驱动压导向的个体化呼气末正压通气对行腹腔镜胃癌根治术老年患者的肺保护作用
钟晓倩 孙高悦 张倩倩 李云


摘要:目的 探討驱动压导向的个体化呼气末正压(PEEP)通气是否会对行腹腔镜胃癌根治术的老年患者的肺起到保护作用。方法 选取择期行腹腔镜胃癌根治术的老年患者64例,按照随机数字表法分为驱动压导向的个体化PEEP组(试验组)和固定PEEP组(对照组),每组32例。对照组PEEP为5 cmH2O;在气腹稳定后,试验组PEEP按照4~16 cmH2O依次递增滴定,每次进行10次呼吸循环并记录各个PEEP值最后1次呼吸循环时的驱动压,滴定结束后选择最低驱动压对应的PEEP持续至拔管。记录插管后5 min(T1)、PEEP滴定后即刻(T2)、手术开始1 h(T3)、手术开始2 h(T4)、气腹释放后10 min(T5)时的气道峰压(Ppeak)、气道平台压(Pplat)、PEEP;计算驱动压、肺动态顺应性(Cdyn);记录患者T1-5时的动脉血氧分压(PaO2),计算氧合指数(OI);评估患者术前及术后第1、3、5天的肺功能,记录术后第2天改良临床肺部感染评分(mCPIS)及术后7 d内肺部并发症(PPCs)的发生情况。结果 与T1时比较,T2-5时2组Ppeak、Pplat、驱动压均升高、Cdyn均降低,T4时对照组OI降低(P<0.05)。与对照组比较,T2-5时试验组Ppeak、Pplat、Cdyn升高,驱动压降低,T3-5时OI升高(P<0.05)。与术前比较,术后1 d、3 d、5 d 2组FVC均降低,术后1 d、3 d 2组FEV1、最大呼气流量(PEF)均降低(P<0.05)。与对照组比较,试验组术后1 d用力肺活量(FVC)、FEV1、PEF升高(P<0.05)。与术前比较,2组患者术后第2天mCPIS评分升高(P<0.05);与对照组比较,试验组术后第2天mCPIS评分降低(P<0.05)。术后7 d内试验组PPCs发生率低于对照组(15.6% vs. 40.6%)。结论 驱动压导向的个体化PEEP可提高肺顺应性,改善氧合功能和术后早期肺功能,并降低驱动压及术后肺部并发症的发生。
关键词:正压呼吸;腹腔镜检查;胃肿瘤;驱动压;个体化;老年
中图分类号:R614.2文献标志码:ADOI:10.11958/20230480
Effect of individualized positive end-expiratory pressure guided by driving pressure on lung protection after laparoscopic radical gastrectomy in elderly patients
Abstract: Objective To explore the effect of individualized positive end expiratory pressure guided by driving pressure on lung protection after laparoscopic radical gastrectomy for elderly patients. Methods A total of 64 patients underwent elective laparoscopic radical gastrectomy for gastric cancer in the Second Affiliated Hospital of Anhui Medical University were selected. According to the random number table method, patients were divided into the driving the pressure guided individualized positive end-expiratory pressure (PEEP) group (experimental group) and the fixed PEEP group (control group), 32 cases in each group. In the control group , PEEP = 5 cmH2O. In the experimental group, PEEP titration was performed according to the increasing method, and the PEEP corresponding to the lowest driving pressure was selected until extubation. Peak airway pressure (Ppeak), plateau airway pressure (Pplat) and PEEP were recorded at 5 min after intubation (T1), immediately after PEEP titration (T2), 1 h after operation (T3), 2 h after operation (T4), and 10 min after pneumoperitoneum release (T5). Driving pressure (ΔP) and lung dynamic compliance (Cdyn) were calculated. Arterial blood was collected at T1-5 for blood gas analysis, arterial partial pressure of oxygen (PaO2) was recorded, and oxygenation index (OI) was calculated. The occurrence of pulmonary complications (PPCs) within 7 days after operation was recorded. Modified clinical pulmonary infection score (mCPIS) was recorded on the second day after operation. The pulmonary function was evaluated before operation, 1 day, 3 days and 5 days after operation. Results Compared with T1, Ppeak, Pplat and ΔP were increased and Cdyn was decreased at T2-5, while OI was decreased at T4 in control group (P<0.05). Compared with the control group , Ppeak, Pplat and Cdyn in the experimental group were increased at T2-5, ΔP was decreased, and OI was increased at T3-5 (P<0.05). Compared with the preoperative results, FVC at 1, 3 and 5 days after surgery was decreased, and FEV1 and maximum expiratory flow (PEF) were decreased 1 and 3 days after surgery in the experimental groups (P<0.05). Compared with the control group, FVC, FEV1 and PEF were higher 1 day after operation in the experimental group (P<0.05). Compared with the preoperative results, mCPIS scores of the two groups were higher on the second day after surgery (P<0.05). Compared with the control group, the mCPIS score was lower on day 2 after surgery in the experimental group (P<0.05). The incidence of PPCs within 7 days after surgery was lower in the experimental group than that in the control group (15.6% vs. 40.6%). Conclusion Individualized PEEP guided by drive pressure can improve lung compliance, reduce drive pressure, improve oxygenation function and early postoperative lung function, reduce the incidence of postoperative lung complications, and has a certain lung protection effect.
