脓毒症所致的ALI/ARDS患者血浆SIRT-1、syndecan-1的表达水平及其对预后的影响
冯颂乔 何业伟 王妍
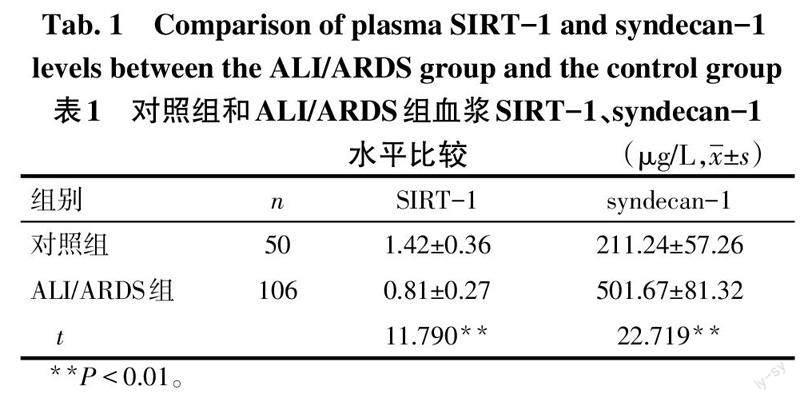
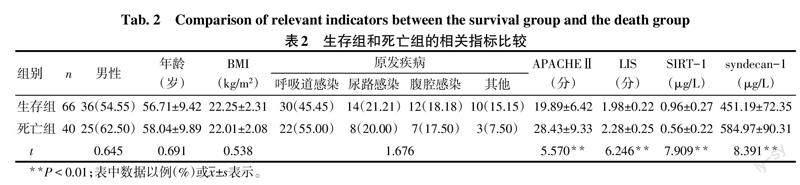
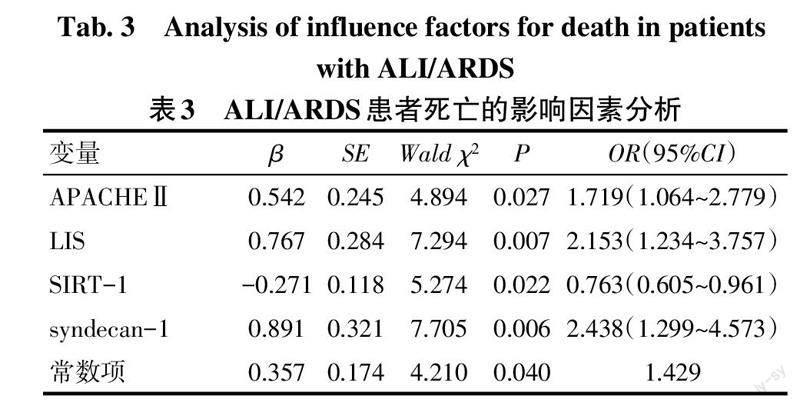
摘要:目的 分析脓毒症所致的急性肺损伤(ALI)/急性呼吸窘迫综合征(ARDS)患者血浆沉默信息调节因子1(SIRT-1)、多配体蛋白聚糖-1(syndecan-1)的表達水平及其对预后的影响。方法 选取ALI/ARDS患者106例作为ALI/ARDS组,统计所有患者入院1个月内的生存情况,并根据其生存情况将其分为生存组66例和死亡组40例。另选取健康志愿者50作为对照组。采用酶联免疫吸附试验检测所有研究对象血浆SIRT-1、syndecan-1的表达,同时收集患者的急性生理学及慢性健康状况评分Ⅱ(APACHEⅡ)以及肺损伤评分(LIS)。结果 ALI/ARDS组的血浆SIRT-1水平低于对照组,血浆syndecan-1水平高于对照组(P<0.05)。死亡组的血浆SIRT-1水平低于生存组,APACHEⅡ、LIS及血浆syndecan-1水平均高于生存组(P<0.05)。ALI/ARDS患者的血浆SIRT-1水平与syndecan-1、APACHEⅡ、LIS均呈负相关,血浆syndecan-1水平与APACHEⅡ、LIS呈正相关(P<0.05)。较高水平APACHEⅡ、LIS及血浆syndecan-1是ALI/ARDS患者死亡的危险因素,而较高水平血浆SIRT-1则是ALI/ARDS患者死亡的保护因素(P<0.05)。血浆SIRT-1、syndecan-1对ALI/ARDS患者预后的评估价值较高,曲线下面积分别为0.860(95%CI:0.791~0.930)、0.870(95%CI:0.800~0.940)。结论 ALI/ARDS患者血浆SIRT-1水平呈异常低表达、syndecan-1水平呈异常高表达,两者的表达水平与患者的预后情况密切相关。
关键词:急性肺损伤;呼吸窘迫综合征;多配体蛋白聚糖1;预后;沉默信息调节因子1
中图分类号:R563.8文献标志码:ADOI:10.11958/20221108
Expression levels of plasma SIRT-1 and syndecan-1 in patients with sepsis-induced
ALI/ARDS and their effect on prognosis
FENG Songqiao, HE Yewei, WANG Yan
Department of Critical Medicine, the Second Affiliated Hospital of Dalian Medical University, Dalian 116044, China
Abstract: Objective To analyze plasma expression levels of silent information regulation 1 (SIRT-1) and syndecan-1 in patients with acute lung injury (ALI)/ associated acute respiratory distress syndrome (ARDS) and their correlation with prognosis. Methods A total of 106 patients with ALI/ARDS were selected as the ALI/ARDS group. The survival status of all patients within one month after admission was counted, and