Effects of mesencephalic astrocyte-derived neurotrophic factor on sepsis-associated acute kidney injury
Saifeng Chen ,Xuewei Hao ,Guo Chen ,Guorong Liu ,Xiaoyan Yuan ,Peiling Shen ,Dongfeng Guo
1 Postgraduate Training Base at Shanghai Gongli Hospital, Ningxia Medical College, Shanghai 200135, China
2 Department of Emergency Medicine, Shanghai Gongli Hospital, Shanghai 200135, China
BACKGROUND: To determine the protective role of mesencephalic astrocyte-derived neurotrophic factor (MANF) in regulating sepsis-associated acute kidney injury (S-AKI).METHODS: A total of 96 mice were randomly divided into the control group,control+MANF group,S-AKI group,and S-AKI+MANF group.The S-AKI model was established by injecting lipopolysaccharide(LPS) at 10 mg/kg intraperitoneally.MANF (200 μg/kg) was administered to the control+MANF and S-AKI+MANF groups.An equal dose of normal saline was administered daily intraperitoneally in the control and S-AKI groups.Serum and kidney tissue samples were obtained for biochemical analysis.Western blotting was used to detect the protein expression of MANF in the kidney,and enzyme-linked immunosorbent assay (ELISA) was used to determine expression of MANF in the serum,pro-infl ammatory cytokines (tumor necrosis factor-α [TNF-α] and interleukin-6 [IL-6]).Serum creatinine (SCr),and blood urea nitrogen (BUN)were examined using an automatic biochemical analyzer.In addition,the kidney tissue was observed for pathological changes by hematoxylin-eosin staining.The comparison between two groups was performed by unpaired Student’s t-test,and statistics among multiple groups were carried out using Tukey’s post hoc test following one-way analysis of variance (ANOVA).A P-value <0.05 was considered statistically significant.RESULTS: At the early stage of S-AKI,MANF in the kidney tissue was up-regulated,but with the development of the disease,it was down-regulated.Renal function was worsened in the S-AKI group,and TNF-α and IL-6 were elevated.The administration of MANF significantly alleviated the elevated levels of SCr and BUN and inhibited the expression of TNF-α and IL-6 in the kidney.The pathological changes were more extensive in the S-AKI group than in the S-AKI+MANF group.CONCLUSION: MANF treatment may significantly alleviate renal injury,reduce the inflammatory response,and alleviate or reverse kidney tissue damage.MANF may have a protective effect on S-AKI,suggesting a potential treatment for S-AKI.
KEYWORDS: Sepsis-associated acute kidney injury;Mesencephalic astrocyte-derived neurotrophic factor;Renal function;Cytokines;Endoplasmic reticulum stress
INTRODUCTION
Sepsis is a systemic reactive syndrome caused by infection that can further lead to septic shock and multiple organ dysfunction syndrome (MODS).[1]Sepsis is also one of the main causes of death in patients hospitalized in the intensive care unit (ICU).[2,3]A meta-analysis in 2019 estimated that the incidence of ICU-treated sepsis was 58 per 100,000 person-years,of which 41.9% died prior to hospital discharge.[4]It should be noted that acute kidney injury (AKI)is often present in ICU or hospitalized patients.[5]Zhang et al[6]found that 40% of critically ill patients develop sepsis after AKI,suggesting that AKI is likely to increase the risk of sepsis.Meanwhile,sepsis-associated acute kidney injury(S-AKI) greatly increases the mortality of sepsis patients.Due to progression to new chronic kidney disease (CKD),some S-AKI patients need therapy for renal function,although they recover from AKI.[7]Nonetheless,to date,effective prevention and treatment are still needed for S-AKI.
The homeostasis of the endoplasmic reticulum (ER) is impaired after unfolded or misfolded proteins accumulate in the ER after sepsis.This phenomenon is called endoplasmic reticulum stress (ERS),and it may be a factor of S-AKI pathogenesis.[8]Mild ERS can be considered a maintenance mechanism,but prolonged ERS can worsen cell function and even lead to apoptosis.[9]In animal models of sepsis,markers of increased ERS were detected in multiple organs,including the heart and the liver,and these markers were directly correlated with the degree of organ dysfunction,which is likely the primary cause of sepsis inducing multiorgan failure.[10]Moreover,the accumulation of inf lammatory factors,reduction of tissue perfusion,ischemia,and hypoxia aggravate ERS in sepsis patients.[11]The transition from the maintenance function of ERS to pathogenesis may cause the organism to enter a vicious cycle.It is suggested that inhibition of ERS is a potential target for the prevention and treatment of sepsis.
