1 (C-10), 32.3 (C-7), 32.2 (C-15), 31.8 (C-8), 31.7(C-23), 30.6 (C-25), 30.1 (C-2), 29.3 (C-24), 21.1 (C-11), 19.4 (C-19), 18.7 (C-6ʺ), 17.4 (C-27), 16.4 (C-18), 15.1 (C-21)。以上数据与文献报道数据基本一致[17-18],故鉴定化合物9 为纤细薯蓣皂苷。
化合物10:无色针状结晶(甲醇),mp 276~278 ℃,分子式为C44H70O16,,ESI-MSm/z: 877 [M+Na]+。1H-NMR (500 MHz, pyridine-d5)δ: 6.28 (1H, d,J= 1.1 Hz, H-1ʺ),5.92 (1H, d,J= 1.5 Hz, H-1ʹʹʹ), 5.29 (1H, brd,J= 5.5 Hz, H-6), 1.76 (3H, d,J= 6.2 Hz, H3-6ʺ), 1.12 (3H, d,J= 6.2 Hz, H3-21), 1.03 (3H, s, H3-19), 0.81 (3H, s, H3-18), 0.68 (3H, d,J= 5.5 Hz, H3-27);13C-NMR (126 MHz, pyridine-d5)δ: 140.8 (C-5), 121.8 (C-6), 109.6(C-1ʹʹʹ), 109.3 (C-22), 102.0 (C-1ʺ), 100.2 (C-1ʹ), 86.7(C-4ʹʹʹ), 82.7 (C-16), 81.1 (C-2ʹʹʹ), 78.1 (C-3), 77.9 (C-5ʹ), 77.7 (C-3ʹʹʹ), 77.4 (C-2ʹ), 77.0 (C-4ʹ), 76.7 (C-3ʹ),74.2 (C-4ʺ), 72.8 (C-3ʺ), 72.5 (C-2ʺ), 69.5 (C-5ʺ), 66.9(C-26), 62.9 (C-17), 62.5 (C-5ʹʹʹ), 61.4 (C-6ʹ), 56.6 (C-14),50.3 (C-9), 42.0 (C-20), 40.5 (C-13), 39.9 (C-12), 39.0 (C-4), 37.5 (C-1), 37.2 (C-10), 32.3 (C-7), 32.2 (C-15), 31.8(C-23), 31.7 (C-8), 30.6 (C-25), 30.2 (C-2), 29.3 (C-24),21.1 (C-11), 19.4 (C-19), 18.7 (C-6ʺ), 17.3 (C-27), 16.4(C-18), 15.1 (C-21)。以上数据与文献报道数据基本一致[19-20],故鉴定化合物10 为重楼皂苷I。
