金合欢素对肝癌HepG2细胞增殖、凋亡和迁移的影响及机制研究
吴琼 李锦源 黄文涛 安娜
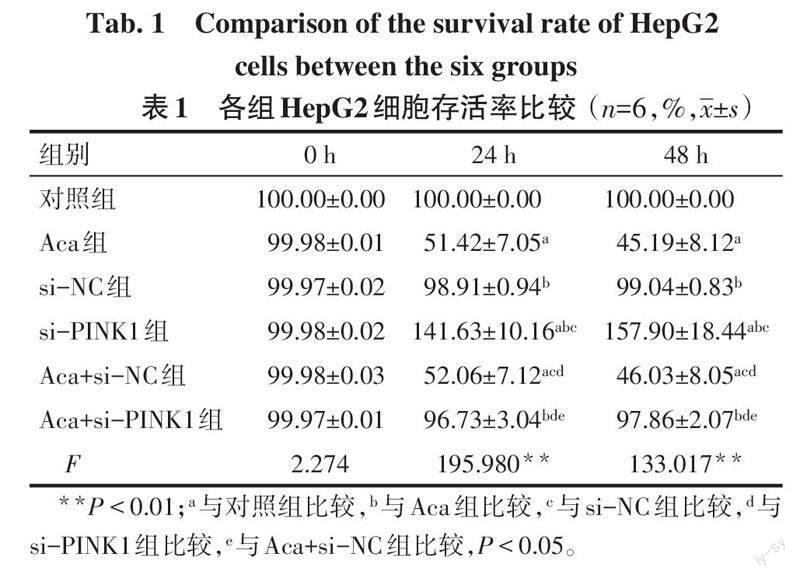
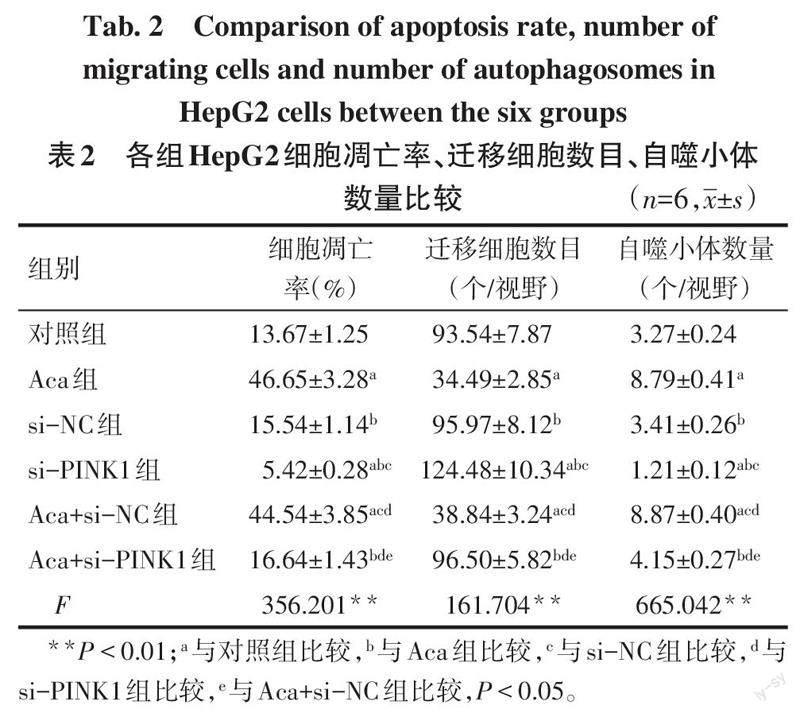
摘要:目的 探討金合欢素(Aca)通过调节PTEN诱导激酶1(PINK1)/E3泛素连接酶(Parkin)通路介导的线粒体自噬对肝癌HepG2细胞增殖、凋亡和迁移的影响。方法 将肝癌HepG2细胞分为对照组(正常培养的HepG2细胞)、Aca组(10 μmol/L Aca)、PINK1小干扰RNA阴性对照(si-NC)组(转染si-NC)、PINK1小干扰RNA(si-PINK1)组(转染si-PINK1)、Aca+si-NC组(转染si-NC后用10 μmol/L Aca处理)、Aca+si-PINK1组(转染si-PINK1后用10 μmol/L Aca处理)。CCK-8法检测细胞增殖;流式细胞术检测细胞凋亡;Transwell实验检测细胞迁移;透射电镜观察自噬小体的形成;Western blot检测细胞中自噬相关蛋白[Beclin-1、微管相关蛋白1轻链3(LC3)-Ⅰ、LC3-Ⅱ]及PINK1/Parkin通路相关蛋白表达。结果 与对照组比较,Aca组HepG2细胞存活率、迁移细胞数目降低,凋亡率、自噬小体数量、Beclin-1、LC3-Ⅱ/LC3-Ⅰ、PINK1、Parkin蛋白表达水平升高(P<0.05),si-PINK1组HepG2细胞存活率、迁移细胞数目升高,凋亡率、自噬小体数量、Beclin-1、LC3-Ⅱ/LC3-Ⅰ、PINK1、Parkin蛋白表达水平降低(P<0.05);与Aca组、Aca+si-NC组比较,Aca+si-PINK1组HepG2细胞存活率、迁移细胞数目升高,凋亡率、自噬小体数量、Beclin-1、LC3-Ⅱ/LC3-Ⅰ、PINK1、Parkin蛋白表达水平降低(P<0.05)。结论 Aca可能通过激活PINK1/Parkin通路介导的线粒体自噬抑制肝癌HepG2细胞增殖、迁移,促进细胞凋亡。
关键词:肝肿瘤,实验性;线粒体,肝;自噬;金合欢素;PINK1/Parkin通路;HepG2细胞
中图分类号:R285文献标志码:ADOI:10.11958/20221115
Effects of acacetin on proliferation, apoptosis and migration of hepatocellular carcinoma HepG2 cells and its mechanism
WU Qiong, LI Jinyuan, HUANG Wentao, AN Na
Department of Pharmacy, Cancer Hospital Affiliated to Guangxi Medical University, Nanning 530021, China
Corresponding Author E-mail: pic1230@qq.com
Abstract: Objective To investigate the effect of acacetin (Aca) on the proliferation, apoptosis and migration of liver cancer HepG2 cells by regulating PTEN induced kinase 1 (PINK1)/E3 ubiquitin protein ligase (Parkin) pathway mediated mitochondrial autophagy. Methods Liver cancer HepG2 cells were divided into the control group (normally cultured HepG2 cells), the Aca group (10 μmol/L Aca), the PINK1 small interfering RNA negative control (si-NC) group (transfected with si-NC), the PINK1 