Mesencephalic astrocyte-derived neurotrophic factor(MANF) is a kind of neurotrophic factor (NTF) that was found recently and is widely expressed in mammalian tissues.[12]We searched the database “Human Protein Atlas” with MANF as a keyword and found that MANF is detectable in the kidney,blood,heart,lung,liver and brain (supplementary Figure 1).MANF localizes in the ER in cells,and evidence further suggests that MANF is important for the maintenance of ER homeostasis.MANF is particularly high in secretory tissues with extensive protein production.[12]Additional evidence suggests that MANF is involved in the ERS-related diseases.[13]For example,when MANF is lacking,ethanol-induced neuronal apoptosis and ERS are greatly increased.[14]MANF can reduce the content of ERS-related proteins to protect neurons from amyloid β-peptide (Aβ) to improve the symptoms of Alzheimer’s disease (AD).[15]Furthermore,in MANF-knocked-out mice,insulin-deficient diabetes gradually occurs.[16]MANF plays a protective role in hepatic-injury-related diseases.[17,18]MANF also prevents alcohol-induced ERS and cell damage in pancreatic acinar cells.[19]
MANF has been reported to protect cells from stressinduced cell death and protect the heart from ischemic injury after secretion.[20]A previous study showed that ERS upregulates MANF in podocytes and renal tubules to protect renal function.[13]As an ERS-related protein,MANF has a positive effect on a number of diseases,but no study has explored the role of MANF in S-AKI.The goals of this study were to examine the expression of MANF in S-AKI,evaluate the relationship between MANF and renal function and inf lammatory factors.
METHODS
Animals and groups
C57/BL6 mice (male,6-8 weeks old) were used for the S-AKI model and purchased from Shanghai Laboratory Animal Co.,Ltd.They were kept in a specific pathogen-free(SPF) room with a light/dark 12:12 (12-hour light and 12-hour dark) condition and free access to food and water.The room temperature was controlled between 20 and 22 °C.
The animals were randomly divided into four groups with 24 mice in each group.Mice in the control group were injected with sterile normal saline into the abdominal cavity at a dose of 10 mg/kg.The sepsis model was induced by injecting lipopolysaccharide (LPS) (Sigma-Aldrich Inc.,USA) at 10 mg/kg intraperitoneally.The S-AKI can be developed in 24 h.[19]After S-AKI induction,200 μg/kg MANF (ICD02HU,Immuno Clone Biosciences Co.,USA)was injected intraperitoneally daily in the S-AKI+MANF and control+MANF groups.Simultaneously,an equal dose of normal saline was administered daily intraperitoneally in the control and S-AKI groups.The serum and kidney tissue specimens were collected at 24 h,36 h,and 48 h after the S-AKI model was established.All animal experimental procedures were reviewed and approved by the Ethical Committee for Animals of Shanghai Gongli Hospital.
Measurement of MANF expression in the kidney
The expression of MANF in the kidney was measured at 0,24,36 and 48 h after induction of S-AKI using Western blotting.The kidney tissue was subjected to protein lysis with a tissue grinder (Tissuelyser-24,Jingxin Industrial Development Co.,China).The protein lysate was quantified with an enhanced BCA protein assay kit (P0010,Beyotime,China).Protein was subjected to electrophoresis and transmembrane in turn.The treated polyvinylidene dif luoride (PVDF) membrane was subjected to blocking and washing at 4 ℃.The treated membrane was exposed to an antibody against MANF (dilution 1:1000,A305-572A-T,Thermo Fisher Scientific Inc.,USA) and then reacted with the secondary antibody.The treated membrane was electrogenerated by chemiluminescence (ChemiQ4600,Clinx Science Instruments,China) to examine protein expression.Finally,the image was processed by ImageJ software (NIH,USA).
Measurement of MANF expression in the serum
We pressed the mice’s neck gently to ensure sufficient congestion of the retroorbital vein after fixing the mice and inserted the needle for serum collection along the inner canthus of the mouse at an angle of 45°.Then,serum samples collected were placed at 4 ℃ for 30 min and centrifuged(3,500 r/min,10 min) with a centrifuge (MIKRO220R,Hettich,Germany).We tested the MANF in the serum using the MANF ELISA kit (MM-21129R1,MEIMIAN,China).
Measurement of pro-inf lammatory cytokines,blood urea nitrogen (BUN),and serum creatinine (SCr)
We also tested inflammatory factors (tumor necrosis factor-α [TNF-α] and interleukin-6 [IL-6]) using enzymelinked immunosorbent assay (ELISA) (MEIMIAN,China).The BUN and SCr were examined using an automatic biochemical analyzer (HITACHI,Japan).The test methods were operated in accordance with the manufacturer’s instructions.
