脱脂对镀锌汽车板涂装性能影响
龙袁,郝玉林,蔡宁,周纪名,姚士聪,曹建平,刘华赛,李学涛,杨建炜
脱脂对镀锌汽车板涂装性能影响
龙袁1,2,郝玉林1,蔡宁1,周纪名3,姚士聪1,曹建平1,刘华赛1,李学涛1,杨建炜1
(1.首钢集团有限公司技术研究院,北京 100043;2.绿色可循环钢铁流程北京市重点实验室,北京 100043;3.首钢京唐钢铁联合有限责任公司制造部,河北 唐山 063210)
研究脱脂影响镀锌钢板磷化反应的机理及电泳漆膜性能。使用扫描电镜、电化学工作站、X射线光电子能谱分析仪、成形试验机和循环式腐蚀试验机等设备研究了磷化膜形貌、表面元素、电化学性能以及电泳漆膜性能。脱脂不良时磷化膜在镀锌钢板表面生长异常,导致磷化膜呈现为明暗相间的花斑状;表面元素和材料在磷化液中的电化学性能说明磷化膜生长异常的原因是残存油膜阻碍磷化反应进行,同时异常磷化膜相对于正常磷化膜在3.5% NaCl溶液中开路电位负、阻抗小、自腐蚀电流大,说明异常磷化膜耐蚀性能差,易腐蚀;电泳漆膜性能表明,脱脂不良对漆膜的划格附着力影响小,评级为0级,但对电泳漆膜的杯突性能影响明显,杯突高度为6 mm时,漆膜发生破裂;电泳耐蚀性能下降明显,电泳漆膜扩蚀宽度从2.4 mm增加到3.9 mm。脱脂不良会导致镀锌板表面磷化膜质量异常,进而影响电泳耐蚀性能,在实际生产过程中,需要对脱脂工序有效管控,结合车身材料用油种类及油量及时调控参数,避免脱脂不良问题出现。
汽车板;脱脂;磷化;电泳;耐蚀
由于锌可以作为牺牲阳极的材料保护钢材不受腐蚀,所以热浸镀锌钢板和电镀镀锌钢板常用于高端汽车品牌外板零件[1-2]。为了延长车身使用寿命,汽车厂会对车身进行涂装来增强车身耐蚀性能。涂装主要包括磷化前处理、电泳和喷漆3个步骤,其中磷化前处理包括脱脂、表调和磷化3个工序。作为电泳的前道工序,磷化前处理十分重要,直接决定了漆膜的附着力和涂装后的耐蚀性能[3-5]。
影响磷化膜性能的因素非常多,从前处理工艺本身到钢铁材料成分及表面性能都会直接影响磷化膜性能。Oh等[6]研究了Zn2+、Mn2+、Ni2+离子对镀锌板磷化膜耐蚀性能的影响,Mn2+、Ni2+离子对磷化膜耐蚀影响明显,Mn2+浓度增加提升耐蚀性能,而Ni2+浓度增加降低耐蚀性能,Zn2+离子浓度增加对磷化膜耐蚀没有明显作用。Furuya等[7]研究了酸洗钢板成分中V元素对磷化膜性能的影响,当V元素含量超过0.47%时,其氧化物容易与水反应,生成较多H+,抑制磷化反应,导致钢板表面难以上膜。事实上,钢板成分Cr、Mn、Si、Ti、Cu等元素超出一定含量[8-11]以及表面的氧化物[12-14]都会抑制磷化反应。Tsai等[15]研究表明Mg2+浓度增大,能够细化磷化膜结晶尺寸,降低磷化膜孔隙率,从而提升镀锌板磷化膜耐蚀性能。钢板表面粗糙度也会对磷化膜及相关转化膜产生影响,相关研究表明:粗糙度在一定范围内,Ra值越小,RPc值越大,能够增加磷化时的活性形核点,降低磷化膜颗粒尺寸,促进磷化进程,提高磷化膜质量[16-17]。