small interfering RNA (si-PINK1) group (transfected with si-PINK1), the Aca+si-NC group (treated with 10 μmol/L Aca after transfection of si-NC) and Aca+si-PINK1 group (treated with 10 μmol/L Aca after transfection of si-PINK1). Cell counting kit-8 (CCK-8) method was used to detect cell proliferation. Flow cytometry was used to detect cell apoptosis. Transwell test was used to detect cell migration. Transmission electron microscope was used to observe the formation of autophagosomes. Western blot assay was used to detect expression levels of autophagy related proteins [Beclin-1, microtubule associated protein 1 light chain 3 (LC3)-Ⅰ and LC3-Ⅱ] and PINK1/Parkin pathway related proteins in cells. Results Compared with the control group, the survival rate and the number of migrating of HepG2 cells were significantly decreased in the Aca group, and the apoptosis rate, number of autophagy bodies, expression levels of Beclin-1, LC3-Ⅱ/LC3-Ⅰ, PINK1 and Parkin protein were increased significantly (P<0.05). The survival rate and the number of migrating of HepG2 cells were significantly increased in the si-PINK1 group, and the apoptosis rate, number of autophagy bodies, expression levels of Beclin-1, LC3-Ⅱ/LC3-Ⅰ, PINK1 and Parkin protein were decreased significantly (P<0.05). Compared with the Aca group and the Aca+si-NC group, the survival rate and the number of migrating of HepG2 cells were significantly increased in the Aca+si-PINK1 group, and the apoptosis rate, number of autophagy bodies, expression levels of Beclin-1, LC3-Ⅱ/LC3-Ⅰ, PINK1 and Parkin protein were decreased significantly (P<0.05). Conclusion Aca may inhibit the proliferation and migration and promote cell apoptosis of liver cancer HepG2 cells by activating mitochondrial autophagy mediated by PINK1/Parkin pathway.