Pathological examination of the kidney tissue
The mice were sacrificed at 48 h after the S-AKI model was established,and the kidney tissue was prepared for pathological examination using hematoxylin-eosin (H&E)staining.After the kidney tissue was fixed in 10% formalin solution (Chulei,China) for 24 h,the kidney was dehydrated with alcohol and embedded in paraffin.Sections were cut and stained in a fully automatic dyeing machine (ST5010XL,Leica,Germany).Images were examined after staining with a microscope (D-CleverEye,Dipath,China).
Statistical analysis
The measured data are expressed as the mean ± standard deviation followed by analysis of significant differences using SPSS software (v23.0,SPSS Inc.,USA). The comparison between two groups was performed by unpaired Student’st-test,and statistics among multiple groups were carried out using Tukey’s post hoc test following oneway analysis of variance (ANOVA).AP-value <0.05 was considered statistically significant.
RESULTS
MANF expression in the kidney tissue
In the S-AKI group,MANF in the kidney tissue was significantly up-regulated at 24 h,but gradually decreased with the prolongation of onset time.After 36 h,MANF returned to a normal level,and it was lower than its baseline level after 48 h (Figure 1A).
Compared with the control group at 48 h,the MANF in the control+MANF group was up-regulated,while the MANF in the S-AKI group was down-regulated.Compared with that in the S-AKI group,the MANF in the S-AKI+MANF group was up-regulated (Figure 1B).
MANF expression in the serum
Compared with the control group,the MANF in the serum of the S-AKI group did not change significantly.The MANF in the serum of the control+MANF and S-AKI+MANF groups was up-regulated after 24 h (Figure 1C).
Changes of pro-inflammatory cytokines,BUN,and SCr
Compared with the control group,there were no significant changes in inflammatory factors (TNF-α and IL-1β) or renal function (BUN and SCr) in the control+MANF group (Figures 2 A-D).In the S-AKI group,SCr and BUN increased gradually,and TNF-α and IL-1β were overexpressed.Compared with the S-AKI group,renal function in the S-AKI+MANF group was gradually improved,and the secretion of the inflammatory factors (TNF-α and IL-1β) was gradually reduced.
Pathological changes of the kidney tissue
The kidney tissue in the control and control+MANF groups was normal.There were no pathological changes in the infiltration of renal tubular epithelial cells or inflammatory cells in the renal cortex and renal interstitium.In the S-AKI and S-AKI+MANF groups,the kidney tissue showed the inf iltration of inf lammatory cells in the renal cortex and stroma,swelling of renal tubular epithelial cells,vacuolar degeneration,and partial brushlike margin shedding.The pathological changes were more extensive in the S-AKI group than in the S-AKI+MANF group (Figure 2E).
DISCUSSION
When the patient suffers from S-AKI,insufficient tissue irrigation,poor renal microcirculation function,and weakening of oxygen and energy intake by the kidney tissue can aggravate ERS and further aggravate renal injury.[21]MANF is an ERS-related protein,and MANF upregulation can reduce and protect pancreatic β cells.[22]Thus,in this study,we detected the MANF in S-AKI mice at different periods.Accordingly,at 24 h after LPS was injected intraperitoneally to induce sepsis,S-AKI occurred in mice.At this time,the MANF in the kidneys increased rapidly.This was likely due to LPS-induced sepsis in mice.The mice had insufficient circulatory perfusion,tissue hypoxia,and massive accumulation of inf lammatory cells,which activated ERS.[23]MANF reactivity was initially up-regulated,as reported by Gao et al,[24]and the expression of MANF in the kidney of mice decreased at 36 h and further decreased at 48 h.The renal function of S-AKI mice gradually deteriorated,and the accumulation of inflammatory factors increased.This change in MANF was similar to the results of Yang et al.[25]This may be caused by the fact that when pathogenic factors appear,the unfolded protein response (UPR) appears in the endoplasmic reticulum,triggering ERS,resulting in the activation of MANF and the up-regulation of MANF.[20]However,with the progression of the disease,the lack of irrigation of kidney tissue has become increasingly obvious,[26,27]and other problems have further aggravated ERS.[28,29]MANF may be insufficient to maintain ER homeostasis.[30]The ESR further intensifies after the reduction of MANF,forming a vicious cycle.