Key words: positive-pressure respiration; laparoscopy; stomach neoplasms; driving pressure; individualized; elderly
胃癌(gastric cancer,GC)是全球常见的恶性肿瘤之一,也是导致肿瘤患者死亡的重要原因[1]。2020年全球GC新增100多万例,死亡约76.9万例;而我国GC新发47.9万例,死亡37.4万例[2]。目前腹腔镜胃癌根治术凭借安全性高、出血量少、恢复时间短,已广泛应用于临床[3]。然而,在腹腔镜胃癌根治术中,由于CO2气腹的影响,使得肺容积减少、肺顺应性降低及气道压和驱动压升高,导致患者术后肺部并发症(postoperative pulmonary complications,PPCs)的发生风险增加[4]。近年有临床研究表明,优化潮气量和呼气末正压(PEEP)设置可通过降低驱动压(driving pressure,DP或ΔP)、改善术中氧合及降低PPCs的发生而发挥肺保护作用[5]。本研究拟探讨以最低驱动压为导向的个体化PEEP通气策略对腹腔镜胃癌根治术老年患者是否具有肺保护作用,并评估其PPCs的发生和肺功能情况,为优化老年患者围手术期肺保护性通气策略提供参考。
1 对象与方法
1.1 研究对象 选择2021年9月—2022年6月安徽医科大学第二附属医院择期行腹腔镜胃癌根治术患者64例。纳入标准:(1)年龄65~80岁。(2)美国麻醉医师协会(ASA)Ⅰ—Ⅲ级。(3)体质量指数(BMI)18.5~30.0 kg/m2。(4)手术时长≥3 h。(5)術前肺功能无明显异常。排除标准:(1)术前血氧饱和度≤0.9。(2)近4周有上呼吸道或肺部感染症状,胸部X线片提示肺炎。(3)肺部严重疾病,如慢性阻塞性肺疾病、胸腔积液、哮喘、气胸、影响肺及胸廓顺应性疾病。(4)既往有肺叶切除术等肺部手术史。(5)合并严重心脑血管疾病、肝肾功能障碍等。本研究已获本院伦理委员会批准(伦理号:YX2021-142),所有患者或家属签署知情同意书。
1.2 分组 采用随机数字表法将患者分为驱动压导向的个体化PEEP组(试验组)和固定PEEP组(对照组),每组32例。
1.3 麻醉方法 患者入室后建立外周静脉通路,连接心电监护,局麻下完成桡动脉及颈内静脉穿刺置管,常规监测心电图、有创血压、心率(HR)、脉搏血氧饱和度(SpO2)、平均动脉压(MAP)及脑电双频谱指数(BIS)。然后静脉注射依托咪酯0.4 mg/kg、舒芬太尼0.5 μg/kg。顺式阿曲库铵0.15 mg/kg行麻醉诱导,气管插管后连接麻醉机,进行机械通气。麻醉维持采用全凭静脉麻醉,丙泊酚4~8 mg/(kg·h)、瑞芬太尼0.1~0.3 μg/(kg·min)、顺式阿曲库铵0.1~0.2 mg/(kg·h)持续泵注,根据麻醉深度调整丙泊酚和瑞芬太尼用量,维持BIS 40~60。