Key words: liver neoplasms, experimental; mitochondria, liver; autophagy; acacetin; PINK1/Parkin pathway; HepG2 cells
肝癌是常见的原发性癌症,也是全球主要的癌症死亡原因之一[1]。尽管化疗与手术、放疗相结合是治疗肝癌的重要方法,但存在耐药性和毒性大等不良反应[2]。同时,由于肝癌起病隐匿、进展快、早期诊断率低,多数患者在确诊时已处于疾病晚期,导致治疗效果不佳[3]。据统计,晚期肝癌患者5年肿瘤复发转移率高达40%~70%[4]。因此,开发可抑制肝癌细胞增殖与转移的有效药物至关重要。金合欢素(acacetin,Aca)是一种天然黄酮类化合物,具有抗氧化、抗炎和抗肿瘤的作用[5]。研究显示,Aca可诱导胃癌细胞凋亡和抑制上皮间质转化[6],但关于Aca对肝癌细胞增殖、凋亡和迁移影响的研究鲜见。有研究表明,线粒体自噬紊乱是肿瘤发生、发展的病理因素,抑制PTEN诱导激酶1(PTEN induced kinase 1,PINK1)/E3泛素连接酶(E3 ubiquitin protein ligase,Parkin)介导的线粒体自噬可促进膀胱肿瘤细胞的生长[7]。但Aca能否通过调节PINK1/Parkin通路介导的线粒体自噬来影响肝癌细胞增殖、凋亡和迁移尚不清楚。因此,本研究旨在探讨Aca对肝癌细胞增殖、凋亡和迁移的影响以及其可能的作用机制。
1 材料与方法
1.1 主要材料 人肝癌细胞株HepG2(批号CSL-00367)购自深圳市百恩维生物科技有限公司。Aca购自上海冠导生物工程有限公司,纯度>98%;PINK1小干扰RNA(si-PINK1)及其阴性对照(si-NC)均由成都瑞芬思生物科技有限公司设计合成;Lipofectamine 2000转染试剂盒、DMEM培养基、胎牛血清(FBS)、磷酸盐缓冲液(PBS)、ECL化学发光试剂盒购自上海慧颖生物科技有限公司;CCK-8、Annexin V-FITC/PI凋亡检测试剂盒、RIPA裂解液、兔源Beclin-1、微管相关蛋白1轻链3(LC3)-Ⅰ、LC3-Ⅱ、PINK1、Parkin、β-肌动蛋白(β-actin)一抗及羊抗兔二抗购自翌圣生物科技上海股份有限公司;细胞培养箱(型号HF180)、HM-96C型酶标仪购自力康生物医疗科技控股有限公司;流式细胞仪、Transwell小室、透射电子显微镜、凝胶成像系統购自上海玉研科学仪器有限公司。
1.2 细胞培养与分组 HepG2细胞在含有10% FBS、100 U/mL青霉素和100 mg/L链霉素的DMEM培养基(37 ℃,5% CO2)中培养。取对数生长期的HepG2细胞分为对照组(正常培养的HepG2细胞)、Aca组(10 μmol/L)[8]、si-NC组(转染si-NC)、si-PINK1组[9](转染si-PINK1)、Aca+si-NC组(转染si-NC后用10 μmol/L Aca处理)、Aca+si-PINK1组(转染si-PINK1后用10 μmol/L Aca处理),转染过程均使用Lipofectamine 2000转染试剂盒进行,每组设置6个平行样本,48 h后收集各组细胞用于后续实验。
1.3 CCK-8法检测各组细胞增殖水平 细胞以5×104个/孔的密度接种在含有10% FBS的DMEM培养基的96孔板中,分别在细胞培养的0、24、48 h时,向每孔加入10 μL CCK-8试剂,在37 ℃、5%CO2加湿培养箱中培养1 h,用酶标仪测量450 nm波长处的光密度(OD)值,计算细胞存活率=(实验组OD值/对照组OD值)×100%。
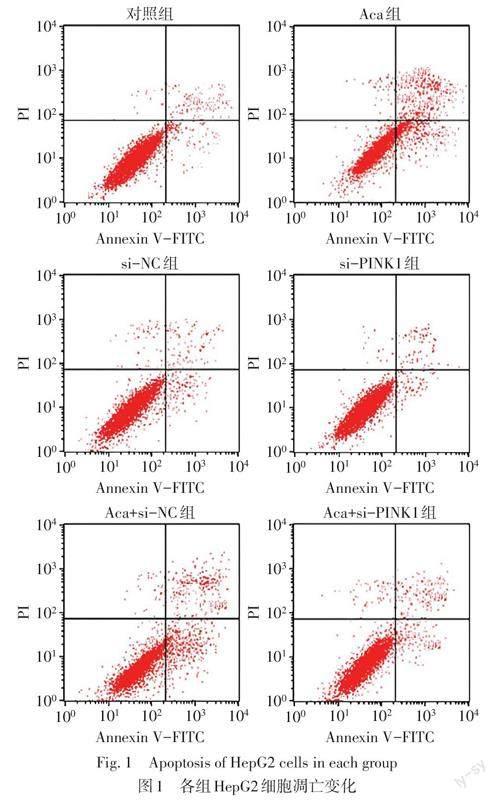
