sTIM-3及其配体Gal-9、HMGB1与2型糖尿病并发冠心病的相关性研究
刘艳秋 范海迪 侯海燕 孙健 林宁
摘要:目的 探討血清中可溶性T细胞免疫球蛋白及黏蛋白分子-3(sTIM-3)、半乳糖凝集素-9(Gal-9)、高迁移率族蛋白B1(HMGB1)水平与2型糖尿病(T2DM)并发冠心病(CHD)的关系。方法 选取T2DM组患者50例、T2DM并发CHD组(T2DM+CHD组)患者52例,同期健康体检人员(Con组)48例。采用酶联免疫吸附试验(ELISA)检测3组血清中sTIM-3、Gal-9及HMGB1的水平。Spearman法分析血清sTIM-3、Gal-9、HMGB1、空腹血糖(FBG)、超敏C-反应蛋白(hs-CRP)间的相关性;使用受试者工作特征(ROC)曲线分析sTIM-3、Gal-9及两者联合检测对T2DM并发CHD的诊断能力;采用Logistic回归分析T2DM并发CHD的危险因素。结果 与Con组相比,T2DM组和T2DM+CHD组sTIM-3、Gal-9、HMGB1升高(P<0.05);与T2DM组相比,T2DM+CHD组sTIM-3和Gal-9升高(P<0.05),HMGB1差异无统计学意义。FBG、hs-CRP与sTIM-3、Gal-9、HMGB1呈正相关,sTIM-3与Gal-9、HMGB1,Gal-9与HMGB1呈正相关(P<0.05)。ROC曲线分析结果显示血清sTIM-3和Gal-9联合诊断T2DM并发CHD曲线下面积为0.812(95%CI:0.743~0.882)。Logistic回归分析显示体质量指数、sTIM-3、Gal-9升高是T2DM并发CHD的危险因素。结论 血清sTIM-3、Gal-9升高是T2DM并发CHD的危险因素。
关键词:糖尿病,2型;冠状动脉疾病;半乳糖凝集素类;高迁移率族蛋白质类;可溶性T细胞免疫球蛋白及黏蛋白分子-3
中图分类号:R392.6文献标志码:ADOI:10.11958/20221307
Correlation research between sTIM-3 and its ligands Gal-9, HMGB1 in patients with type 2 diabetes complicated with coronary heart disease
LIU Yanqiu FAN Haidi HOU Haiyan SUN Jian LIN Ning
1 Department of Clinical Laboratory, Branch Hospital of Huai'an First People's Hospital, Huai'an 223002, China;
2 Department of Clinical Laboratory, Qinhuai District Center for Disease Control and Prevention; 3 Department of
Clinical Laboratory, Huai'an First Hospital Affiliated to Nanjing Medical University
△Corresponding Author E-mail: hayyln@126.com
Abstract: Objective To investigate the relationship between serum soluble T cell immunoglobulin and mucin domain-3 (sTIM-3), galectin-9 (Gal-9) and high mobility group protein B1 (HMGB1) levels in patients with type 2 diabetes mellitus (T2DM) complicated with coronary heart disease (CHD). Methods Fifty patients with T2DM were used as the T2DM group, and fifty-two patients with T2DM complicated with CHD were selected as the T2DM+CHD group. Forty-eight healthy physical examination subjects were selected as the control group (Con group). Serum levels of sTIM-3, Gal-9 and HMGB1 were detected by enzyme-linked immunosorbent assay (ELISA). Spearman correlation analysis was used to analyze the correlation between serum sTIM-3, Gal-9, HMGB1, fasting blood glucose (FBG) and high-sensitivity C-reactive protein (hs-CRP). Receiver operating characteristic curve (ROC) was used to analyse the diagnostic capability of sTIM-3, Gal-9 and their combination in T2DM complicated with CHD. Logistic regression was used to investigate the risk factors of T2DM complicated with CHD. Results Compared with the Con group, sTIM-3, Gal-9 and HMGB1 were significantly increased in the T2DM group and the T2DM+CHD group (P<0.05). Compared with the T2DM group, sTIM-3 and Gal-9 were significantly increased in the T2DM+CHD group (P<0.05), and there was no significant difference in HMGB1. FBG and hs-CRP were positively correlated with sTIM-3, Gal-9 and HMGB1, sTIM-3 was positively correlated with Gal-9 and HMGB1, and Gal-9 was positively correlated with HMGB1 (P<0.05). ROC curve analysis showed that the area under the curve of serum sTIM-3 and Gal-9 in the diagnosis of T2DM complicated with CHD was 0.812 (95%CI: 0.743-0.882). Logistic regression analysis showed that increased body mass index, sTIM-3 and Gal-9 were risk factors for T2DM complicated with CHD. Conclusion Increased serum levels of sTIM-3 and Gal-9 are risk factors for T2DM complicated with CHD.