以上的研究主要集中在磷化液成分及钢铁材料表面元素对材料磷化性能的影响,而针对磷化工序中的脱脂对磷化膜和电泳后的性能研究较少。因此本研究以脱脂工序为主,重点分析脱脂不良时,对影响磷化反应的机理、磷化膜耐蚀性以及电泳漆膜的附着力和耐蚀性能展开研究。
1 试验
1.1 试验材料
试验材料选用工业化生产的热浸镀锌钢板CR4+Z,规格0.65 mm,涂覆某厂550型号防锈油,涂油量为0.5~0.9 g/m2,镀层重量为双面100 g/m2。表面粗糙度Ra(2.5)在0.6~1.0 µm,RPc在60~100 cm–1。
材料前处理在实验室进行,电泳在汽车厂随线完成。脱脂工艺参数:pH=12,游离碱11 pt,温度50~55 ℃。脱脂时间分为2种:4 min(脱脂不良),5 min(脱脂正常);表调工艺:pH=10;磷化工艺:磷化液总酸22 pt,游离酸0.7 pt,促进剂3.0 pt,磷化温度35~45 ℃,磷化时间120 s;电泳工艺:电压200~350 V,灰分18.5%~21.5%,pH为6.0~6.6,烘干温度180~210 ℃。实验室前处理流程为:脱脂®水洗®水洗®表调®磷化®水洗®水洗。汽车厂前处理+电泳流程为:脱脂®水洗®水洗®表调®磷化®水洗®水洗®电泳®烘干。
1.2 试验方法
1)使用日本电子公司JSM-7001F扫描电镜分析钢板表面及截面磷化膜形貌。
2)钢板表面活性使用美国普林斯顿公司生产的PARSTAT2273型电化学工作站进行分析,测试介质为常温磷化液或3.5% NaCl溶液,测量系统为三电极体系,铂电极为辅助电极,饱和甘汞电极(SCE)为参比电极,研究试样为工作电极(暴露面积为1 cm2)。
3)表面元素采用美国塞默飞世尔公司ESCALAB 250Xi型X射线光电子能谱分析仪进行分析,设备采用AlKα(1 486.6 eV)作为射线激发源,光斑直径为650 µm,用C1s的结合能(284.8 eV)作为内标,对测得的谱峰进行校正。
4)按照《ISO 2409:2020 Paints and varnishes- Cross-cut test》标准,使用上海现代环境工程技术有限公司QFH划格器进行漆膜划格测试,划格间距为1 mm;
5)按照《ISO 1520:2006 Paints and varnishes- Cupping test》标准,使用德国ZWICK公司BUP1000型号成型试验机进行漆膜杯突性能测试,冲头形状为直径20 mm的半球形,冲头速度为0.2 mm/s。
6)使用美国Q-Lab公司Q-FOG循环式腐蚀试验机进行电泳漆膜耐蚀性能分析,采用的腐蚀标准为《ISO 11997-1-2017 Paints and varnishes-Determination of resistance tocyclic corrosion conditions-Part 1: Wet (salt fog)/dry/humid》中的Cycle B腐蚀标准,漆面划线破坏及扩蚀宽度测量分别采用《ISO17872: 2007 Paints and varnishes-Guidelines