1.4 流式细胞术检测各组细胞凋亡 收获各组细胞并用预冷的PBS洗涤2次后重悬,将5 μL Annexin V-FITC和10 μL PI添加到100 μL细胞(5×104个)中,使用CellQuest Pro 0.9.13软件通过流式细胞仪进行分析。细胞凋亡率(%)=(凋亡细胞数目/总细胞数目)×100%。
1.5 Transwell检测各组细胞迁移 将细胞以2×105个/孔的密度接种于无血清DMEM培养液的Transwell上室,下室填充含有12% FBS的DMEM培养液,孵育48 h后,用棉签小心擦去膜上表面的细胞,将通过膜的细胞用4%多聚甲醛固定,并用0.1%结晶紫染色,在光学显微镜下随机读取5个视野进行观察并计算迁移细胞数目。
1.6 透射电镜观察自噬小体的形成 将细胞用2.5%戊二醛和1%锇酸固定,经脱水,制片,醋酸双氧铀、柠檬酸铅各复染15 min后,利用透射电子显微镜观察细胞中自噬小体的形成情况并拍照。
1.7 Western blot检测各组细胞中自噬及PINK1/Parkin通路相关蛋白表达 使用RIPA裂解缓冲液裂解并提取细胞中的总蛋白,定量蛋白质浓度后,将等量的蛋白质(50 ?g)进行电泳分离并转移到聚偏二氟乙烯膜上,5%脱脂奶粉封闭1 h后,加入一抗Beclin-1(1∶800)、LC3-Ⅰ(1∶800)、LC3-Ⅱ(1∶800)、PINK1(1∶1 000)、Parkin(1∶1 000)、β-actin(1∶1 500)在4 ℃下孵育过夜,然后与羊抗兔二抗(1∶2 000)在37 ℃下孵育2 h,利用ECL化学发光试剂盒观察蛋白条带显色情况,使用Image J(v1.8.0)软件分析蛋白质条带灰度值。
1.8 统计学方法 采用SPSS 24.0软件进行数据分析。符合正态分布的计量资料以x±s表示,多组间比较用单因素方差分析,组间多重比较用SNK-q法,P<0.05为差异有统计学意义。
2 结果
2.1 各组HepG2细胞增殖能力比较 0 h时,各组HepG2细胞存活率差异无统计学意义(P>0.05)。24 h和48 h时,与对照组比较,Aca组HepG2细胞存活率降低,si-PINK1组HepG2细胞存活率升高(P<0.05);与Aca组、Aca+si-NC组比较,Aca+si-PINK1组HepG2细胞存活率升高(P<0.05),见表1。
2.2 各组HepG2细胞凋亡率、迁移能力、自噬小体形成情况比较 与对照组比较,Aca组HepG2细胞凋亡率、自噬小体数量升高,迁移细胞数目降低(P<0.05),si-PINK1组HepG2细胞凋亡率、自噬小体数量降低,迁移细胞数目升高(P<0.05);与Aca组、Aca+si-NC组比较,Aca+si-PINK1组HepG2细胞凋亡率、自噬小体数量降低,迁移细胞数目升高(P<0.05),见表2,图1~3。
2.3 各组HepG2细胞中相关蛋白相对表达水平比较 与对照组比较,Aca组HepG2细胞中Beclin-1、LC3-Ⅱ/LC3-Ⅰ、PINK1、Parkin蛋白表达水平升高(P<0.05),si-PINK1组HepG2细胞中Beclin-1、LC3-Ⅱ/LC3-Ⅰ、PINK1、Parkin蛋白表达水平降低(P<0.05);与Aca组、Aca+si-NC组比较,Aca+si-PINK1组HepG2细胞中Beclin-1、LC3-Ⅱ/LC3-Ⅰ、PINK1、Parkin蛋白表达水平降低(P<0.05),见图4、表3。
3 讨论
Aca是一种在癌症、炎症、感染和其他代谢紊乱方面具有多种治疗潜力的黄酮[10]。研究显示,Aca可诱导前列腺癌[11]和结直肠癌[12]细胞凋亡,抑制恶性乳腺上皮细胞迁移[13]。本研究结果显示,与对照组比较,Aca组HepG2细胞存活率、迁移细胞数目降低,凋亡率升高,推测Aca可能通过抑制细胞增殖、迁移,诱导细胞凋亡的方式抑制肝癌的进展。