they were divided into the survival group (66 cases) and the death group (40 cases) according to their survival status. In addition, 50 healthy volunteers were selected as the control group. Expression levels of plasma SIRT-1 and syndecan-1 of all subjects were detected by enzyme-linked immunosorbent assay. Clinical data of acute physiology and chronic health score Ⅱ (APACHE Ⅱ) and lung injury score (LIS) were collected. Results The plasma SIRT-1 level was lower in the ALI/ARDS group than that in the control group, and the plasma syndecan-1 level was higher than that in the control group (P<0.05). The plasma SIRT-1 level was lower in the death group than that in the surviva group, and APACHE Ⅱ score, LIS score and plasma syndecan-1 level were significantly higher than those in the survival group (P<0.05). The plasma level of SIRT-1 in patients with ALI/ARDS was negatively correlated with syndecan-1, APACHE Ⅱ score and LIS score, the plasma level of syndecan-1 was positively correlated with APACHEⅡ score and LIS score (P<0.05). Higher APACHEⅡ score, LIS score and plasma syndecan-1 were the risk factors of death in patients with ALI/ARDS, while higher plasma SIRT-1 was the protective factor of death in patients with ALI/ARDS (P<0.05). Values of plasma SIRT-1 and syndecan-1 in evaluating the prognosis of patients with ALI/ARDS were high, and the areas under the curve were 0.860 (95%CI: 0.791-0.930) and 0.870 (95%CI: 0.800-0.940) respectively. Conclusion The plasma SIRT-1 level is abnormally low and syndecan-1 level is abnormally high in patients with ALI/ARDS. The expression levels of SIRT-1 and syndecan-1 are closely related to the prognosis of patients.