for the introduction of scribe marks through coatings on metallic panels for corrosion testing》和《ISO 4628-8-2012 Paints and varnishes-Evaluation of degradation of coatings-Designation of quantity and size of defects, and of intensity of uniform changes in appearance-Part 8: Assessment of degree of delamination and corrosion around a scribe or other artificial defect》标准,加速腐蚀时间为10个周期,共计1 680 h。
2 结果及分析
2.1 磷化膜分析
CR4+Z在脱脂不良和正常脱脂条件下的磷化膜宏观形貌如图1所示,磷化膜的微观形貌如图2所示。材料脱脂不良时,材料表面水膜不连续,存在滴状水流,如图1a红色箭头指示处;材料脱脂正常时,表面水膜连续不破裂,如图1b所示。材料脱脂不良和正常脱脂经过磷化处理后,呈现为不同的宏观形貌,脱脂不良时,材料的磷化膜表现为花斑状,明暗相间,如图1c所示;脱脂正常时,材料的磷化膜均匀、致密、连续,呈灰色,表面无锈蚀、花斑,无大的磷化渣附着,也无严重挂灰,外观质量良好,如图1d所示。

图1 材料脱脂和磷化膜宏观照片
磷化膜的微观形貌如图2所示。图2a、2b分别为图1c中1和2位置的磷化膜电镜图,1位置处磷化膜为扁平化生长,平铺在钢板表面,2位置处磷化膜为团簇状,少量磷化膜垂直于钢板方向生长,大部分存在倒伏现象,近似于1位置处磷化膜为扁平化生长,图2c为图1d正常磷化膜的微观形貌,整体为团簇状,垂直于钢板方向生长,倒伏少。从微观形貌分析可知,磷化膜在钢板表面存在状态的差异是导致了磷化膜在宏观外观上存在区别的原因。磷化膜存在扁平生长和较多倒伏的状态下,对光线的镜面反射率较高,磷化膜外观表现比较亮,而正常磷化膜对光线漫反射比例较高,外观表现颜色较暗。
由图3材料磷化处理后的截面图像可见,镀层的厚度为6~8mm,磷化膜存在扁平生长和较多倒伏的状态下,磷化膜的厚度为0.5~1mm,磷化膜正常时,厚度在1~2mm,磷化膜异常处厚度明显低于正常处。
按照《ISO 3892:2000 Conversion coatings on metallic materials-Determination of coating mass per unit area-Gravimetric methods》标准将磷化膜溶解去除,再进行元素的X射线光电子能谱分析,结果如图4所示。磷化膜去除后,计算得到磷化膜异常时膜重为1.67 g/m2,磷化膜正常时膜重为2.79 g/m2,说明磷化膜异常时膜厚偏薄,与图3的分析结果是一致的。材料表面主要以Zn和O元素为主,含有少量P和Al元素。