化合物 11:白色无定型粉末,分子式为C51H82O20,,ESI-MSm/z:1037 [M+Na]+。1H-NMR (500 MHz, pyridine-d5)δ:6.41 (1H, brs, H-1ʺ), 6.30 (1H, brs, H-1ʹʹʹʹ), 5.85 (1H,brs, H-1ʹʹʹ), 5.31 (1H, brd,J= 5.0 Hz, H-6), 4.95 (1H,d,J= 7.0 Hz, H-1ʹ), 1.77 (3H, d,J= 6.2 Hz, H3-6ʺ),1.60 (1H, d,J= 2.7 Hz, H3-6ʹʹʹ), 1.59 (1H, d,J= 2.8 Hz,H3-6ʹʹʹʹ), 1.14 (3H, d,J= 6.9 Hz, H3-21), 1.05 (3H, s,H3-19), 0.83 (3H, s, H3-18), 0.69 (3H, d,J= 5.2 Hz, H3-27);13C-NMR (125 MHz, pyridine-d5)δ: 140.8 (C-5),121.9 (C-6), 103.4 (C-1ʹʹʹ), 102.3 (C-1ʺ), 102.3 (C-1ʹʹʹʹ),100.4 (C-1ʹ), 81.2 (C-16), 80.5 (C-2ʹ), 78.1 (C-5ʹ), 78.0(C-4ʹʹʹ), 77.8 (C-4ʹ), 77.7 (C-3), 77.1 (C-3ʹ), 74.2 (C-4ʺ), 74.1 (C-4ʹʹʹʹ), 73.4 (C-2ʺ), 72.9 (C-2ʹʹʹʹ), 72.7 (C-3ʺ), 72.6 (C-3ʹʹʹʹ), 70.5 (C-3ʹʹʹ), 69.6 (C-5ʺ), 68.4 (C-5ʹʹʹ), 66.9 (C-26), 62.9 (C-17), 61.3 (C-6ʹ), 56.7 (C-14),50.3 (C-9), 42.0 (C-20), 40.5 (C-13), 39.9 (C-12), 39.0(C-4), 37.6 (C-1), 37.2 (C-10), 32.4 (C-7), 32.3 (C-15),31.9 (C-8), 31.7 (C-23), 30.7 (C-25), 30.2 (C-2), 29.3(C-24), 21.2 (C-11), 19.5 (C-19), 19.0 (C-6ʺ), 18.7 (C-6ʹʹʹ), 18.5 (C-6ʹʹʹʹ), 17.4 (C-27), 16.4 (C-18), 15.1 (C-21)。以上数据与文献报道数据基本一致[21],故鉴定化合物11 为重楼皂苷Ⅱ。
化合物12:白色针状结晶(甲醇),mp 261~264 ℃,分子式为C39H62O13,,ESI-MSm/z: 761 [M+Na]+。1H-NMR (500 MHz, pyridine-d5)δ: 6.38 (1H, brs, H-1ʺ), 5.28 (1H,brd,J= 5.0 Hz, H-6), 1.77 (3H, d,J= 6.3 Hz, H3-6ʺ),1.20 (3H, d,J= 7.1 Hz, H3-21), 1.08 (3H, s, H3-19),0.95 (3H, s, H3-18), 0.68 (3H, d,J= 5.6 Hz, H3-27);13C-NMR (125 MHz, pyridine-d5)δ: 140.8 (C-5), 121.8(C-6), 