术中控制MAP波动幅度不超过基础值±20%,术中输液以晶体液为主,胶体液为羟乙基淀粉(晶体︰胶体=2︰1);必要时单次静脉注射去氧肾上腺素40 μg维持循环稳定。术中气腹压力维持10~12 mmHg(1 mmHg=0.133 kPa),术毕前30 min停用顺式阿曲库铵,并静脉注射舒芬太尼0.2 μg/kg镇痛及甲氧氯普胺10 mg预防术后恶心呕吐。待患者自主呼吸恢复后静脉注射阿托品0.5 mg+新斯的明1 mg拮抗残余肌松;达到拔管指征后拔除气管导管。术后患者均行自控静脉镇痛(PCIA),配方为舒芬太尼150 μg+甲氧氯普胺20 mg+生理盐水配成100 mL,首次剂量2 mL,背景剂量2 mL/h,单次剂量0.8 mL,锁定时间15 min。
1.4 术中通气管理 2组机械通气模式均为压力控制-容量保证通气(pressure-controlled ventilation-volume guaranteed,PCV-VG)模式,潮气量(VT)6 mL/kg,吸入氧(FiO2)60%,吸呼比1︰2,呼吸频率12~20次/min,通过调整呼吸频率控制呼气末二氧化碳分压(PETCO2)在35~45 mmHg。气腹后2组均行手法肺复张,设置可调压力限制阀为40 cmH2O(1 cmH2O=0.098 kPa),通过挤压球囊使肺泡复张,重复3次,每次持续30 s。试验组:肺复张后PEEP按照4、6、8、10、12、14、16 cmH2O依次递增,每个PEEP水平维持10次呼吸循环,记录最后1次呼吸循环对应的驱动压,选择最低驱动压对应的PEEP[6],直至拔管。对照组:肺复张后PEEP设置为5 cmH2O。
1.5 观察指标 记录2组患者插管后5 min(T1)、PEEP滴定后即刻(T2)、手术开始1 h(T3)、手术开始2 h(T4)、气腹释放10 min(T5)时的气道峰压(Ppeak)、气道平台压(Pplat)、PEEP;计算驱动压(ΔP=Pplat-PEEP)、肺动态顺应性Cdyn=VT/ΔP;分别于T1-5采集动脉血进行血气分析,记录动脉血氧分压(PaO2),计算氧合指数(OI=PaO2/FiO2);评估术前及术后第1、3、5天肺功能,包括用力肺活量(FVC)、第1秒用力呼气容积(FEV1)及最大呼气流量(PEF);记录术后第2天改良临床肺部感染评分(mCPIS);记录术后7 d内PPCs发生情况。
1.6 统计学方法 采用SPSS 24.0软件进行数据分析。服从正态分布的计量资料以均数±标准差([x]±s)表示,组间比较采用两独立样本t检验,重复测量数据采用重复测量资料的方差分析;计数资料以例(%)表示,组间比较采用χ2检验。P<0.05为差异有统计学意义。
2 结果
2.1 一般情况比较 2组患者的基线特征、手术时间、术中输液量、血管活性药物使用情况等比较,差异均无统计学意义(P>0.05),见表1。
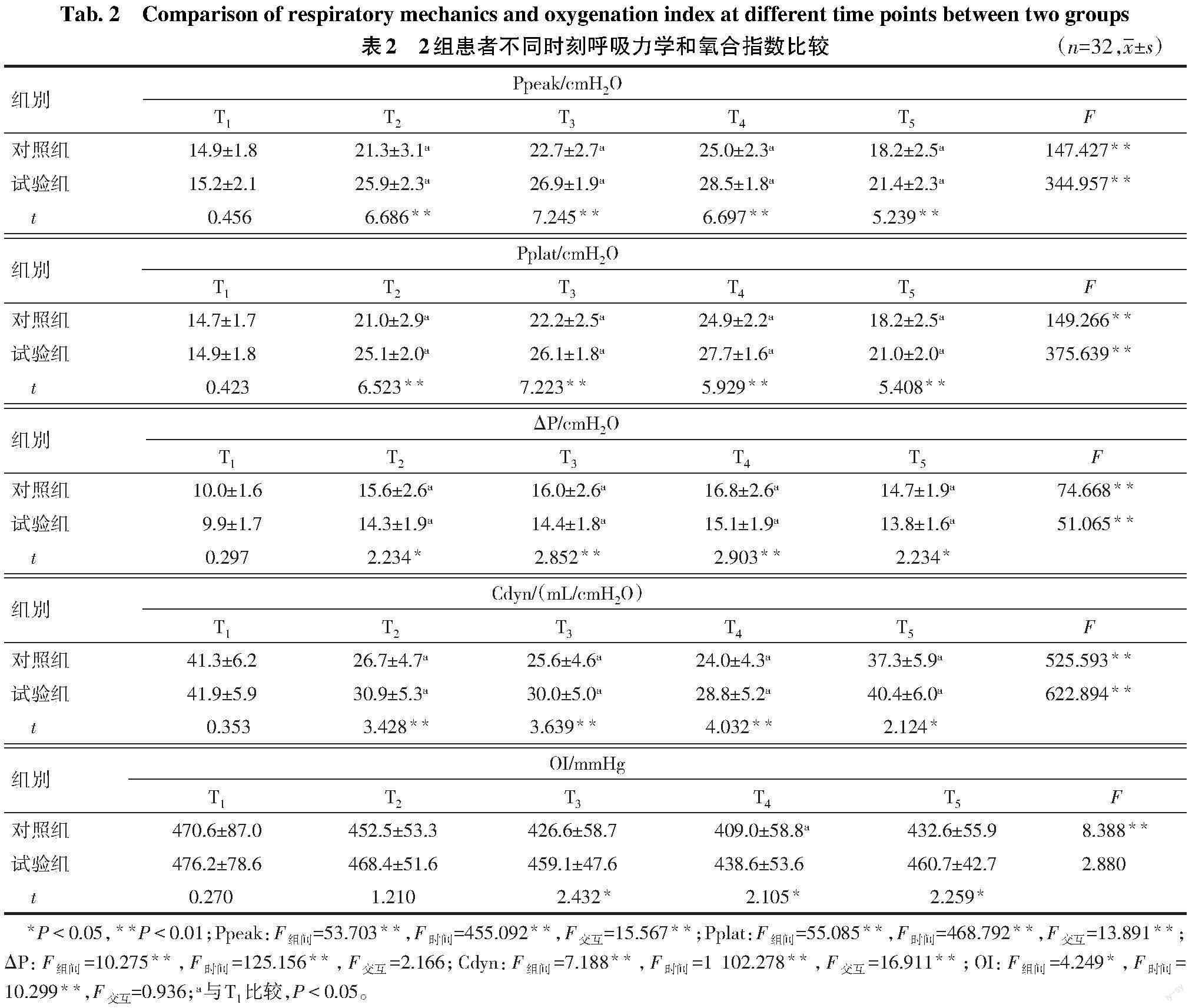