因为镀锌锌锅中含有质量分数小于0.5%的Al,所以Al元素来自于Zn镀层。脱脂好坏对磷化膜去除后材料表面元素种类影响较小。从P 2p结合能为134.2 eV[18],可以判断P以磷酸盐的形式存在于材料表面,Al 2p结合能为74.6 eV[19],说明Al元素以氧化物形式存在,而Zn 2p1/2及2p3/2的结合能分别为1 021.9 eV和1 045.0 eV,说明Zn在材料表面存在ZnO及ZnAl2O4[20]。由于磷酸盐和金属氧化物中都存在氧元素,结合锌和铝的氧化物及磷酸盐中O元素的结合能分别为530.3 eV[21-22]和531.7 eV[18],分峰拟合后计算得到磷化膜异常处的金属氧化物和磷酸盐含量分别为39%和61%,磷化膜正常处的金属氧化物和磷酸盐含量分别为21%和79%。磷化膜异常处金属氧化物含量明显高于正常处,这说明脱脂不良时,材料表面存在的油膜阻碍了磷化液与镀锌层表面的反应,镀锌层表面的氧化物溶解减缓,从而阻碍镀锌层的溶解,导致Zn2+溶解量较少,抑制了磷化反应,从而导致磷化膜生长异常。
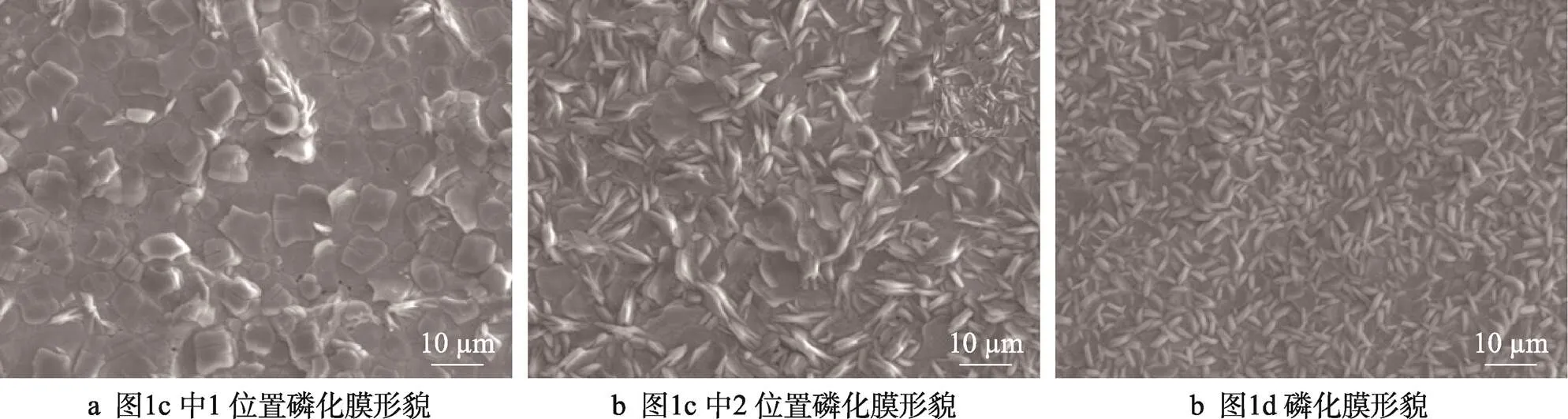
图2 不同位置磷化膜微观形貌
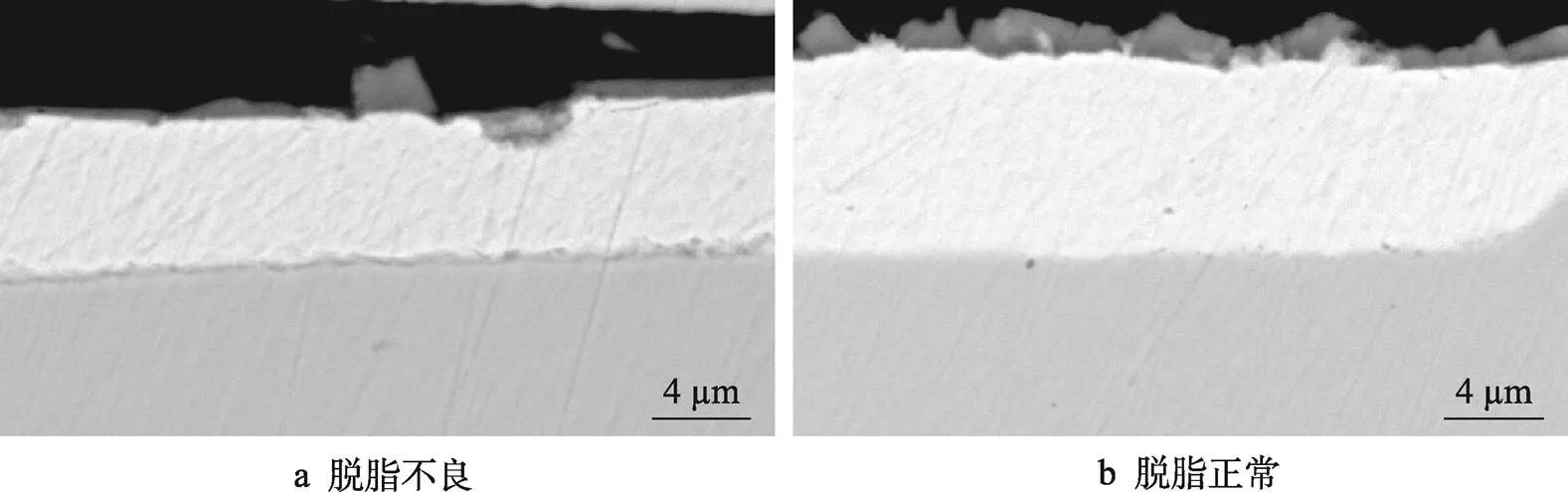
图3 不同脱脂条件下的磷化膜截面图
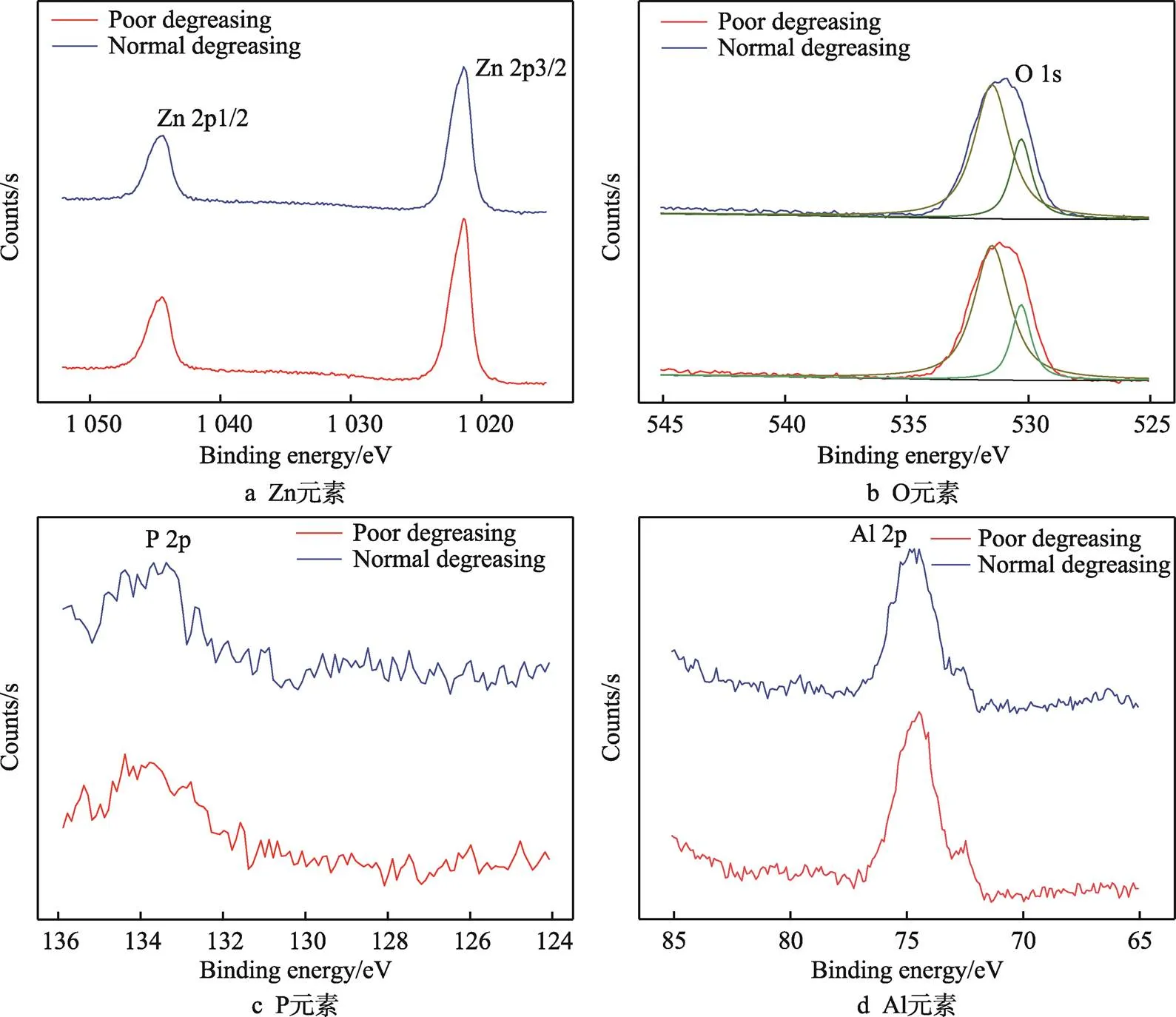
图4 不同脱脂条件下磷化膜溶解后钢板表面元素XPS结果
2.2 磷化膜电化学性能分析
镀锌钢板在磷化液中的反应过程如式(1)~(4)[23]。磷化液中磷酸发生解离,生成H+,镀锌钢板进入磷化液后,Zn与H+发生反应,形成Zn2+,同时消耗H+,使反应(4)正向进行,在钢板表面形成磷化膜Zn3(PO4)2×4H2O。
通过电位-时间的测定,可以监测磷化膜的生长过程。图5展示了在脱脂不良和脱脂正常的条件下,镀锌板经过表调之后在磷化液中的电位变化。明显发现,在脱脂正常的条件下,镀锌板在磷化液中的开路电位分为4个阶段[24-25],第Ⅰ阶段(0~5 s)电位从初始值位置轻微升高,对应镀锌板表面Zn层初期溶解活化过程;由于溶解速度加快,在局部阳极区域产生的Zn(H2PO4)2浓度迅速增加而达到饱和,并在局部阴极溶解以不可逆的无定形形态沉积在Zn层表面,导致电位下降,即第Ⅱ阶段磷化膜早期快速萌发和生长过程(5~25 s);随着磷化膜在钢板表面逐渐形成,磷化膜覆盖率逐渐提高,Zn层裸露面积逐渐减少,锌的溶解速度减慢,阳极过程逐渐减慢,导致电位逐渐上升,90 s时为反应平衡节点,即第Ⅲ阶段(25~90 s);在磷化反应的最后第Ⅳ阶段(>90 s),Zn层表面全部覆盖磷化膜,同时抑制了阳极过程(Zn层溶解)和阴极过程(析氢反应),处于反应平衡阶段,最终电位保持稳定。依据上述磷化膜的生长过程,将磷化时间定为120 s是相对合理的,一方面可以避免在90 s反应平衡节点附近磷化膜质量的波动,确保磷化膜质量,另一方面可以避免磷化时间过长导致涂装生产效率下降。

图5 镀锌板在磷化液中的开路电位