109.8 (C-22), 102.1 (C-1ʺ), 100.3 (C-1ʹ), 90.2(C-17), 90.0 (C-16), 79.7 (C-2ʹ), 78.3 (C-5ʹ), 77.9 (C-3), 77.8 (C-3ʹ), 74.2 (C-4ʺ), 72.9 (C-2ʺ), 72.6 (C-3ʺ),71.8 (C-4ʹ), 69.5 (C-5ʺ), 66.7 (C-26), 62.7 (C-6ʹ) , 53.0(C-14), 50.2 (C-9), 45.2 (C-13), 44.8 (C-20), 39.0 (C-4), 37.6 (C-1), 37.2 (C-10), 32.5 (C-7), 32.4 (C-8), 32.1(C-15), 31.8 (C-12), 31.8 (C-23), 30.4 (C-25), 30.2 (C-2), 28.8 (C-24), 21.0 (C-11), 19.5 (C-19), 18.7 (C-6ʺ),17.3 (C-27), 17.2 (C-18), 9.8 (C-21)。以上数据与文献报道数据基本一致[22],故鉴定化合物12 为重楼皂苷Ⅵ。
按表1皮肤反应分级标准记录反应结果,32例受试者中出现1级皮肤不良反应的人数多于5例,或2级皮肤不良反应的人数多于2例,或出现任何1例3级或3级以上皮肤不良反应时,判定受试物对人体有皮肤不良反应。
化合物 13:白色无定型粉末,分子式为C45H72O17,,ESI-MSm/z:907 [M+Na]+。1H-NMR (500 MHz, pyridine-d5)δ:6.35 (1H, brs, H-1ʺ), 5.81 (1H, brs, H-1ʹʹʹ), 1.71 (3H, d,J= 6.2 Hz, H3-6ʺ), 1.60 (3H, d,J= 6.1 Hz, H3-6ʹʹʹ), 1.21(3H, d,J= 7.1 Hz, H3-27), 1.05 (3H, s, H3-19), 0.93(3H, s, H3-18), 0.66 (3H, d,J= 5.7 Hz, H3-21);13CNMR (125 MHz, pyridine-d5)δ: 140.8 (C-5), 121.9 (C-6), 109.9 (C-22), 102.9 (C-1ʹʹʹ), 102.1 (C-1ʺ), 100.2 (C-1ʹ), 90.2 (C-17), 90.0 (C-16), 78.6 (C-2ʹ),78.1 (C-4ʹ),78.1 (C-5ʹ), 77.9 (C-3), 76.9 (C-3ʹ), 74.1 (C-4ʹʹʹ), 73.9(C-4ʺ), 72.8 (C-2ʺ), 72.8 (C-3ʺ), 72.7 (C-2ʹʹʹ), 72.5 (C-3ʹʹʹ), 70.4 (C-5ʹʹʹ), 69.5 (C-5ʺ), 66.7 (C-26), 62.5 (C-6ʹ),53.0 (C-14), 50.2 (C-9), 45.2 (C-13), 44.8, (C-20), 38.9(C-4), 37.5 (C-10), 37.1 (C-1), 32.4 (C-12), 32.3 (C-7),32.1 (C-8), 32.1 (C-23), 31.8 (C-15), 30.4 (C-25), 30.1(C-2), 28.8 (C-24), 20.9 (C-11), 19.4 (C-19), 18.6 (C-6ʹʹʹ), 18.5 (C-6ʺ), 17.3 (C-27), 17.2 (C-18), 9.8 (C-21)。以上数据与文献报道数据基本一致[23],故鉴定化合物13 为pennogenin 3-O-β-chacotrioside。
化合物 14:白色无定型粉末,分子式为C44H70O17,,ESI-MSm/z:893 [M+Na]+。1H-NMR (500 MHz, pyridine-d5)δ:6.28 (1H, brs, H-1ʺ), 5.92 (1H, brs, H-1ʹʹʹ), 5.28 (1H,brd,J= 3.8 Hz, H-6), 4.94 (1H,J= 7.6 Hz, H-1ʹ), 1.75(3H,J= 6.3 Hz, H3-6ʺ), 1.22 (3H, d,J= 7.2 Hz, H3-21), 1.08 (3H, s, H3-19), 0.94 (3H, s, H3-18), 0.67 (3H,d,J= 5.9 Hz, H3-27);13C-NMR (125 MHz, pyridined5)δ: 140.8 (C-5), 121.9 (C-6), 109.9 (C-1ʹʹʹ), 109.7 (C-22), 102.0 (C-1ʺ), 100.2 (C-1ʹ), 90.2 (C-17), 90.1 (C-16), 86.7 (C-4ʹʹʹ), 82.7 (C-2ʹʹʹ), 78.1 (C-3ʹʹʹ), 77.9 (C-3),77.8 (C-3ʹ), 77.5 (C-2ʹ) , 77.0 (C-5ʹ), 76.8 (C-4ʹ), 74.2(C-4ʺ), 72.9 (C-3ʺ), 72.5 (C-2ʺ), 69.6 (C-5ʺ), 66.8 (C-26), 62.5 (C-5ʹʹʹ), 61.4 (C-6ʹ), 53.1 (C-14), 50.3 (C-9),45.2 (C-13), 44.8 (C-20), 39.0 (C-4), 37.6 (C-1), 37.2(C-10), 32.5 (C-15), 32.4 (C-7), 32.1 (C-12), 32.1 (C-23), 31.9 (C-8), 30.5 (C-25), 30.2 (C-2), 28.9 (C-24),21.0 (C-11), 19.5 (C-19), 18.7 (C-6ʺ), 17.4 (C-27), 17.2(C-18), 9.8 (C-21)。以上数据与文献报道数据基本一致[24],故鉴定化合物14 为重楼皂苷H。