线粒体自噬是一种选择性自噬,可促进线粒体更新并防止功能失调线粒体的积累以维持细胞稳态[14]。越来越多的证据表明,线粒体自噬对于癌症的生长和转移至关重要[15],适度激活自噬可延缓肿瘤的恶性进展。如激活线粒体自噬可抑制三阴性乳腺癌细胞增殖与转移[16];黄芪多糖可通过激活线粒体自噬来抑制肝癌细胞增殖[17]。自噬体的形成依赖于Beclin-1[18]、LC3等各种标志物的表达。LC3是自噬的标志性蛋白,由细胞质中的LC3-Ⅰ和自噬体膜上的LC3-Ⅱ组成,LC3蛋白从LC3-Ⅰ到LC3-Ⅱ的转化被广泛认为是自噬激活的标志[19]。本研究结果显示,与对照组比较,Aca组HepG2细胞中自噬小体数量、Beclin-1、LC3-Ⅱ/LC3-Ⅰ蛋白表达水平升高,推测Aca可能通过激活线粒体自噬来抑制HepG2细胞的增殖、迁移,促进其凋亡。
PINK1/Parkin通路是细胞应激下线粒体自噬的主要通路,在细胞应激条件下,PINK1会在线粒体外膜上积聚,进而磷酸化并激活Parkin,活化的Parkin可以诱导各种线粒体外膜蛋白的泛素化,泛素标记的外膜蛋白被自噬受体蛋白p62(p62/SQSTM1)识别,导致被自噬体包裹并最终被自噬溶酶体降解[20]。相关研究显示,PINK1和Parkin的低表达可以降低腮腺多形性腺瘤线粒体自噬活性,且与肿瘤的发生、发展密切相关[21]。δ-缬草甜菜碱通过激活PINK1/Parkin通路来诱导线粒体自噬,进而抑制结肠癌细胞增殖,促进结肠癌细胞凋亡[22]。本研究结果显示,与对照组比较,Aca组HepG2细胞中PINK1、Parkin蛋白表达水平升高,推測Aca对HepG2细胞增殖、迁移的抑制以及细胞凋亡的促进作用可能与PINK1/Parkin通路介导的线粒体自噬有关。笔者进一步通过转染si-PINK1以干扰PINK1/Parkin通路,发现沉默PINK1后HepG2细胞中PINK1、Parkin蛋白表达水平降低,HepG2细胞增殖、迁移能力增强,凋亡能力、线粒体自噬减弱,提示PINK1/Parkin通路确实参与了HepG2细胞增殖、迁移、凋亡、线粒体自噬过程。为了进一步验证上述推测,本研究在Aca作用的基础上以si-PINK1干预HepG2细胞,结果显示,si-PINK1减弱了Aca对HepG2细胞增殖、迁移的抑制作用,以及对细胞凋亡及线粒体自噬的促进作用,证实了Aca可能通过激活PINK1/Parkin通路介导的线粒体自噬抑制肝癌HepG2细胞增殖、迁移,促进细胞凋亡。
综上所述,Aca可通过激活PINK1/Parkin通路介导的线粒体自噬,从而抑制肝癌HepG2细胞的增殖、迁移,促进肝癌细胞凋亡。然而,Aca是否还通过其他通路介导的线粒体自噬调控肝癌HepG2细胞的生物学行为,有待后续实验进一步探究。
参考文献
[1] AMINI M,LOOHA M A,ZAREAN E,et al. Global pattern of trends in incidence,mortality, and mortality-to-incidence ratio rates related to liver cancer,1990-2019:a longitudinal analysis based on the global burden of disease study[J]. BMC Public Health,2022,2(1):604-629. doi:10.1186/s12889-022-12867-w.
[2] 李彦南,刘瑞宝. 肝癌介入与免疫联合治疗的机制与进展[J]. 现代肿瘤医学,2022,30(14):2658-2661. LI Y N,LIU R B. Mechanism and progress of combined intervention and immunotherapy for liver cancer[J]. Journal of Modern Oncology,2022,30(14):2658-2661. doi:10.3969/j.issn.1672-4992.2022. 14.039.