2.2 不同时刻呼吸力学和OI比较 与T1时比较,T2-5时2组Ppeak、Pplat、ΔP均升高,Cdyn均降低,T4时对照组OI降低(P<0.05)。与对照组比较,T2-5时试验组Ppeak、Pplat、Cdyn升高,ΔP降低,T3-5时OI升高(P<0.05),见表2。
2.3 术后肺功能比较 2组患者在术前FVC、FEV1、PEF差异均无统计学意义(P>0.05)。与术前比较,术后1 d、3 d、5 d 2组患者FVC均降低,术后1 d、3 d 2组FEV1、PEF均降低(P<0.05)。与对照组比较,试验组术后1 d FVC、FEV1及PEF升高(P<0.05),见表3。
2.4 术后第2天mCPIS比较 2组患者在术前mCPIS评分差异无统计学意义(P>0.05)。与术前相比,2组患者术后第2天mCPIS评分均升高(P<0.05);与对照组比较,试验组术后第2天mCPIS评分降低(P<0.05),见表4。
2.5 术后7 d内PPCs发生率 术后7 d内试验组肺不张及总体PPCs发生率低于对照组(P<0.05),见表5。
3 讨论
3.1 应用个体化PEEP的必要性 PPCs是导致患者预后不良的一个重要因素。在接受普通外科手术人群中PPCs发生率为2%~5.6%,在上腹部和胸部外科手术为20%~70%[7]。值得注意的是,术后第1周发生死亡的病例中有接近25%与PPCs相关[8]。全麻手术中零呼气末正压会导致呼气末肺容积显著减少和肺不张面积增加[9]。腹腔镜手术过程中CO2气腹使膈肌上移,可能使上述影响更为明显,因此术中PEEP的合理应用十分必要。國际专家共识[10]建议初始PEEP设置为5 cmH2O,之后的PEEP应进行个体化选择。本研究以ΔP为导向探索行腹腔镜胃癌根治术老年患者的最佳PEEP。
3.2 ΔP指导个体化PPEP滴定的可行性 ΔP是驱动呼吸系统的直接动力,对于机械通气患者,ΔP=Pplat-PEEP[11],CRS=VT/(Pplat-PEEP)。在潮气量不变的前提下,当ΔP最低时,肺顺应性达到最高。以最低ΔP通气可以避免肺泡过度扩张或通气不足,提高肺顺应性。荟萃分析显示全麻患者术中高ΔP与较高的PPCs发生率独立相关[12]。Park等[6]在一项胸科患者的前瞻性随机对照试验中发现,以ΔP为导向的个体化PEEP可降低PPCs发生率。曹鹏等[13]发现ΔP指导的个体化PEEP可以降低腹腔镜结肠癌根治术患者术后24 h白细胞介素6的浓度。因此,以ΔP为目标设置个体化PEEP具有一定的可行性。
3.3 与既往研究结果的比较 全麻机械通气期间施加适当的PEEP可使萎陷的肺泡重新扩张,增加功能残气量和肺顺应性,改善通气和氧合[14]。本研究中,在PEEP设定后的各时间点,试验组OI及Cydn均高于对照组、ΔP均低于对照组,这与Fernandez-Bustamante等[15]的研究结果相符。上述结果可能是因为在肺复张之后,5 cmH2O PEEP未能在CO2气腹存在的情况下维持肺泡的扩张状态,开放的肺泡再次塌陷,导致对照组Cydn降低、ΔP升高及OI下降。