Key words: acute lung injury; respiratory distress syndrome; syndecan-1; prognosis; silent information regulator 1
急性肺损伤(acute lung injury,ALI)/急性呼吸窘迫综合征(associated acute respiratory distress syndrome,ARDS)是由感染、烧伤、严重创伤等因素引起的危急重症,其主要临床特征为肺血管内皮细胞受损、肺水肿、进行性低氧血症和呼吸窘迫[1-2]。ALI/ARDS起病急、进展快、治疗难,患者预后普遍较差,具有较高的病死率,如何在疾病早期有效地评估患者病情以及预测其预后情况成为难点[3]。沉默信息调节因子1(silent information regulation 1,SIRT-1)是Sir2超蛋白家族一员,生物学功能丰富,对衰老、炎症反应、糖脂代谢、氧化应激等均有重要的调节作用[4]。SIRT-1可减弱急性呼吸窘迫综合征动物模型体内的炎症反应,抑制疾病进展[5]。多配体蛋白聚糖-1(syndecan-1)是一种具有多种生物学功能的跨膜硫酸乙酰肝素蛋白多糖,在恶性肿瘤、心血管疾病中均发挥着重要的调节作用[6]。此外,syndecan-1还是血管内皮屏障的重要组成部分,且其表达水平升高与肺血管内皮细胞受损关系密切[7]。目前,关于SIRT-1、syndecan-1与ALI/ARDS相关的临床研究鲜见。本研究旨在探讨脓毒症所致的ALI/ARDS患者血浆SIRT-1、syndecan-1的表达情况,并进一步分析了两者的表达与患者预后的关系。
1 资料与方法
1.1 一般资料 选取2020年4月—2022年4月于大连医科大学附属第二医院就诊的因脓毒症所致的ALI/ARDS患者(ALI/ARDS组)106例,诊断标准参照文献[8-9],男61例,女45例,年龄(57.21±9.65)岁,体质量指数(BMI)为(22.16±2.13)kg/m2。纳入标准:患者的性别、年龄、是否原发疾病等记录完整。排除标准:孕妇及哺乳期女性;合并有间质性肺病、肺栓塞等肺部疾病;合并自身免疫性疾病或近期服用过免疫抑制剂者;合并有恶性肿瘤、严重心脑血管疾病、严重原发性肝肾功能疾病者;入院后存活时间<48 h者。另选取同期在我院体检的健康志愿者50例为对照组,男26例,女24例,年龄(56.87±8.32)岁,BMI(22.51±2.09)kg/m2。2组性别(χ2=0.424)、年龄(t=0.214)及BMI(t=0.963)比较差异无统计学意义(P>0.05)。本次研究通过我院伦理委员会的批准(LL2020-03-014),患者或家属对本次研究的内容知情并签署同意书。
1.2 研究方法 (1)比较2组血浆SIRT-1、syndecan-1水平。所有研究对象在入院时抽取静脉血6 mL,参照说明书,采用酶联免疫吸附试验检测血浆SIRT-1、syndecan-1的水平,试剂盒均购于生工生物工程(上海)股份有限公司。(2)根据ALI/ARDS患者入院1个月内的生存情况,分为生存组(n=66)和死亡组(n=40)。分析2组研究对象的性别、年龄、BMI、原发疾病构成、血浆SIRT-1、血浆syndecan-1、患者入院时的急性生理学及慢性健康状况评分Ⅱ(APACHEⅡ)以及肺损伤评分(LIS)的差异。(3)分析ALI/ARDS患者血浆SIRT-1、syndecan-1水平与APACHEⅡ、LIS的相关性,分析影响ALI/ARDS患者死亡的因素及相关指标对ALI/ARDS患者预后的评估价值。
1.3 统计学方法 采用SPSS 22.0软件进行数据分析。符合正态分布的计量资料以均数±标准差(x±s)表示,2组间比较采用t检验。计数资料以例或例(%)表示,组间比较用χ2检验,相关性分析采用Pearson相關,危险因素分析采用多因素Logistic回归,预后价值评估采用受试者工作特征(ROC)曲线分析。检验标准ɑ=0.05。
2 结果
2.1 对照组和ALI/ARDS组血浆SIRT-1、syndecan-1水平比较 与对照组比较,ALI/ARDS组血浆SIRT-1水平降低,血浆syndecan-1水平升高(P<0.05),见表1。
2.2 生存组和死亡组的相关指标比较 2组性别、年龄、BMI及原发疾病构成差异无统计学意义。与生存组比较,死亡组血浆SIRT-1水平降低,APACHEⅡ、LIS以及血浆syndecan-1水平升高(P<0.05),见表2。