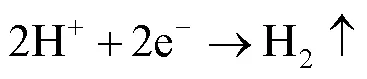
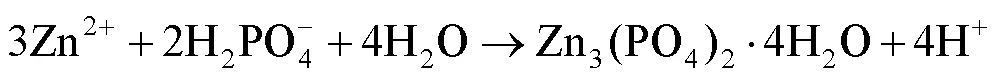
脱脂不良时,由于表面残存油脂阻碍镀层与磷化液的接触,抑制了阳极反应,所以第Ⅰ、Ⅱ阶段相对于脱脂正常时不明显,第Ⅲ阶段磷化膜生长速度也相对较慢,最后阶段Ⅳ电位稳定时的电位也相对较负,说明脱脂不良时获得的磷化膜质量相对较差,耐蚀性能弱。
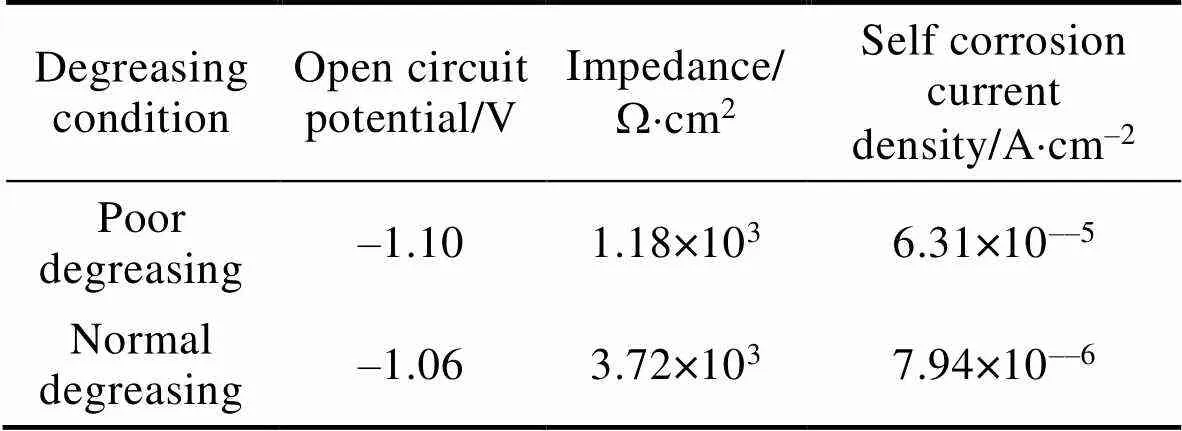
为了进一步分析磷化膜的耐蚀性能,在3.5%的NaCl溶液中分析了不同脱脂条件下磷化膜的开路电位,阻抗和极化性能,结果如图6及表1所示。脱脂不良和脱脂正常时磷化膜阻抗图均为双阻抗弧,脱脂正常时磷化膜阻抗为3.72×103W×cm2,脱脂异常时磷化膜阻抗为1.18×103W×cm2,正常磷化膜阻抗大于异常磷化膜阻抗;从开路电位和极化曲线分析可知,脱脂正常时磷化膜开路电位为–1.06 V,自腐蚀电流密度为7.94×10–6A×cm–2,脱脂异常时磷化膜开路电位为–1.10 V,自腐蚀电流密度为6.31×10–5A×cm–2,说明脱脂异常时的磷化膜容易发生腐蚀,异常的磷化膜腐蚀速率会比正常的磷化腐蚀速率快,因此脱脂不良时获得的磷化膜耐蚀性能差,易腐蚀。
2.3 电泳漆膜性能分析
电泳涂装是在电泳涂料胶体中,将具有导电性的被涂物作为阴极(或阳极),在电泳槽的两侧另设置与其对应的阳极(或阴极),在两电极间通直流电,在直流电场作用下,带电荷的涂料胶体粒子向工件移动,在被涂物表面上析出均一、绝缘、水不溶的涂膜的一种方法。电泳一共包括电解、电泳、电沉积和电渗4个物理化学作用。汽车厂目前主要使用的是阴极电泳,也就是白车身或者汽车零件作为阴极。阴极电泳涂料主要成分为带正电荷的胺盐,阴阳极电解反应及电沉积反应如式(5)~(7):

图6 不同脱脂条件下的磷化膜在3.5%NaCl溶液中的开路电位、阻抗和极化曲线
表1 不同脱脂条件下的磷化膜在3.5%NaCl溶液中的开路电位、阻抗值和自腐蚀电流密度
Tab.1 Open circuit potential, impedance and self-corrosion current density of phosphating films in 3.5%NaCl solution under different degreasing conditions


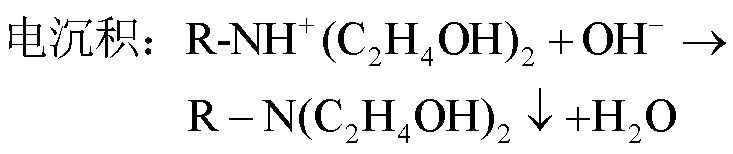
磷化作为汽车板涂装前处理工序,其主要目的是提高钢板漆膜的附着力和膜下耐蚀性能。因此,以下内容将主要分析镀锌钢板在不同脱脂工艺条件下获得的磷化膜对电泳之后的漆膜性能和耐蚀性能的影响。
按照《ISO 2409:2020 Paints and varnishes-Cross- cut test》国际标准进行电泳漆膜的划格实验,结果如图7所示。根据标准0~5级的评级分级,脱脂不良和脱脂正常时,划格处的切割边缘完全平滑,网格内没有漆膜剥落,评级均为0级,这说明钢板在一定程度脱脂不良条件下,对电泳漆膜划格附着力的影响较小。