Key words: diabetes mellitus, type 2; coronary disease; galectins; high mobility group proteins; sTIM-3
据国际糖尿病联合会统计,目前全世界约有5.37亿人患糖尿病,其中90%左右为2型糖尿病(T2DM)[1]。冠状动脉粥样硬化性心脏病(coronary heart disease,CHD)简称为冠心病,是糖尿病的大血管并发症之一,动脉粥样硬化是CHD的病理基础,与非糖尿病患者比较,糖尿病患者发生动脉粥样硬化斑块的风险更大,程度也更重[2]。T细胞免疫球蛋白及黏蛋白分子-3(T cell immunoglobulin domain and mucin domain protein-3,TIM-3)是一种膜蛋白,表达在活化的辅助性T细胞(Th)1、自然杀伤细胞、树突状细胞、巨噬细胞、单核细胞等细胞表面[3]。TIM-3还存在可溶性蛋白的形式,即可溶性TIM-3(soluble TIM-3,sTIM-3)。sTIM-3来源于TIM-3基因表达水平升高与TIM-3从细胞膜表面脱落增加两种情况[4]。TIM-3与其配体半乳糖凝集素-9(galectin-9,Gal-9)相互作用,抑制T细胞功能[5]。高迁移率族蛋白B1(high mobility group protein 1,HMGB1)是TIM-3的另一个重要配体[6]。研究发现,TIM-3表达与一些慢性疾病如糖尿病、动脉粥样硬化、慢性病毒感染、类风湿性关节炎等相关性较强[7]。但sTIM-3及其配体Gal-9、HMGB1在T2DM并发CHD中的作用尚不明确。本研究通过检测T2DM并发CHD患者血清中sTIM-3、Gal-9、HMGB1的变化,旨在发现T2DM并发CHD疾病的新型检测标志物。
1 对象与方法
1.1 研究对象 选取2019年2月—2021年12月于淮安市第一人民医院分院确诊并住院治疗的单纯T2DM患者(T2DM组)、T2DM并发CHD患者(T2DM+CHD组)和同期健康体检者(Con组)。T2DM的诊断符合2018年美国糖尿病协会糖尿病诊疗标准;CHD诊断符合中华医学会心血管病分会制定的《慢性稳定性心绞痛诊断与治疗指南》。排除标准:(1)患CHD外的T2DM其他并发症者。(2)患急性心肌炎、严重心力衰竭者。(3)患自身免疫性疾病、血液系统疾病及其他重要脏器功能不全者。最终纳入T2DM组50例,男26例,女24例,年龄31~76岁,平均(52.74±8.90)岁;T2DM+CHD组52例,男25例,女27例,年龄28~72岁,平均(53.15±8.62)岁;Con组48例,男23例,女25例,年龄34~77岁,平均(51.62±9.31)岁。本研究经患者知情同意,通过淮安市第一人民医院分院伦理委员会批准(伦理批号:HAYYFY2019-KY002)。
1.2 资料收集 一般资料:收集年龄、性别、体质量指数(BMI)。实验室资料:采集所有研究对象空腹静脉血,使用酶联免疫吸附试验(ELISA)试剂盒(上海双赢生物科技有限公司)检测血清sTIM-3、Gal-9、HMGB1含量;使用日立全自动生化分析仪7600检测空腹血糖(FBG)、超敏C-反应蛋白(hs-CRP)、三酰甘油(TG)、总胆固醇(TC)、低密度脂蛋白胆固醇(LDL-C)与高密度脂蛋白胆固醇(HDL-C);使用伯乐糖化血红蛋白分析仪D-10检测糖化血红蛋白(HbA1c)。