2.3 血浆SIRT-1、syndecan-1与APACHEⅡ、LIS的相关性分析 ALI/ARDS患者血浆SIRT-1水平与syndecan-1、APACHEⅡ、LIS均呈负相关(r分别为 -0.451、-0.372、-0.393,均P<0.05);血浆syndecan-1水平与APACHEⅡ、LIS呈正相关(r分别为0.437、0.485,均P<0.05)。
2.4 ALI/ARDS患者死亡的危险因素分析 以表2中2组间差异有统计学意义的SIRT-1、syndecan-1、APACHEⅡ、LIS为自变量,以入院1个月内患者生存情况为因变量(死亡=1,生存=0)。多因素Logistic回归分析结果显示,较高的APACHEⅡ、LIS以及血浆syndecan-1水平是ALI/ARDS患者死亡的危险因素,而较高水平的血浆SIRT-1则是ALI/ARDS患者死亡的保护因素(P<0.05),见表3。
2.5 各指标对ALI/ARDS患者预后的评估价值分析 经ROC曲线分析显示,相较APACHEⅡ和LIS,血浆SIRT-1、syndecan-1水平评估ALI/ARDS患者预后有一定优势,敏感度和特异度较均较高,见图1、表4。
3 讨论
SIRT-1是一种NAD+依赖性Ⅲ类组蛋白去乙酰化酶,可以通过除去乙酰基促使DNA链紧密缠绕,进而发挥基因沉默的功能[10]。在众多Sir2超蛋白家族成员中,有关SIRT-1的研究最为广泛,但既往研究多集中在衰老、糖尿病方面。近年来有研究发现,SIRT-1对重症呼吸疾病亦有保护作用,如Fu等[11]研究发现,SIRT-1能够通过改善肺血管内皮细胞通透性,从而缓解脂多糖诱导的小鼠模型肺损伤。另有研究发现,miRNA-499-5p可加重ALI模型小鼠的肺损伤程度,而靶向抑制SIRT-1的表达是其主要的作用机制之一[12]。Yang等[13]研究发现,miRNA-146a-3p可通过上调SIRT-1的表达来改善小鼠的肺损伤。本研究结果显示,ALI/ARDS组血浆SIRT-1水平低于对照组,死亡组血浆SIRT-1水平低于生存组,且较高水平血浆SIRT-1是ALI/ARDS患者死亡的保护因素,提示SIRT-1在ALI/ARDS患者血浆中呈异常低表达,且其表达水平降低与患者预后不良关系密切。考虑SIRT-1可能主要是通过以下两个方面改善ALI/ARDS患者的肺损伤:一方面,ALI/ARDS本质上是由于肺毛细血管通透性增加导致的非心源性肺水肿,而SIRT-1可改善肺血管内皮细胞通透性,这是其保护ALI/ARDS患者的重要作用机制[11];另一方面,ALI/ARDS患者存在明显的炎症反应,炎症反应可促进疾病的进展,而SIRT-1可通过抑制核因子-κB信号通路的活化,抑制炎症因子的分泌,进而改善患者的肺损伤程度[14-15]。
syndecan-1分子质量约为85 ku,其定位于2p23,在心血管疾病和肺部疾病中均发挥着重要作用[16]。位于血管内皮细胞管腔表面的内皮细胞糖萼是血管屏障的重要组成部分,内皮细胞糖萼降解可破坏血管屏障,进而促进ALI/ARDS的发生、发展[17]。syndecan-1是内皮细胞糖萼降解的标志物,可敏感地反映肺损伤的严重程度。相关研究发现,脂多糖诱导的ALI模型小鼠血清syndecan-1水平明显升高,在注射脂多糖3 h后即可到达峰值,且在24 h内血清syndecan-1水平均维持在较高水平[18]。Li等[19]研究显示,减轻脂多糖所致的脓毒症小鼠肺内皮细胞糖萼降解后,小鼠血清syndecan-1水平也随之降低,且小鼠的存活率得到了明显的提升。本研究结果亦显示,ALI/ARDS组的血浆syndecan-1水平高于对照组,死亡组血浆syndecan-1水平高于生存组,且血浆syndecan-1水平过高是ALI/ARDS患者死亡的危险因素,证实了syndecan-1在ALI/ARDS患者血浆中呈异常高表达,且其表达水平升高与患者预后不良有关。液体正平衡引起的液体过负荷可导致重症患者死亡[20]。Kajita等[21]研究发现,脓毒症相关的急性呼吸窘迫综合征患者血清syndecan-1水平明显升高,且血清syndecan-1高表达与液体正平衡有关,可见syndecan-1表达还可以反映机体累积液体平衡情况,这可能是其能评估患者预后的原因之一。本研究ROC分析结果显示,血浆SIRT-1、syndecan-1对患者预后的评估价值较高,有潜力作为临床预测评估患者预后的新型生物标志物。