化合物15:白色针状结晶(甲醇),mp 263~265 ℃,化学式为C51H82O21,,ESI-MSm/z: 1053 [M+Na]+。1H-NMR (500 MHz, pyridine-d5)δ: 6.41 (1H, brs, H-1ʺ), 6.29 (1H,brs, H-1ʹʹʹʹ), 5.85 (1H, brs, H-1ʹʹʹ), 5.32 (1H, brd,J= 6.0 Hz, H-6), 1.76 (3H, d,J= 6.3 Hz, H3-6ʺ), 1.60 (3H, d,J= 6.0 Hz, H3-6ʹʹʹʹ), 1.59 (3H, d,J= 6.0 Hz, H3-6ʹʹʹ),1.22 (3H, d,J= 7.3 Hz, H3-21), 1.08 (3H, s, H3-19),0.95 (3H, s, H3-18), 0.67 (1H, d,J= 5.6 Hz, H3-27);13C-NMR (125 MHz, pyridine-d5)δ: 140.8 (C-5), 121.9(C-6), 109.8 (C-22), 103.4 (C-1ʹʹʹ), 102.2 (C-1ʺ), 102.2(C-1ʹʹʹʹ), 100.3 (C-1ʹ), 90.2 (C-17), 90.0 (C-16), 80.4(C-4ʹʹʹ), 78.0 (C-2ʹ), 78.0 (C-4ʹ), 77.8 (C-3), 77.7 (C-3ʹ),77.0 (C-5ʹ), 74.2 (C-4ʺ), 74.1 (C-4ʹʹʹʹ), 73.3 (C-3ʹʹʹ),72.9 (C-3ʺ), 72.9 (C-2ʹʹʹ), 72.9 (C-3ʹʹʹʹ), 72.7 (C-2ʹʹʹʹ),72.6 (C-2ʺ), 70.5 (C-5ʺ"), 69.6 (C-5"), 68.3 (C-5ʹʹʹ),66.7 (C-26), 61.2 (C-6ʹ), 53.1 (C-14), 50.3 (C-9), 45.2(C-13), 44.8 (C-20), 39.0 (C-4), 37.6 (C-1), 37.6 (C-12), 37.2 (C-10), 32.5 (C-7), 32.4 (C-15), 32.1 (C-23),31.8 (C-8), 30.5 (C-25), 30.2 (C-2), 28.8 (C-24), 21.0(C-11), 19.5 (C-19), 18.9 (C-6""), 18.7 (C-6"), 18.5 (C-6ʹʹʹ), 17.3 (C-27), 17.3 (C-18), 9.8 (C-21)。以上数据与文献报道数据基本一致[25],故鉴定化合物15 为重楼皂苷Ⅶ。
化合物 16:白色无定型粉末,分子式为C27H44O7,,ESI-MSm/z:503 [M+Na]+, 984 [2M+Na]+。1H-NMR (500 MHz,pyridine-d5)δ: 6.24 (1H, d,J=2.2 Hz, H-7), 4.22 (1H,m, H-3), 4.19 (1H, m, H-2), 3.87 (1H, m, H-22), 3.58(1H, m, H-9), 3.00 (1H, m, H-5), 2.99 (1H, m, H-17),1.58 (3H, s, H3-21), 1.36 (6H, s, H3-26,27), 