To determine whether MANF can produce drug toxicity and side effects in normal mice,we first injected MANF.It was found that the indexes of renal function and inf lammation in mice treated with MANF had no significant changes compared with normal mice.These results indicated that during homeostasis,the increase of MANF would not cause damage to cells.In this study,the MANF in the kidney tissue and serum of normal mice treated with MANF was significantly higher than that of normal mice.This indicates that MANF can be injected intraperitoneally,circulated into the serum,and then be absorbed into the kidney tissue.In contrast,after injection of MANF into S-AKI mice,the renal function and inf lammatory indexes of mice did not improve immediately,which may be that time is required to absorb the drug after injection.[31]The renal function of the MANF-treated mice gradually improved,and the inf lammatory factors decreased,indicating that after exogenous supplementation with MANF protein,the MANF in kidney tissue increased,thus alleviating the stress state of the endoplasmic reticulum and reducing cell damage.This is consistent with the phenomenon of MANF supplementation in other ERS-related diseases.[32,33]
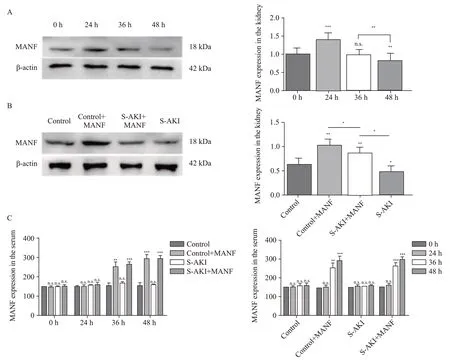
Figure 1.Expression of MANF in the kidney and serum of mice.A: Western blotting analysis of MANF in protein extracts from the kidney tissue of the S-AKI mice at different time;B: Western blotting analysis of MANF levels in protein extracts from the kidney tissue among different groups at 48 h;C: MANF in the serum at different time detected by the MANF ELISA kit.MANF: mesencephalic astrocyte-derived neurotrophic factor;S-AKI: sepsis-associated acute kidney injury;n.s.: no significance.*P<0.05,**P<0.01,***P<0.001.
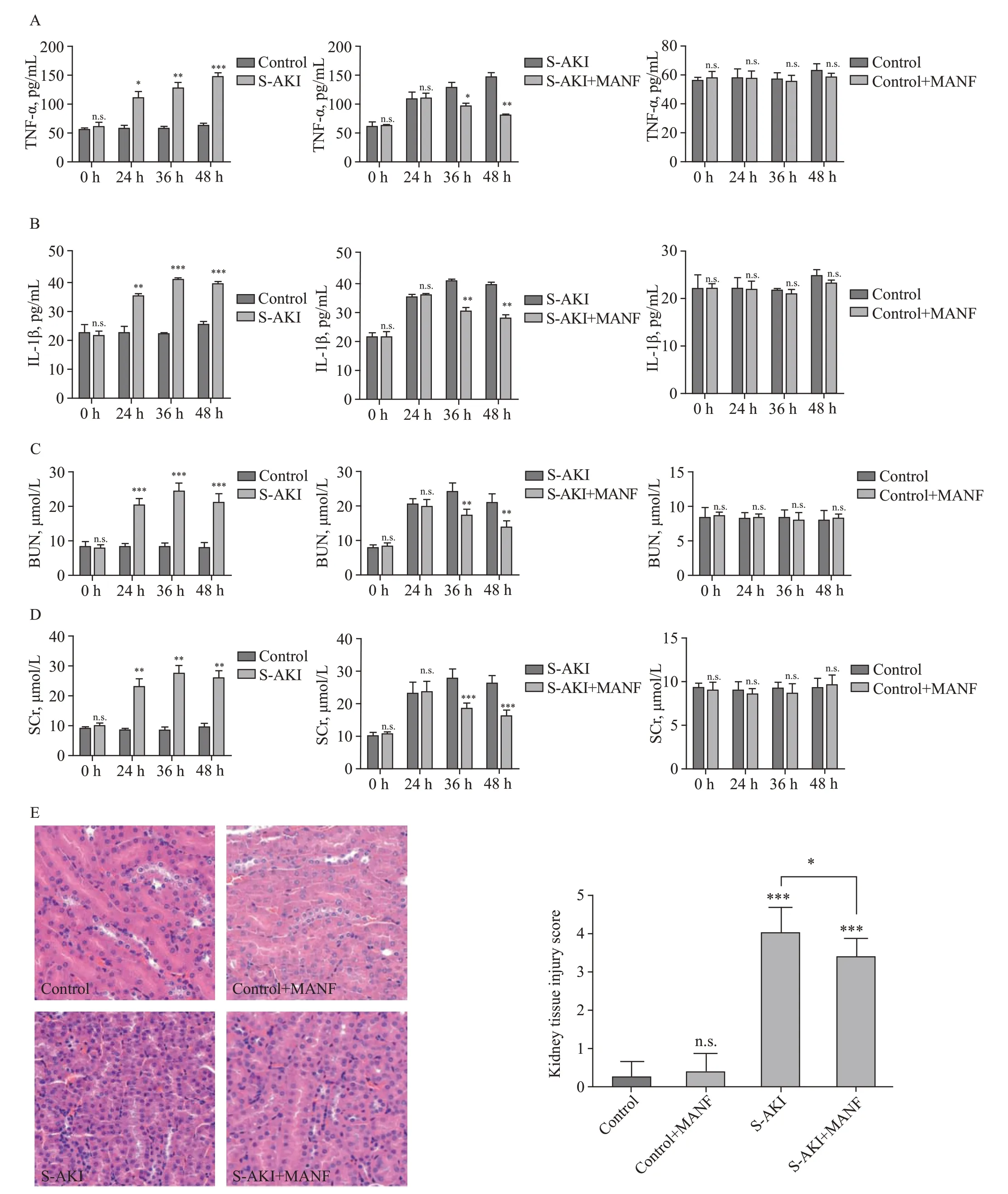
Figure 2.Changes of pro-inf lammatory cytokines,BUN,SCr,and histological findings among different groups.A: changes of TNF-α;B: changes of IL-1β;C: changes of BUN;D: changes of SCr;E: histological findings of the kidney tissue detected by hematoxylin-eosin staining (H&E,×40)staining.TNF-α: necrosis factor-α;IL-1β: interleukin-1β;MANF: mesencephalic astrocyte-derived neurotrophic factor;S-AKI: sepsis-associated acute kidney injury;BUN: blood urea nitrogen;SCr: serum creatinine.*P<0.05,**P<0.01,***P<0.001.