有研究发现,12 cmH2O PEEP相较于4 cmH2O PEEP未能降低肥胖患者PPCs的发生率[16],说明针对特殊类型手术(如腹腔镜手术)和特殊类型患者(如肥胖患者),固定高水平PEEP相较于固定低水平PEEP在降低PPCs发生率方面未显示出明显优势。然而,个体化PEEP相较于固定PEEP更有利于扩张萎陷的肺泡,同时避免肺泡的过度扩张,进而改善氧合及肺顺应性。目前认为术中ΔP安全边界的上限应小于18 cmH2O[5]。曲宗阳等[17]研究结果显示,ΔP>18 cmH2O是术后肺部并发症的危险因素。本研究中ΔP均在安全范围内。本研究结果显示2组术中输液量和去氧肾上腺素使用情况差异无统计学意义,表明个体化PEEP水平对术中各时间点血流动力学未产生明显影响。这与Nestler等[18]研究结果一致,分析原因可能与PEEP的滴定范围和患者术前心功能有关。本研究结果显示,个体化PEEP可降低术后7 d内PPCs的发生率,与Liu等[19]结果一致,且在发生PPCs的患者中,肺不张的发生率最高。闭玉华等[20]以ΔP为导向探讨个体化PEEP对腹腔镜结肠癌根治术老年患者的影响,其观察指标主要包括肺超声评分、炎性因子水平、术后呼吸相关并发症等,结果显示,在机械通气后5 min、干预后30 min及拔管前,观察组肺超声评分均低于对照组,且术后呼吸相关并发症总发生率低于对照组,与本研究结果一致。本研究中2组患者术后1 d FVC、FEV1、PEF均较术前降低,这可能与全身麻醉药物使用、CO2气腹建立、术后肌松残余、膈肌功能障碍及患者高龄等因素有关。但试验组术后1 d FVC较对照组升高,说明ΔP导向的个体化PEEP能在一定程度上减轻机械通气造成的肺损伤,进而改善术后早期肺功能。
临床肺部感染评分是以临床表现、影像学和微生物学等指标评估感染的测评方案,也用于评估肺部感染严重程度、评估疗效及感染预后[21]。本研究显示,2组术后第2天mCPIS评分均高于术前;但相较于对照组,ΔP导向的个体化PEEP可降低临床肺部感染评分。这与刘静等[22]研究结果一致;试验组术后7 d PPCs的发生率降低,说明个体化PEEP对腹腔镜胃癌根治术老年患者PPCs有一定的预防作用。
3.4 局限性 本研究尚存在一定的局限性:(1)通过胸X线片评估术后肺部情况不如胸部CT精确。(2)未对相关炎性因子进行定量检测。(3)在PEEP滴定过程中未使用电阻抗断层成像设备进行监测,无法观察在滴定过程中肺部通气分布的动态变化过程。(4)本研究为单中心、小样本量试验,且手术类型单一,故研究结果的普遍性有待进一步验证。
综上所述,ΔP导向的个体化PEEP可提高肺顺应性、改善氧合功能和术后早期肺功能,并能降低驱动压及术后肺部并发症的发生。
参考文献
[1] BRAY F,FERLAY J,SOERJOMATARAM I,et al. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2018,68(6):394-424. doi:10.3322/caac.21492.