1.3 统计学方法 采用SPSS 23.0软件进行数据分析。符合正态分布的计量资料用x±s表示,多组间比较采用单因素方差分析,若方差不齐,组间多重比较采用Tamhane's T2检验;若方差齐,则采用LSD-t检验。非正态分布的计量资料用M(P25,P75)表示,多组间比较采用Kruskal-Wallis H检验,组间多重比较采用Bonferroni法。计数资料采用例(%)表示,组间比较用χ2检验。相关性分析采用Spearman法。采用受试者工作特征(ROC)曲线分析指标对T2DM并发CHD的诊断能力。使用多因素Logistic回归分析T2DM并发CHD的影响因素。P<0.05为差异有统计学意义。
2 结果
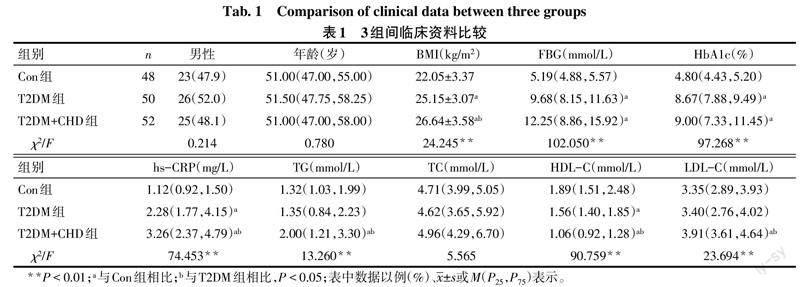
2.1 3组间临床资料比较 3组间性别、年龄、TC差异无统计学意义。与Con组相比,T2DM组BMI、FBG、HbA1c、hs-CRP升高,HDL-C降低(P<0.05);T2DM+CHD组BMI、FBG、HbA1c、hs-CRP、TG、LDL-C升高,HDL-C降低(P<0.05)。与T2DM组相比,T2DM+CHD组BMI、hs-CRP、TG、LDL-C升高,HDL-C降低(P<0.05)。见表1。
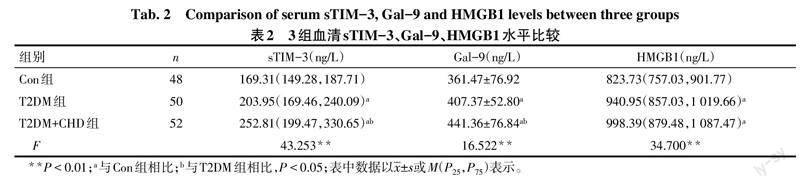
2.2 3组间sTIM-3、Gal-9和HMGB1比较 与Con组相比,T2DM组和T2DM+CHD组sTIM-3、Gal-9和HMGB1升高(P<0.05);与T2DM相比,T2DM+CHD组sTIM-3和Gal-9升高(P<0.05),HMGB1差异无统计学意义。见表2。
2.3 sTIM-3、Gal-9、HMGB1和FBG、hs-CRP的相关性分析 在所有受试者中,FBG与sTIM-3、Gal-9、HMGB1呈正相关,rs分别为0.613、0.472、0.619(P<0.05)。hs-CRP與sTIM-3、Gal-9、HMGB1呈正相关,rs分别为0.596、0.466、0.476(P<0.05)。sTIM-3与Gal-9、HMGB1呈正相关,rs分别为0.527和0.690(P<0.05);Gal-9与HMGB1呈正相关(rs=0.466,P<0.05)。
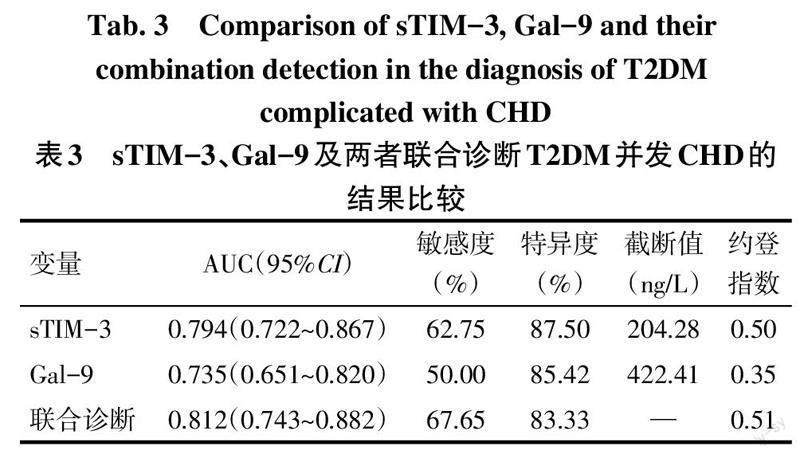
2.4 sTIM-3、Gal-9及两者联合检测对T2DM并发CHD的诊断能力 ROC曲线结果显示,sTIM-3、Gal-9及两者联合检测诊断T2DM并发CHD的ROC曲线下面积(AUC)分别是0.794、0.735、0.812,联合诊断效能更高,但sTIM-3诊断特异度较高,见表3、图1。
2.5 T2DM并发CHD发生的影响因素 以T2DM是否合并CHD为因变量(否=0,是=1),以BMI、年龄、sTIM-3、Gal-9、HMGB1为自变量,进行Logistic回归分析。结果显示BMI、sTIM-3、Gal-9升高是T2DM并发CHD的危险因素(P<0.05),见表4。
3 讨论
T2DM以高血糖、胰岛素抵抗和慢性T细胞活化为特征,伴有低度慢性炎症[8]。研究证实,人体血糖稳态紊乱后会激活T细胞,CD4+T细胞与CHD、颈动脉粥样硬化等疾病发展有关[9]。Wang等[10]研究发现,T2DM患者NK细胞表面TIM-3表达水平显著增加,且与HbA1c和FBG水平呈正相关。还有学者发现,与健康对照组相比,T2DM患者血清中sTIM-3水平显著升高[11]。本研究结果显示,与Con组相比,T2DM组患者血清中sTIM-3显著升高,且血清sTIM-3的水平和hs-CRP呈正相关,表明sTIM-3可能参与了T2DM导致的低度慢性炎症的发展。