[3] RUMGAY H,FERLAY J,MARTEL C,et al. Global,regional and national burden of primary liver cancer by subtype[J]. Eur J Cancer,2022,161(1):108-118. doi:10.1016/j.ejca.2021.11.023.
[4] ZHANG X,XIN Y,YANG Y,et al. Aspartate aminotransferase-to-platelet ratio index for predicting late recurrence of hepatocellular carcinoma after radiofrequency ablation[J]. Int J Hyperthermia,2022,39(1):1437-1445. doi:10.1080/02656736.2022.2043457.
[5] 马纳,李亚静,范吉平. 金合欢素药理研究进展[J]. 中国现代应用药学,2018,35(10):1591-1595. MA N,LI Y J,FAN J P. Advances in pharmacological research of acacetin[J]. Chinese Journal of Modern Applied Pharmacy,2018,35(10):1591-1595. doi:10.13748/j.cnki.issn1007-7693.2018.10.035.
[6] ZHANG G,LI Z,DONG J,et al. Acacetin inhibits invasion,migration and TGF-β1-induced EMT of gastric cancer cells through the PI3K/Akt/Snail pathway[J]. BMC Complement Med Ther,2022,22(1):10-21. doi:10.1186/s12906-021-03494-w.
[7] LOU Y,MA C,LIU Z,et al. Antimony exposure promotes bladder tumor cell growth by inhibiting PINK1-Parkin-mediated mitophagy[J]. Ecotoxicol Environ Saf,2021,221(1):112420-112429. doi:10.1016/j.ecoenv.2021.112420.
[8] ZENG W,ZHANG C,CHENG H,et al. Targeting to the non-genomic activity of retinoic acid receptor-gamma by acacetin in hepatocellular carcinoma[J]. Sci Rep,2017,7(1):348-360. doi:10.1038/s41598-017-00233-5.
[9] 王婧,楊雯,涂琦,等. 灯盏花素通过调节PINK1/Parkin信号通路介导的线粒体功能诱导人宫颈癌HeLa细胞凋亡[J]. 实用医学杂志,2020,36(24):3333-3337. WANG J,YANG W,TU Q,et al. Breviscapine induces apoptosis of human cervical cancer HeLa cells by regulating mitochondrial function mediated by PINK1/Parkin signaling pathway[J]. The Journal of Practical Medicine,2020,36(24):3333 -3337. doi:10.3969/j.issn.1006-5725.2020.24.006.