[2] SUNG H,FERLAY J,SIEGEL R L,et al. Global Cancer Statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2021,71(3):209-249. doi:10.3322/caac.21660.
[3] KINOSHITA T,UYAMA I,TERASHIMA M,et al. Long-term outcomes of laparoscopic versus open surgery for clinical stage II/III gastric cancer:a multicenter cohort study in Japan (LOC-A Study)[J]. Ann Surg,2019,269(5):887-894. doi:10.1097/SLA.0000000000002768.
[4] MISKOVIC A,LUMB A B. Postoperative pulmonary complications[J]. Br J Anaesth,2017,118(3):317-334. doi:10.1093/bja/aex002.
[5] AMATO M B,MEADE M O,SLUTSKY A S,et al. Driving pressure and survival in the acute respiratory distress syndrome[J]. N Engl J Med,2015,372(8):747-755. doi:10.1056/NEJMsa1410639.
[6] PARK M,AHN H J,KIM J A,et al. Driving pressure during thoracic surgery: a randomized clinical trial[J]. Anesthesiology,2019,130(3):385-393. doi:10.1097/ALN.0000000000002600.
[7] LAKSHMINARASIMHACHAR A,SMETANA G W. Preoperative evaluation:estimation of pulmonary risk[J]. Anesthesiol Clin,2016,34(1):71-88. doi:10.1016/j.anclin.2015.10.007.
[8] DIAZ-FUENTES G,HASHMI H R,Venkatram S. Perioperative evaluation of patients with pulmonary conditions undergoing non-cardiothoracic surgery[J]. Health Serv Insights,2016,9(Suppl 1):9-23. doi:10.4137/HSI.S40541.
[9] OSTBERG E,THORISSON A,ENLUND M,et al. Positive end-expiratory pressure alone minimizes atelectasis formation in nonabdominal surgery:a randomized controlled trial[J]. Anesthesiology,2018,128(6):1117-1124. doi:10.1097/ALN.0000000000002134.
[10] YOUNG C C,HARRIS E M,VACCHIANO C,et al. Lung-protective ventilation for the surgical patient:international expert panel-based consensus recommendations[J]. Br J Anaesth,2019,123(6):898-913. doi:10.1016/j.bja.2019.08.017.
[11] 王金龍,黄英姿. 驱动压在急性呼吸窘迫综合征肺保护性通气中的研究进展[J]. 中华内科杂志,2018,57(10):766-768. WANG J L,HUANG Y Z. Research progress of driving pressure in lung protective ventilation in acute respiratory distress syndrome[J]. Chin J Intern Med,2018,57(10):766-768. doi:10.3760/cma.j.issn.0578-1426.2018.10.016.
[12] NETO A S,HEMMES S N,BARBAS C S,et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia:a meta-analysis of individual patient data[J]. Lancet Respir Med,2016,4(4):272-280. doi:10.1016/S2213-2600(16)00057-6.