Zhang等[12]研究发现,TIM-3在CHD患者外周血CD4+T细胞表面高表达,且随着CHD病情的加重而升高。Hou等[13]研究发现,冠状动脉粥样硬化患者外周血NK细胞表面TIM-3表达增加,且TIM-3与CRP和肿瘤坏死因子-α(TNF-α)水平相关。本研究中,与Con组、T2DM组相比,T2DM+CHD组患者血清中sTIM-3水平显著升高,且sTIM-3与FBG呈正相关。推测人体血糖代谢紊乱导致外周血细胞膜表面sTIM-3脱落增加,影响了动脉粥样硬化相关的炎症微环境,进一步导致T2DM并发CHD的进展。
Gal-9是半乳糖结合蛋白家族的一员,最初作为T淋巴细胞分泌的一种嗜酸性粒细胞趋化因子而被发现,其在免疫调节中发挥重要作用[14]。Gal-9是TIM-3的配体,参与细胞聚集、黏附、分化[15]、凋亡[16]和炎症反应[17]等。Sun等[18]发现,肥胖合并糖尿病患者血浆Gal-9水平显著高于健康对照组和单纯肥胖组,且肥胖合并糖尿病组的Gal-9水平与空腹胰岛素和C肽呈正相关。本研究发现,与Con组相比,T2DM组患者血清中Gal-9水平显著升高,推测原因为高糖状态下,Gal-9通过与Th1细胞上表达的TIM-3结合,调节Th1免疫反应,从而调节T2DM的疾病进展。Kurose等[19]发现,T2DM合并慢性肾病的患者肾小球滤过率越低,血清Gal-9水平越高。本研究中,与T2DM组相比,T2DM+CHD组患者血清中Gal-9水平显著升高,且Gal-9与sTIM-3、FBG、hs-CRP呈正相关。推测可能为T2DM患者机体内升高的Gal-9水平加剧了机体的慢性炎症和应激反应,参与了动脉粥样硬化的发生和进展。
HMGB1是一种高度保守的核蛋白,由坏死细胞被动释放,由炎性细胞和一些非免疫细胞主动分泌[20]。Behl等[21]发现,HMGB1通过促进晚期糖化终产物受体和toll样受体结合,进而激活核因子(NF)-κB通路,刺激与糖尿病相关的炎性因子产生,加重糖尿病相关的免疫和代谢反应。Chen等[22]发现,T2DM患者血清中HMGB1上调,且HMGB1水平与血清白细胞介素(IL)-6和TNF-α相关。本研究中T2DM患者血清HMGB1水平明显高于Con组,且HMGB1与sTIM-3、FBG、hs-CRP呈正相關,推测可能是机体的高糖环境导致HMGB1水平持续升高,激活NF-κB信号通路,诱导炎性因子的产生和分泌,加重T2DM引起的慢性炎症状态。Benlier等[23]发现,与健康受试者相比,冠状动脉疾病患者的血清HMGB1水平显著增加。但在本研究中,T2DM组和T2DM+CHD组患者血清HMGB1水平差异无统计学意义,且HMGB1不是T2DM并发CHD发生的影响因素,推测高血糖可诱导HMGB1水平升高,但T2DM并发CHD疾病状态下血清HMGB1水平并没有持续升高。
本研究ROC曲线显示,sTIM-3和Gal-9联合诊断的效能较高,表明sTIM-3与Gal-9联合检测能够提高对T2DM并发CHD的诊断能力,但是血清sTIM-3的诊断特异度更佳。Logistic回归分析结果显示,高水平的BMI、sTIM-3、Gal-9增加了T2DM并发CHD的发病风险。
综上所述,sTIM-3、Gal-9可能通过影响炎症的发生发展参与T2DM并发CHD的发病机制,有望成为T2DM并发CHD的新型检验标志物。由于本研究样本量有限,未对T2DM合并CHD患者进行亚组分析,存在一定局限,且血清中sTIM-3与其配体Gal-9相互作用的具体机制尚不完全明确,还需深入研究。
参考文献
[1] GUO J,SMITH S M. Newer drug treatments for type 2 diabetes[J]. BMJ,2021,373:n1171. doi:10.1136/bmj.n1171.