杯突实验可以测试漆膜在变形后的碎裂或与金属底材分离的程度,反映漆膜在基材上的附着力大小。漆膜的杯突性能按照《ISO 1520:2006 Paints and varnishes-Cupping test》国际标准进行测试。一般情况下,汽车厂对电泳板杯突的要求为:当杯突高度为6 mm时,漆膜不发生破裂即认为合格[26]。因此本研究杯突高度设置为6 mm。
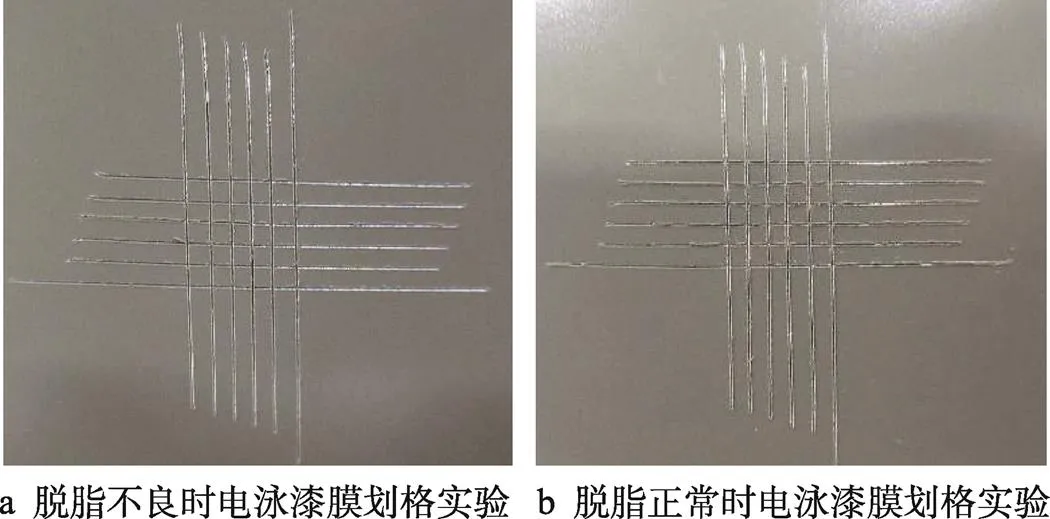
图7 电泳漆膜划格实验
如图8所示,当杯突高度为6 mm时,脱脂不良电泳板漆膜发生破裂,而脱脂正常时,杯突6 mm高度时,漆膜完整未破裂,说明磷化膜异常会导致电泳板的杯突性能下降,同时也说明漆膜与基板的附着力发生下降。
电泳板耐蚀性能一般采用加速腐蚀实验进行评估,结果表明脱脂不良时,电泳板单边扩蚀宽度为3.9 mm;脱脂正常时,电泳板单边扩蚀宽度为2.4 mm,如图9所示。出现差别的原因是脱脂不良引起磷化膜膜重低,微观形貌异常,导致其磷化膜本身耐蚀性能下降、电泳漆膜附着力下降,在膜下腐蚀过程中,漆膜破坏处的磷化膜优先发生腐蚀,产生的腐蚀产物体积膨胀,撑破漆膜,造成划线处扩蚀宽度变大[27]。因此,脱脂不良会导致电泳板耐蚀性能明显下降,不利于车身防腐,缩短车身寿命,若涉及关键结构件,甚至会危害行车安全。
在涂装产线生产过程中,一旦发生脱脂不良的现象,如图10a红色圈内所示,就会影响到车身磷化膜外观,出现明暗相间的花斑,如图10b红色圈内所示。出现此类问题时,若继续进行电泳及后续喷漆,车身耐蚀性能难以保障,需要对车身花斑处打磨处理,重新进行前处理,确保车身电泳后的耐蚀性能。同时需要反查脱脂工艺是否出现不合理变动以及材料涂油状态,确保脱脂正常,避免出现磷化膜花斑问题。

图10 涂装线脱脂不良照片及磷化膜外观
3 结论
脱脂作为涂装工序的第一步,对涂装质量有决定性影响。通过以上研究发现:
1)镀锌板脱脂不良,油膜的残留阻碍了锌层表面与磷化液充分反应,抑制了磷化膜成膜,引起磷化膜偏平化生长,磷化膜生长异常是材料外观上呈现花斑状缺陷的根本原因。
2)磷化膜异常时,会直接影响电泳漆膜质量,造成电泳漆膜杯突性能和耐蚀性能下降。
3)在实际生产中,若发现磷化膜出现花斑状态,需要对脱脂工艺或者材料的涂油种类和涂油量进行反查,从而解决脱脂不良问题。
[1] KANCHARLA H, MANDAL G K, SINGH S S, et al. Effect of Prior Copper-Coating on the Microstructural Development and Corrosion Behavior of Hot-Dip Galvanized Mn Containing High Strength Steel Sheet[J]. Surface and Coatings Technology, 2022, 437: 128347.