[13] 曹鹏,宋正杰,程靜林,等. 驱动压导向的个体化呼气末正压通气对腹腔镜手术患者的肺保护作用[J]. 实用临床医药杂志,2021,25(10):40-44. CAO P,SONG Z J,CHENG J L,et al. Effect of individualized positive end expiratory pressure guided by driving pressure on lung protection in patients undergoing laparoscopic surgery[J]. Journal of Clinical Medicine in Practice,2021,25(10):40-44. doi:10.7619/jcmp.20210459.
[14] PEREIRA S M,TUCCI M R,MORAIS C C A,et al. Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis[J]. Anesthesiology,2018,129(6):1070-1081. doi:10.1097/ALN.0000000000002435.
[15] FERNANDEZ-BUSTAMANTE A,SPRUNG J,PARKER R A,et al. Individualized PEEP to optimise respiratory mechanics during abdominal surgery:a pilot randomised controlled trial[J]. Br J Anaesth,2020,125(3):383-392. doi:10.1016/j.bja.2020.06.030.
[16] Writing Committee for The PCGOTPVNFTCTNOTESOA,Bluth T,Serpa Neto A,Writing Committee for the PROBESE Collaborative Group of the PROtective VEntilation Network (PROVEnet) for the Clinical Trial Network of the European Society of Anaesthesiology,BLUTH T,SERPA NETO A,et al. Effect of intraoperative high positive end-expiratory pressure (PEEP) with recruitment maneuvers vs low PEEP on postoperative pulmonary complications in obese patients:a randomized clinical trial[J]. JAMA,2019,321(23):2292-2305. doi:10.1001/jama.2019.7505.
[17] 曲宗阳,周淑珍,包杰,等. 老年腹部大手术患者术后肺部并发症及相关因素分析[J]. 中华老年医学杂志,2020,39(9):1034-1037. QU Z Y,ZHOU S Z,BAO J,et al. Analysis of pulmonary complications and related factors in elderly patients following major abdominal surgery[J]. Chin J Geriatr,2020,39(9):1034-1037. doi:10.3760/cma.j.issn.0254-9026.2020.09.010.
[18] NESTLER C,SIMON P,PETROFF D,et al. Individualized positive end-expiratory pressure in obese patients during general anaesthesia: a randomized controlled clinical trial using electrical impedance tomography[J]. Br J Anaesth,2017,119(6):1194-1205. doi:10.1093/bja/aex192.
[19] LIU J,MENG Z,LV R,et al. Effect of intraoperative lung-protective mechanical ventilation on pulmonary oxygenation function and postoperative pulmonary complications after laparoscopic radical gastrectomy[J]. Braz J Med Biol Res,2019,52(6):e8523. doi:10.1590/1414-431X20198523.
[20] 闭玉华,黄俊萍. 以驱动压为导向个体化滴定式呼气末正压通气在老年腹腔镜结肠癌患者中的应用效果[J]. 重庆医学,2023,52(3):348-356. BI Y H,HUANG J P. Application effect of individualized titrated positive end-expiratory pressure ventilation guided by driving pressure in elderly patients with laparoscopic colon cancer[J]. Chongqing Med J,2023,52(3):348-356. doi:10.3969/j.issn.1671-8348.2023.03.006.
[21] 李华,任冬冬,王振涛. 降钙素原与临床肺部感染评分评估呼吸机相关性肺炎预后的研究[J]. 中华医院感染学杂志,2014,24(12):2901-2903. LI H,REN D D,WANG Z T. Clinical research on procalcitonin and pulmonary infection score in the evaluation of ventilator-associated pneumonia prognosi[J]. Chin J Nosocomio,2014,24(12):2901-2903. doi:10.11816/cn.ni.2014-135777.
[22] 刘静,孟志鹏,颜伟,等. 肺保护性通气策略对腹腔镜胃癌根治手术老年患者肺氧合功能及术后肺部并发症的影响[J]. 临床麻醉学杂志,2019,35(4):344-347. LIU J,MENG Z P,YAN W,et al. Effect of intraoperative lung protective mechanical ventilation on pulmonary oxygenation function and postoperative pulmonary complications after laparoscopic radical gastrectomy for elderly patients[J]. J Clin Anesthesiol,2019,35(4):344-347. doi:10.12089/jca.2019.04.008.