[2] THOMAS M C. Type 2 diabetes and heart failure:Challenges and solutions[J]. Curr Cardiol Rev,2016,12(3): 249-255. doi:10.2174/1573403x12666160606120254.
[3] DIXON K O,DAS M,KUCHROO V K. Human disease mutations highlight the inhibitory function of TIM-3[J]. Nat Genet,2018,50(12):1640-1641. doi:10.1038/s41588-018-0289-3.
[4] GROSSMAN T B,MINIS E,LOEB-ZEITLIN S E,et al. Soluble T cell immunoglobulin mucin domain 3(sTim-3)in maternal sera:A potential contributor to immune regulation during pregnancy[J]. J Matern Fetal Neonatal Med,2021,34(24):4119-4122. doi:10.1080/14767058.2019.1706471.
[5] HASTINGS W D,ANDERSON D E,KASSAM N,et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines[J]. Eur J Immunol,2009,39:2492-2501. doi:10.1002/eji.200939274.
[6] CHEN S,DONG Z,YANG P,et al. Hepatitis B virus X protein stimulates high mobility group box 1 secretion and enhances hepatocellular carcinoma metastasis[J]. Cancer Lett,2017,394:22-32. doi:10.1016/j.canlet.2017.02.011.
[7] 鐘玉梅,陈洋,罗小超,等. Tim-3调控巨噬细胞极化在类风湿性关节炎中的研究进展[J]. 天津医药,2020,48(9):898-902. ZHONG Y M,CHEN Y,LUO X C,et al. Research progress of Tim-3 regulating the polarization of macrophage in rheumatoid arthritis[J]. Tianjin Med J,2020,48(9):898-902. doi:10.11958/20200625.
[8] KAMATA Y,TAKANO K,KISHIHARA E,et al. Distinct clinical characteristics and therapeutic modalities for diabetic ketoacidosis in type 1 and type 2 diabetes mellitus[J]. J Diabetes Complications,2017,31(2):468-472. doi:10.1016/j.jdiacomp.2016. 06.023.