[2] 李研, 李铁军, 滕华湘, 等. 热镀锌汽车板波纹度的控制技术[J]. 金属热处理, 2019, 44(4): 207-210. LI Yan, LI Tie-jun, TENG Hua-xiang, et al. Control Technology of Waviness on Galvanized Auto Sheet[J]. Heat Treatment of Metals, 2019, 44(4): 207-210.
[3] ZIMMERMANN D, MUÑOZ A G, SCHULTZE J W. Microscopic Local Elements in the Phosphating Process[J]. Electrochimica Acta, 2003, 48(20-22): 3267-3277.
[4] JIANG L, VOLOVITCH P, WOLPERS M, et al. Activation and Inhibition of Zn-Al and Zn-Al-Mg Coatings on Steel by Nitrate in Phosphoric Acid Solution[J]. Corrosion Science, 2012, 60: 256-264.
[5] NARAYANAN S. Surface Pretretament by Phosphate Conversion Coatings - a Review[J].Reviews on advanced materials science, 2005, 9(2):130-177.
[6] OH J E, KIM Y H. The Corrosion Resistance Characteristics of Ni, Mn, and Zn Phosphates in Automotive Body Panel Coatings[J]. Journal of Industrial and Engineering Chemistry, 2012, 18(3): 1082-1087.
[7] FURUYA S, YAMAZAKI K, MASUOKA H, et al. Effects of Solid-Solute V on the Phosphatability of Hot- Rolled Steel Sheets[J]. ISIJ International, 2020, 60(11): 2512-2518.
[8] USUKI N, SAKOTA A, WAKANO S, et al. Effects of Ti and Mn Addition on the Surface State and Phosphatability of Cold Rolled Steel Sheets[J]. Tetsu-to-Hagane, 1991, 77(3): 398-405.
[9] AUGUSTSSON P E, OLEFJORD I, OLEFJORD G Y. The Influence of Annealing on the Phosphatability of Steel Sheets[J]. Materials and Corrosion, 1983, 34(11): 563-569.
[10] GHALI E I, POTVIN R J A. The Mechanism of Phosphating of Steel[J]. Corrosion Science, 1972, 12(7): 583- 594.
[11] KIM H J. Variation of Phosphatability with Chemical Composition and Surface Roughness of Steel Sheet[J]. Surface Engineering, 1998, 14(3): 265-267.
[12] MASUOKA H, FURUYA S, TAKEYAMA H, et al. Relationship between Morphology of Mn Oxides Simulated by Ion Plating and Phosphatability of Mn-Added High-Strength Cold-Rolled Steel Sheets[J]. ISIJ International, 2020, 60(11): 2519-2524.
[13] NOMURA M, HASHIMOTO I, KAMURA M, et al. Development of High Strength Cold-Rolled Steel-Sheets with Excellent Phosohatability[J]. KOBELCO TECHNOLOGY REVIEW, 2008, 10(28):44-48.
[14] TEGEHALL P E, VANNERBERG N G. Nucleation and Formation of Zinc Phosphate Conversion Coating on Cold-Rolled Steel[J]. Corrosion Science, 1991, 32(5-6): 635-652.
[15] TSAI C Y, LIU J S, CHEN Pei-li, et al. Effect of Mg2+on the Microstructure and Corrosion Resistance of the Phosphate Conversion Coating on Hot-Dip Galvanized Sheet Steel[J]. Corrosion Science, 2010, 52(12): 3907-3916.
[16] KIM H Y, NOH Y T, JEON J H, et al. Effect of Surface Roughness on Crystal Size of Manganese Phosphate Coating of Carbon Steel[J]. Journal of Nanoscience and Nanotechnology, 2020, 20(7): 4312-4317.
[17] 赵联. ESP热轧板的涂装行为与涂装性研究[D]. 武汉: 武汉理工大学, 2020. ZHAO Lian. Study on Coating Behaviors and Coating Properties of ESP Hot Rolled Sheet[D]. Wuhan: Wuhan University of Technology, 2020.
[18] DOBBELAERE T, MINJAUW M, AHMAD T, et al. Plasma-Enhanced Atomic Layer Deposition of Zinc Phosphate[J]. Journal of Non-Crystalline Solids, 2016, 444: 43-48.
[19] ISLAM M S, SAKAIRI M. Corrosion Behavior of A6061 Aluminum Alloy in Cation Containing Aqueous Medium [J]. Corrosion Communications, 2022, 5: 39-48.
[20] ZHANG Li, YAN Jian-hui, ZHOU Min-jie, et al. Fabrication and Photocatalytic Properties of Spheres-in- Spheres ZnO/ZnAl2O4Composite Hollow Microspheres [J]. Applied Surface Science, 2013, 268: 237-245.
[21] ZÄHR J, OSWALD S, TÜRPE M, et al. Characterisation of Oxide and Hydroxide Layers on Technical Aluminum Materials Using XPS[J]. Vacuum, 2012, 86(9): 1216- 1219.
[22] MAR L G, TIMBRELL P Y, LAMB R N. An XPS Study of Zinc Oxide Thin Film Growth on Copper Using Zinc Acetate as a Precursor[J]. Thin Solid Films, 1993, 223(2): 341-347.
[23] 郝玉林, 蔡宁, 姚士聪, 等. 高强双相钢表面选择性氧化行为对磷化性能的影响[J]. 表面技术, 2020, 49(8): 309-315, 341. HAO Yu-lin, CAI Ning, YAO Shi-cong, et al. Effect of Selective Oxidation Behavior of High Strength Dual- Phase Steel Surface on Phosphating Properties[J]. Surface Technology, 2020, 49(8): 309-315, 341.