综上所述,脓毒症所致的ALI/ARDS患者血浆SIRT-1水平呈异常低表达、syndecan-1水平呈异常高表达,两者的表达水平与患者的预后情况关系密切。临床可通过检测ALI/ARDS患者血浆SIRT-1、syndecan-1的表达情况来预测评估患者的预后,对预后不良的高风险患者进行重点监测,或可在一定程度上降低患者的病死率。
参考文献
[1] LONG M E,MALLAMPALLI R K,HOROWITZ J C. Pathogenesis of pneumonia and acute lung injury[J]. Clin Sci (Lond),2022,136(10):747-769. doi:10.1042/CS20210879.
[2] MCVEY M J,STEINBERG B E,GOLDENBERG N M. Inflammasome activation in acute lung injury[J]. Am J Physiol Lung Cell Mol Physiol,2021,320(2):L165-L178. doi:10.1152/ajplung.00303.2020.
[3] MOKRA D. Acute lung injury-from pathophysiology to treatment[J]. Physiol Res,2020,69(Suppl 3):S353-S366. doi:10.33549/physiolres.934602.
[4] CHEN C,ZHOU M,GE Y,et al. SIRT1 and aging related signaling pathways[J]. Mech Ageing Dev,2020,187(4):111215. doi:10.1016/j.mad.2020.111215.
[5] 趙维,刘俊彦,李玉英. SIRT1减弱对脂多糖致急性呼吸窘迫综合征小鼠的促炎作用[J]. 中华肺部疾病杂志(电子版),2017,10(2):163-167. ZHAO W,LIU J Y,LI Y Y. Effect of SIRT1 weakening on inflammation in mice with acute respiratory distress syndrome induced by lipopolysaccharide[J]. Chin J Lung Dis(Electronic Edition),2017,10(2):163-167. doi:10.3877/cma.j.issn.1674-6902.2017.02.010.
[6] RANGARAJAN S,RICHTER J R,RICHTER R P,et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression[J]. J Histochem Cytochem,2020,68(12):823-840. doi:10.1369/0022155420937087.
[7] LAMBERT J,MAKIN K,AKBAREIAN S,et al. ADAMTS-1 and syndecan-4 intersect in the regulation of cell migration and angiogenesis[J]. J Cell Sci,2020,133(7):jcs235762. doi:10.1242/jcs.235762.
[8] SHANKAR-HARI M,PHILLIPS G S,LEVY M L,et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3)[J]. JAMA,2016,315(8):775-787. doi:10.1001/jama.2016.0289.
[9] 中華医学会重症医学分会. 急性肺损伤/急性呼吸窘迫综合征诊断和治疗指南(2006)[J]. 中华急诊医学杂志,2007,16(4):343-349. Critical Care Branch of CMA. Guidelines for diagnosis and treatment of acute lung injury/acute respiratory distress syndrome(2006)[J]. Chin J Emerg Med,2007,16(4):343-349. doi:10.3760/j.issn:1671-0282.2007.04.002.