1.21 (3H, s,H3-18), 1.06 (3H, s, H3-19);13C-NMR (125 MHz,pyridine-d5)δ: 203.6 (C-6), 166.2 (C-8), 121.7 (C-7),84.2 (C-14), 77.6 (C-22), 76.9 (C-20), 69.6 (C-25), 68.2(C-2), 68.1 (C-3), 51.5 (C-5), 50.2 (C-17), 48.2 (C-13),42.7 (C-24), 38.7 (C-10), 38.0 (C-1), 34.5 (C-9), 32.5(C-4) ,32.1 (C-12), 31.8 (C-15), 30.2 (C-27), 30.0 (C-26), 27.5 (C-23), 24.5 (C-19), 21.7 (C-21), 21.5 (C-16),21.2 (C-11), 18.0 (C-18)。以上数据与文献报道数据基本一致[26],故鉴定化合物16 为β-蜕皮激素。
4 抗细菌、真菌活性实验
对分到的化合物进行抗细菌和抗真菌活性实验,参照文献中的方法进行活性评价[27]。
4.1 抗细菌实验
将单体化合物溶于DMSO 中,放入96 孔板中稀释,在各孔中加入细菌菌液(大肠埃希氏菌ATCC25922、金黄色葡萄球菌金黄亚种ATCC29213、铜绿假单胞菌ATCC27853、耐甲氧西林金黄色葡萄球菌ATCC43300),终浓度为5×105CFU/mL;设置培养基为空白对照、细菌对照以及头孢他啶、青霉素G 钠阳性药对照。37 ℃培养24 h,酶标仪检测625 nm 吸光度(A)值,计算细菌抑制率。
细菌抑制率=(A空白对照-A实验)/A空白对照
结果显示,化合物1 和3~16 均对大肠埃希氏菌ATCC25922、金黄色葡萄球菌金黄亚种ATCC29213、铜绿假单胞菌ATCC27853、耐甲氧西林金黄色葡萄球菌ATCC43300 无明显抑制作用,在化合物浓度为100 μmol/L 时,其抑制率低于50%。
4.2 抗真菌实验
将化合物溶于DMSO 中,放入96 孔板中稀释,在各孔中加入真菌菌液(絮状表皮癣菌CBS 566.94、红色毛癣菌ATCC4438、石膏样小孢子菌CBS118893、白色念珠菌氟康唑耐药株),终浓度为1×105CFU/mL(白色念珠菌氟康唑耐药株)和5×105CFU/mL(丝状真菌);设置培养基为空白对照、真菌对照以及两性霉素B、盐酸特比萘芬阳性药对照。白色念珠菌氟康唑耐药株37 ℃培养24 h,丝状真菌25 ℃培养5 d,酶标仪检测625 nmA值,计算真菌抑制率。
真菌抑制率=(A空白对照-A实验)/A空白对照