[9] NYAMBUYA T M,DLUDLA P V,MXINWA V,et al. T-cell activation and cardiovascular risk in adults with type 2 diabetes mellitus:A systematic review and meta-analysis[J]. Clin Immunol,2020,210:108313. doi:10.1016/j.clim.2019.108313.
[10] WANG H,CAO K,LIU S,et al. Tim-3 expression causes NK cell dysfunction in type 2 diabetes patients[J]. Front Immunol,2022,13:852436. doi:10.3389/fimmu.2022.852436.
[11] 石新慧. 可溶性Tim-3在疾病中的表达及其意义的研究[D]. 北京:中国人民解放军军事医学科学院基础医学研究所,2016. SHI X H. Expression and clinical significance of soluble Tim-3 in different diseases[D]. Beijing:Institute of Basic Medical Sciences,Academy of Military Medical Sciences,2016.
[12] ZHANG J,ZHAN F,LIU H L. Expression level and significance of Tim-3 in CD4+ T lymphocytes in peripheral blood of patients with coronary heart disease[J]. Braz J Cardiovasc Surg,2022,37(3):350-355. doi:10.21470/1678-9741-2020-0509.
[13] HOU N,ZHAO D,LIU Y,et al. Increased expression of T cell immunoglobulin- and mucin domain-containing molecule-3 on natural killer cells in atherogenesis[J]. Atherosclerosis,2012,222(1):67-73. doi:10.1016/j.atherosclerosis.2012.02.009.
[14] HAO H,HE M,LI J,et al. Upregulation of the Tim-3/Gal-9 pathway and correlation with the development of preeclampsia[J]. Eur J Obstet Gynecol Reprod Biol,2015,194:85-91. doi:10.1016/j.ejogrb.2015.08.022.
[15] HIRASHIMA M,KASHIO Y,NISHI N,et al. Galectin-9 in physiological and pathological conditions[J]. Glycoconj J,2002,19(7/8/9):593-600. doi:10.1023/B:GLYC.0000014090.63206.2f.
[16] SAKAI K,KAWATA E,ASHIHARA E,et al. Galectin-9 ameliorates acute GVH disease through the induction of T-cell apoptosis[J]. Eur J Immunol,2011,41(1):67-75. doi:10.1002/eji.200939931.
[17] MANSOUR A A,RAUCCI F,SAVIANO A,et al. Galectin-9 regulates monosodium urate crystal-induced gouty inflammation through the modulation of Treg/Th17 ratio[J]. Front Immunol,2021,12:762016. doi:10.3389/ fimmu.2021.762016.
[18] SUN L,ZOU S,DING S,et al. Circulating T cells exhibit different TIM3/Galectin-9 expression in patients with obesity and obesity-related diabetes[J]. J Diabetes Res,2020,2020:2583257. doi:10.1155/2020/2583257.
[19] KUROSE Y,WADA J,KANZAKI M O,et al. Serum galectin-9 levels are elevated in the patients with type 2 diabetes and chronic kidney disease[J]. BMC Nephrol,2013,14:23. doi:10.1186/1471-2369-14-23.
[20] SU Z,WANG T,ZHU H,et al. HMGB1 modulates Lewis cell autophagy and promotes cell survival via RAGE-HMGB1-Erk1/2 positive feedback during nutrient depletion[J]. Immunobiology,2015,220(5):539-544. doi:10.1016/j.imbio.2014.12.009.
[21] BEHL T,SHARMA E,SEHGAL A,et al. Expatiating the molecular approaches of HMGB1 in diabetes mellitus:Highlighting signalling pathways via RAGE and TLRs[J]. Mol Biol Rep,2021,48(2):1869-1881. doi:10.1007 /s11033-020-06130-x.
[22] CHEN Y,QIAO F,ZHAO Y,et al. HMGB1 is activated in type 2 diabetes mellitus patients and in mesangial cells in response to high glucose[J]. Int J Clin Exp Pathol,2015,8(6):6683-6691.
[23] BENLIER N,ERDO?AN M B,KE?IO?LU S,et al. Association of high mobility group box 1 protein with coronary artery disease[J]. Asian Cardiovasc Thorac Ann,2019,27(4):251-255. doi:10.1177/0218492319835725.
(2022-08-22收稿 2022-10-12修回)
(本文編辑 李志芸)