Regardless of the factors that lead to AKI,even mild AKI patients are at risk of CKD.[34,35]After AKI,the small arterioles and capillaries of the kidney are affected.The reduction in renal blood flow[36]and inflammatory cell infiltration[37]may be the factors affecting the prognosis of patients.There was no obvious damage to the kidney tissue in the control and control+MANF groups.The pathological changes in the kidney in S-AKI and AKI are similar.[38]Kidney injury was significantly less severe in the S-AKI+MANF group than in the S-AKI group.This indicates that MANF not only protects kidney function and reduces inflammation but also appears to protect kidney tissue and slow or reverse kidney damage.However,the precise molecular mechanisms by which MANF affects ERS during kidney injury deserve additional investigation.
CONCLUSION
MANF may play a role in the occurrence and development of S-AKI.For normal mice,the increase in MANF did not lead to injury or kidney tissue changes.For S-AKI mice,MANF treatment may significantly alleviate renal injury,reduce the inf lammatory response,and alleviate or reverse kidney tissue damage.This conclusion not only supports the theory of the protective effect of MANF on ERS-related diseases but also shows that MANF may be a new treatment for S-AKI.
Funding:The study was supported by the Health Commission Clinical Characteristic Discipline Construction Program of Pudong New Area,Shanghai (PW Yts2021-17) and Youth Science and Technology Project Health and Family Planning Commission of Pudong New Area,Shanghai (PWRq2020-35).
Ethical approval:The stusy was approved by the Ethical Committee for Animals of Shanghai Gongli Hospital.
Conf licts of interest:The authors declare no conf lict of interest.
Contributors:SFC and XWH contributed equally to this study.SFC analyzed the data and wrote the article.XWH performed the experiments and organized the data;XYY guided the design of the project.DFG guided and reviewed the article.PLS,GC,and GRL participated in the collection of data for this study.
All the supplementary files in this paper are available at http://wjem.com.cn.
 World journal of emergency medicine2023年5期
World journal of emergency medicine2023年5期
- World journal of emergency medicine的其它文章
- Emergency department approach to monkeypox
- The neuro-prognostic value of the ion shift index in cardiac arrest patients following extracorporeal cardiopulmonary resuscitation
- A prospective cohort study on serum A20 as a prognostic biomarker of aneurysmal subarachnoid hemorrhage
- Mendelian randomization study to investigate the causal relationship between plasma homocysteine and chronic obstructive pulmonary disease
- Cardiopulmonary prognosis of prophylactic endotracheal intubation in patients with upper gastrointestinal bleeding undergoing endoscopy
- Synchronized ventilation during resuscitation in pigs does not necessitate high inspiratory pressures to provide adequate oxygenation