[24] LIN B I, LU Jin-tang, KONG Gang, et al. Growth and Corrosion Resistance of Molybdate Modified Zinc Phosphate Conversion Coatings on Hot-Dip Galvanized Steel[J]. Transactions of Nonferrous Metals Society of China, 2007, 17(4): 755-761.
[25] 冯立明, 管勇. 涂装工艺学[M]. 北京: 化学工业出版社, 2017. FENG Li-ming, GUAN Yong. Painting Technology[M]. Beijing: Chemical Industry Press, 2017.
[26] 龙袁, 刘斌, 姚士聪, 等. 锌铝镁镀层钢板前处理与电泳性能研究[J]. 电镀与涂饰, 2021, 40(20): 1585-1590. LONG Yuan, LIU Bin, YAO Shi-cong, et al. Study on Pretreatment and Electrophoresis Performance of Zn-Al-Mg Alloy Coated Steel Sheet[J]. Electroplating & Finishing, 2021, 40(20): 1585-1590.
[27] AMIRUDIN A, THIERRY D. Corrosion Mechanisms of Phosphated Zinc Layers on Steel as Substrates for Automotive Coatings[J]. Progress in Organic Coatings, 1996, 28(1): 59-75.
Effect of Degreasing on Coating Performance of Galvanized Automobile Sheet
1,2,1,1,3,1,1,1,1,1
(1. Technology Research Institute of Shougang Group Co., Ltd., Beijing 100043, China; 2. Beijing Key Laboratory of Green Recyclable Process for Iron & Steel Production Technology, Beijing 100043, China; 3. Manufacturing Department, Shougang Jingtang United Iron and Steel Co., Ltd., Hebei Tangshan 063210, China)
Degreasing is one of the important processes of coating pretreatment. Its main function is to clean and remove oil stains on the surface of steel sheet, ensure the quality of subsequent phosphating process, and then ensure the film properties of steel sheet after electrophoretic coating, such as film adhesion and corrosion resistance. Since most of the previous studies focus on the composition of phosphating solution and the surface properties of steel sheet, there are few studies on degreasing. Therefore, the work aims to study the mechanism of the effect of degreasing process on the quality of phosphating film and the performance of electrophoretic film.
Hot-dip galvanized steel sheet (CR4+Z) was used in this study. Under laboratory conditions, the discontinuous water film on the surface of the steel sheet indicated poor degreasing when the steel sheet was degreased for 4 min. The results of electron microscopy of phosphating film showed that when the degreasing was poor, the phosphating film grew abnormally and appeared flat, resulting in the appearance of bright and dark patches of phosphating film. The cross-section results showed that the thickness of the flat phosphating film was 0.5~1 µm, which was obviously thinner. After removal of the phosphating film according to ISO 3892:2000, XPS results showed that metal oxides and phosphate mainly existed on the surface of the steel sheet, and the content of metal oxides on the surface of the steel sheet was 39% when the phosphating film was abnormal, which was significantly higher than that when the phosphating film was normal. According to the change of open circuit potential of the steel sheet in phosphating solution, the first, second and third stages of the phosphating reaction were obviously inhibited when the degreasing was not good, which reduced the production efficiency of Zn2+on the surface of the steel sheet, left more metal oxides, weakened the growth rate of the phosphating film, and resulted in the poor corrosion resistance of the phosphating film. The open circuit potential, impedance and polarization curves of phosphating films obtained under different degreasing states in 3.5% NaCl solution showed that the open circuit potential of phosphating films under abnormal degreasing condition was –1.10 V, which was negative to –1.06 V of normal phosphating films. The impedance of 1.18×103W×cm2was smaller than 3.72×103W×cm2of normal phosphating film and the self-corrosion current density of 6.31×10–5A×cm–2was larger than 7.94×10–6A×cm–2of normal phosphating film, indicating that abnormal phosphating film would lead to poor corrosion resistance and easy corrosion. The electrophoretic film properties showed that poor degreasing to a certain extent had little effect on the scratch adhesion of the film, which was rated as 0. However, it had a significant impact on the performance of the cupping of the electrophoretic paint film. When the cupping height was 6 mm, the paint film was broken, which could not meet the quality requirements of the automobile factory. The corrosion resistance of electrophoretic paint film decreased obviously, and the corrosion width of electrophoretic paint film increased from 2.4 mm to 3.9 mm.
In a word, poor degreasing will lead to the abnormal quality of phosphating film on the surface of galvanized sheet, which will affect the electrophoretic corrosion resistance. In the actual production process, it is necessary to effectively control degreasing process temperature, alkalinity, surfactant parameters and spray pressure, and timely control parameters based on the type and amount of oil used in body materials to avoid poor degreasing problems
automobile sheet; degreasing; phosphating; electrophoresis; corrosion resistance
2022-09-26;
2023-02-24
TG147
A
1001-3660(2023)10-0259-08
10.16490/j.cnki.issn.1001-3660.2023.10.021
2022-09-26;
2023-02-24
龙袁, 郝玉林, 蔡宁, 等. 脱脂对镀锌汽车板涂装性能影响[J]. 表面技术, 2023, 52(10): 259-266.
LONG Yuan, HAO Yu-lin, CAI Ning, et al. Effect of Degreasing on Coating Performance of Galvanized Automobile Sheet[J]. Surface Technology, 2023, 52(10): 259-266.
责任编辑:马梦遥