[10] SONG H,CHEN Q,XIE S,et al. GDF-15 prevents lipopolysaccharide-mediated acute lung injury via upregulating SIRT1[J]. Biochem Biophys Res Commun,2020,526(2):439-446. doi:10.1016/j.bbrc.2020.03.103.
[11] FU C,HAO S,XU X,et al. Activation of SIRT1 ameliorates LPS-induced lung injury in mice via decreasing endothelial tight junction permeability[J]. Acta Pharmacol Sin,2019,40(5):630-641. doi:10.1038/s41401-018-0045-3.
[12] YANG F,YAN J,LU Y,et al. MicroRNA-499-5p targets SIRT1 to aggravate lipopolysaccharide-induced acute lung injury[J]. Free Radic Res,2021,55(1):71-82. doi:10.1080/10715762.2020.1863393.
[13] YANG Y,LI L. Depleting microRNA-146a-3p attenuates lipopolysaccharide-induced acute lung injury via up-regulating SIRT1 and mediating NF-κB pathway[J]. J Drug Target,2021,29(4):420-429. doi:10.1080/1061186X.2020.1850738.
[14] LEI J,SHEN Y,XV G,et al. Aloin suppresses lipopolysaccharide-induced acute lung injury by inhibiting NLRP3/NF-κB via activation of SIRT1 in mice[J]. Immunopharmacol Immunotoxicol,2020,42(4):306-313. doi:10.1080/08923973.2020.1765373.
[15] AN N,YANG T,ZHANG X X,et al. Bergamottin alleviates LPS-induced acute lung injury by inducing SIRT1 and suppressing NF-κB[J]. Innate Immun,2021,27(7/8):543-552. doi:10.1177/17534259211062553.
[16] SOLIMAN N A,YUSSIF S M,SHEBL A M. Syndecan-1 could be added to hormonal receptors and HER2/neu in routine assessment of invasive breast carcinoma, relation of its expression to prognosis and clinicopathological parameters[J]. Pathol Res Pract,2019,215(5):977-982. doi:10.1016/j.prp.2019.02.003.
[17] IBA T,LEVY J H. Derangement of the endothelial glycocalyx in sepsis[J]. J Thromb Haemost,2019,17(2):283-294. doi:10.1111/jth.14371.
[18] 齐颖,李真玉,王斌,等. Wnt5a与Syndecan-1在小鼠脓毒症致急性肺损伤中的表达研究[J]. 中国急救医学,2017,37(1):43-48,封3. QI Y,LI Z Y,WANG B,et al. The expression and significance of Wnt5a and Syndecan-1 in sepsis-induced acute lung injury of mice[J]. Chin J Crit Care Med,2017,37(1):43-48,Cover 3. doi:10.3969/j.issn.1002-1949.2017.01.010.
[19] LI H,HAO Y,YANG L L,et al. MCTR1 alleviates lipopolysaccharide-induced acute lung injury by protecting lung endothelial glycocalyx[J]. J Cell Physiol,2020,235(10):7283-7294. doi:10.1002/jcp.29628.
[20] VIGNON P,EVRARD B,ASFAR P,et al. Fluid administration and monitoring in ARDS: which management?[J]. Intensive Care Med,2020,46(12):2252-2264. doi:10.1007/s00134-020-06310-0.
[21] KAJITA Y,TERASHIMA T,MORI H,et al. A longitudinal change of syndecan-1 predicts risk of acute respiratory distress syndrome and cumulative fluid balance in patients with septic shock: A preliminary study[J]. J Intensive Care,2021,9(1):27. doi:10.1186/s40560-021-00543-x.
(2022-07-14收稿 2022-10-31修回)
(本文編辑 陆荣展)