当浓度为100 μmol/L 时,化合物3 对絮状表皮癣菌和石膏样小孢子菌抑制率为70%~74%;化合物5、6、8、9 对絮状表皮癣菌、红色毛癣菌和石膏样小孢子菌的抑制率为65%~96%;化合物7、10~15 对白色念珠菌氟康唑耐药株、絮状表皮癣菌、红色毛癣菌和石膏样小孢子菌的抑制率为54%~100%(表1)。故进一步对絮状表皮癣菌、红色毛癣菌和石膏样小孢子菌有抑制活性的化合物进行50%最低抑菌浓度(50% minimun inhibitory concentration,MIC50)测试,结果显示,化合物3、5~15 对絮状表皮癣菌有较强抑制作用(表2),MIC50为0.07~93.60 μmol/L;化合物5~15 对红色毛癣菌有较强抑制作用(表3),MIC50为0.06~73.08 μmol/L;化合物3、5~8、10~15 对石膏样小孢子菌有很强的抑制作用(表4),MIC50为0.04~69.92 μmol/L。

表1 腾冲重楼化合物对4 种真菌的抑制作用 (n = 3)Table 1 Inhibitory effects of compounds from P.tengchongensis on four strains of fungi (n = 3)
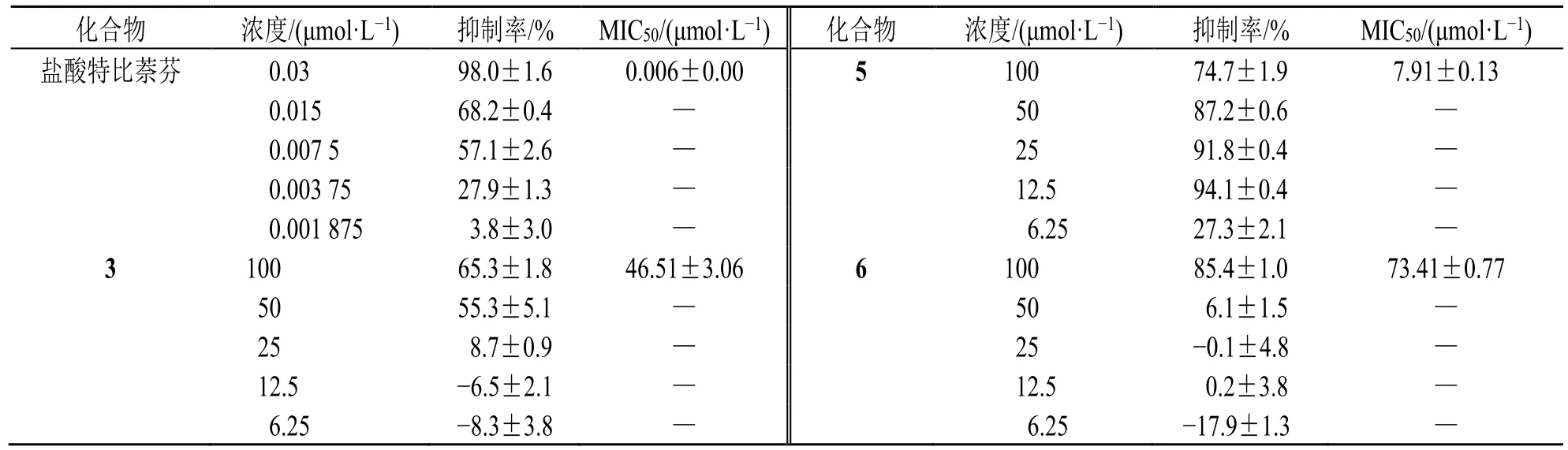
表2 化合物对絮状表皮癣菌的MIC50 值 (n = 3)Table 2 MIC50 values of compounds against Epidermophyton floccosum (n = 3)
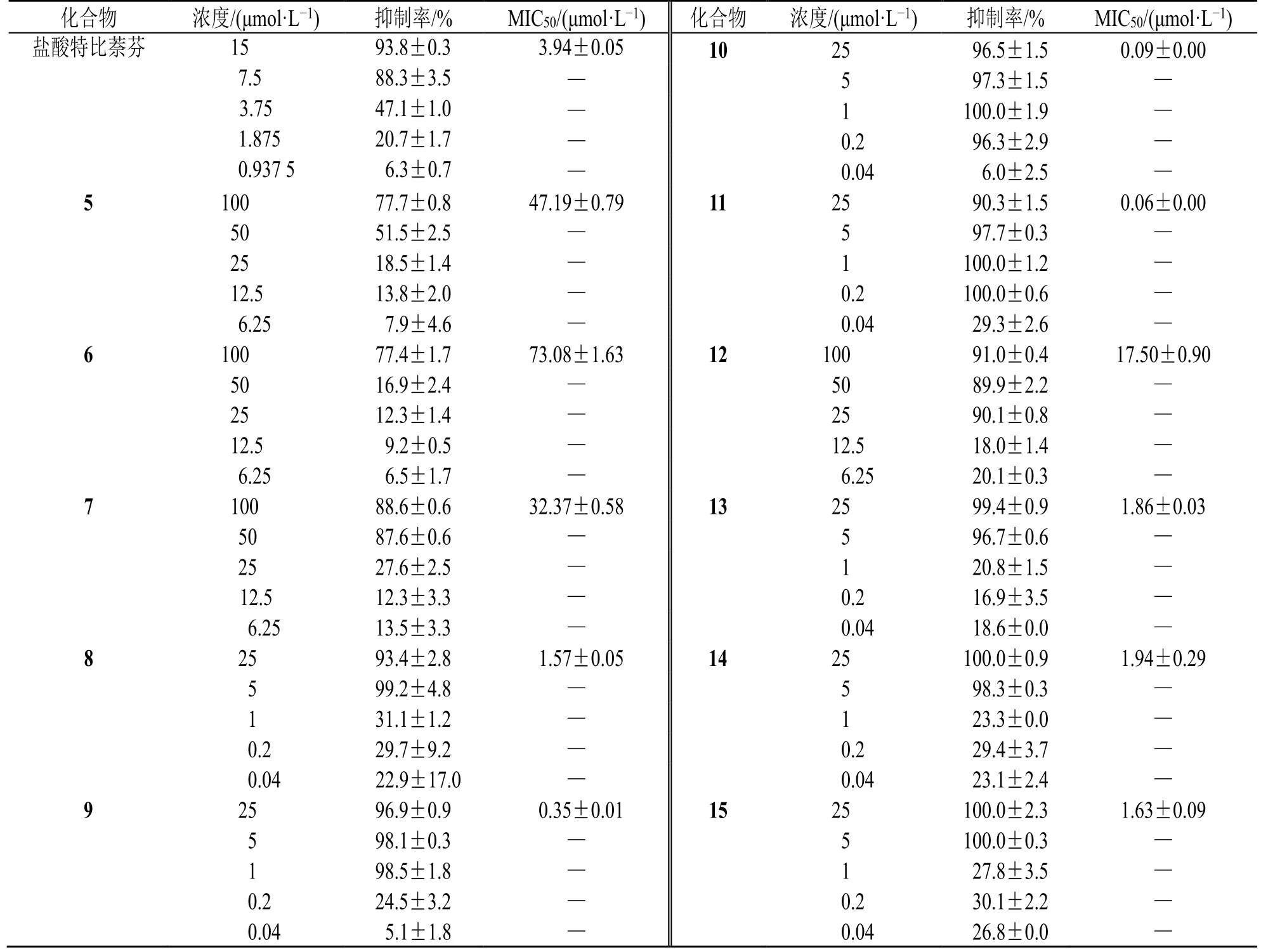
表3 化合物对红色毛癣菌的MIC50 值 (n = 3)Table 3 MIC50 values of compounds against Trichophyton rubrum of (n = 3)
5 讨论
为了更进一步发掘其利用价值,本研究对腾冲重楼正丁醇萃取物进行系统的研究。通过对腾冲重楼根茎的提取、分离,共得到16 个化合物,其中化合物1~7 为首次从腾冲重楼中分离得到,丰富了腾冲重楼的化学成分类型。
前人仅报道了腾冲重楼中化合物的细胞毒活性[9],本研究较系统地评价了该物种中化合物的抗细菌和抗真菌活性。研究显示滇重楼甲醇提取物可抑制宋内氏痢疾杆菌、粘质沙雷氏菌、大肠杆菌、金黄色葡萄球菌[28]。实验结果发现16 个化合物均对大肠埃希氏菌、金黄色葡萄球菌金黄亚种、铜绿假单胞菌、耐甲氧西林金黄色葡萄球菌无明显抑制作用。皮肤癣菌是常见的临床病原真菌,会引起人类头发、皮肤及指趾甲的感染,可用两性霉素B、盐酸特比萘芬、伊曲康唑、伏立康唑、泊沙康唑、艾沙康唑等药物进行治疗[29],但重楼皂苷进行抗皮肤癣菌方面的研究较少,故进行抗皮肤癣菌方面的研究。陆克乔等[30]的研究发现,滇重楼的正丁醇提取物能有效抑制体外白色念珠菌的生物膜形成,可抑制白色念珠菌。化合物7、12~15 对白色念珠菌氟康唑耐药株抑制率可达100%。化合物11 对絮状表皮癣菌、红色毛癣菌、石膏样小孢子菌抑菌效果最好,MIC50值分别为0.07、0.06、0.04 μmol/L。目前只对腾冲重楼进行抗菌活性的研究,后续可进行细胞毒活性等的测试,使对腾冲重楼的研究更加全面。腾冲重楼化合物对深部真菌白色念珠菌氟康唑耐药株和3 种皮肤癣菌有较好的抑制作用,为今后对腾冲重楼治疗皮肤及深部真菌感染疾病方面的开发利用提供参考。
利益冲突所有作者均声明不存在利益冲突