[10] SINGH S,GUPTA P,MEENA A,et al. Acacetin,a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders[J]. Food Chem Toxicol,2020,145(1):111708-111725. doi:10.1016/j.fct.2020.111708.
[11] YUN S,LEE Y J,CHOI J,et al. Acacetin inhibits the growth of STAT3-activated DU145 prostate cancer cells by directly binding to signal transducer and activator of transcription 3 (STAT3)[J]. Molecules,2021,26(20):6204-6223. doi:10.3390/molecules26206204.
[12] PRASAD N,SHARMA J R,YADAV U C S. Induction of growth cessation by acacetin via β-catenin pathway and apoptosis by apoptosis inducing factor activation in colorectal carcinoma cells[J]. Mol Biol Rep,2020,47(2):987-1001. doi:10.1007/s11033-019-05191-x.
[13] JONES A A,GEHLER S. Acacetin and pinostrobin inhibit malignant breast epithelial cell adhesion and focal adhesion formation to attenuate cell migration[J]. Integr Cancer Ther,2020,19(1):1-12. doi:10.1177/1534735420918945.
[14] YAO N,WANG C,HU N,et al. Inhibition of PINK1/Parkin-dependent mitophagy sensitizes multidrug-resistant cancer cells to B5G1,a new betulinic acid analog[J]. Cell Death Dis,2019,10(3):232-247. doi:10.1038/s41419-019-1470-z.
[15] PANIGRAHI D P,PRAHARAJ P,BHOL C S,et al. The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics[J]. Semin Cancer Biol,2020,66(1):45-58. doi:10.1016/j.semcancer.2019.07.015.
[16] XIA J,CHU C, LI W,et al. Mitochondrial protein UCP1 inhibits the malignant behaviors of triple-negative breast cancer through activation of mitophagy and pyroptosis[J]. Int J Biol Sci, 2022,18(7):2949-2961. doi:10.7150/ijbs.68438.
[17] 張慧蓉,钱敏,李萌. 黄芪多糖通过线粒体自噬抑制肝癌肿瘤细胞[J]. 西北药学杂志,2021,36(3):426-429. ZHANG H R,QIAN M,LI M. Astragalus polysaccharide inhibits liver cancer tumor cells through mitophagy[J]. Northwest Pharmaceutical Journal,2021,36(3):426-429. doi:10.3969/j.issn.1004-2407.2021.03.017.
[18] CHANG S N,KHAN I,KIM C G,et al. Decursinol angelate arrest melanoma cell proliferation by initiating cell death and tumor shrinkage via induction of apoptosis[J]. Int J Mol Sci,2021,22(8):4096-4114. doi:10.3390/ijms22084096.
[19] YOU Y,LI J,CHEN L,et al. Photothermal killing of A549 cells and autophagy induction by bismuth selenide particles[J]. Materials(Basel),2021,14(12):3373-3388. doi:10.3390/ma14123373.
[20] WANG Y,TANG C,CAI J,et al. PINK1/Parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury[J]. Cell Death Dis,2018,9(11):1113-1126. doi:10.1038/s41419-018-1152-2.
[21] 丁高峰,郭雷鸣,柯少瑞,等. 腮腺多形性腺瘤及癌在多形性腺瘤中PINK1和Parkin的表达及临床意义[J]. 肿瘤防治研究,2021,48(5):470-473. DING G F,GUO L M,KE S R,et al. Expression and clinical significance of PINK1 and Parkin in pleomorphic adenoma and carcinoma of parotid gland in pleomorphic adenoma[J]. Cancer Research on Prevention and Treatment,2021,48(5):470-473. doi:10.3971/j.issn.1000-8578.2021.20.0923.
[22] D'ONOFRIO N,MARTINO E,MELE L,et al. Colorectal cancer apoptosis induced by dietary δ-valerobetaine involves PINK1/Parkin dependent-mitophagy and SIRT3[J]. Int J Mol Sci,2021,22(15):8117-8133. doi:10.3390/ijms22158117.
(2022-07-15收稿 2022-09-21修回)
(本文编辑 陆荣展)

