A prospective cohort study on serum A20 as a prognostic biomarker of aneurysmal subarachnoid hemorrhage
Tian Yan ,Ziyin Chen ,Shengdong Zou ,Zefan Wang ,Quan Du ,Wenhua Yu ,Wei Hu,Yongke Zheng,Keyi Wang,Xiaoqiao Dong,Shuangyong Dong
1 The Fourth School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou 322000, China
2 Department of Neurosurgery, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine,Hangzhou 310006, China
3 Department of Intensive Care Unit, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine,Hangzhou 310006, China
4 Clinical Laboratory Center, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine,Hangzhou 310006, China
5 Emergency Department, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou 310006, China
BACKGROUND: A20 may be a neuroprotective factor.Herein,we aimed to investigate whether serum A20 levels were associated with disease severity,delayed cerebral ischemia (DCI),and outcome after aneurysmal subarachnoid hemorrhage (aSAH).METHODS: In this prospective cohort study containing 112 aSAH patients and 112 controls,serum A20 levels were quantified.At 90 d poststroke,Modified Rankin Scale (MRS) scores ≥3 were defined as a poor outcome.All correlations and associations were assessed using multivariate analysis.RESULTS: Compared with controls,there was a significant elevation of serum A20 levels in patients (median 123.7 pg/mL vs.25.8 pg/mL; P<0.001).Serum A20 levels were independently correlated with Hunt-Hess scores (β 9.854;95% confidence interval [95% CI] 2.481-17.227,P=0.009) and modified Fisher scores (β 10.349,95% CI 1.273-19.424, P=0.026).Independent associations were found between serum A20 levels and poor outcome (odds ratio [OR] 1.015,95%CI 1.000-1.031,P=0.047) and DCI (OR 1.018,95% CI 1.001-1.035,P=0.042).Areas under the curve for predicting poor outcome and DCI were 0.771 (95% CI 0.682-0.845) and 0.777 (95% CI 0.688-0.850),respectively.Serum A20 levels ≥128.15 pg/mL predicted poor outcome,with a sensitivity of 73.9% and specificity of 74.2%,and A20 levels ≥160.55 pg/mL distinguished the risk of DCI with 65.5% sensitivity and 89.2% specificity.Its ability to predict poor outcome and DCI was similar to those of Hunt-Hess scores and modified Fisher scores (both P>0.05).CONCLUSION: Enhanced serum A20 levels are significantly associated with stroke severity and poor clinical outcome after aSAH,implying that serum A20 may be a potential prognostic biomarker for aSAH.
KEYWORDS: Subarachnoid hemorrhage;Aneurysm;A20;Delayed cerebral ischemia;Outcome;Biomarkers
INTRODUCTION
Aneurysmal subarachnoid hemorrhage (aSAH),often occurring in people between 40 and 60 years of age,is the third most common type of stroke and has a worse clinical outcome.[1]The Hunt-Hess scale and modified Fisher scale,as the two conventional severity indicators,are preferred in clinical work to evaluate the clinical outcome of aSAH patients.[2,3]Delayed cerebral ischemia(DCI),mainly attributed to cerebral vasospasm,has been identified as a major clinical syndrome after aneurysm rupture,frequently leading to neurological impairments and poor outcome in aSAH patients.[4,5]Early brain injury is an important pathophysiological process following aSAH and clearly involves neuroinflammation.[6]In recent years,some inf lammatory biomarkers have been broadly noted with respect to predicting DCI occurrence and poor outcomes after aSAH.[7,8]
A20,also known as tumor necrosis factor α-inducible protein 3,possesses inflammation-inhibitory effects by depressing the activation of nuclear factor-kappa B (NFκB)[9]and may act as an endogenous protective factor in some inflammatory diseases,including acute myocardial infarction,chronic hepatitis B,and rheumatoid arthritis.[10-12]A20 expression was dramatically up-regulated in damaged brain tissues following experimental head trauma or intracerebral hemorrhage.[13,14]Exogenous supplementation with A20 could significantly inhibit neuroinflammation,reduce brain edema,decrease blood-brain barrier disruption,and improve neurological function in rats after hemorrhagic stroke or traumatic brain injury,indicating that A20 may have a neuroprotective effect on acute brain injury (ABI).A20 expression by peripheral blood mononuclear cells was substantially increased in patients with intracerebral hemorrhage.[13]This study was designed to assess the prognostic role of serum A20 in aSAH.
METHODS
Study design and participant selection
We conducted a prospective cohort study from December 2020 to December 2022 at the Affiliated Hangzhou First People’s Hospital,Zhejiang University School of Medicine (Hangzhou,China).The main subjects of the study were consecutively recruited patients with first-time non-traumatic subarachnoid hemorrhage.All patients were enrolled according to the following inclusion criteria: (1) patients were over 18 years old;(2) rupture of a single intracranial aneurysm was conf irmed by computed tomography angiography (CTA) or digital subtraction angiography (DSA);(3) patients were hospitalized within 24 h of symptom onset;and (4) ruptured intracranial aneurysms were secured by surgical clipping or endovascular intervention within 48 h after admission.
We further excluded patients with (1) other neurological disorders,such as ischemic or hemorrhagic stroke,moyamoya disease,arteriovenous malformation,intracranial tumors,or severe head trauma;(2) some special aneurysm conditions,such as rebleeding or pseudoaneurysm;and (3) other specific diseases,such as severe infection,malignant tumors,uremia,immune deficiency syndrome,liver cirrhosis,chronic heart or lung diseases.Alternatively,controls were recruited from healthy volunteers who were free of chronic diseases and had normal results in conventional tests.
All procedures of this study were completed in accordance with theDeclaration of Helsinki,and the protocol of the study was approved by the Institutional Review Committee of Affiliated Hangzhou First People’s Hospital,Zhejiang University School of Medicine (Opinion No: 058-01).Written informed consent to join the study was obtained from relatives of patients and controls themselves.
Data collection
Demographic data,medical history,vital signs,and imaging information were recorded.The Hunt-Hess scale and modified Fisher scale were evaluated by at least two clinicians.When the two scores differed,they were re-evaluated by a third senior clinician.The location,shape,and diameter of the aneurysms were observed using CTA or DSA.Head computed tomography scans were performed at days 1,7,14 and 30 after surgery to investigate acute hydrocephalus and DCI.[15]Via a telephone inquiry,poststroke 90-day follow-up was performed by an uninformed physician.Modif ied Rankin Scale (MRS) scores ≥3 were def ined as a poor outcome.
Sample collection and immune analysis
The collection time of fresh blood samples from aSAH patients was 1.5 to 25.5 h after stroke (median 10.0 h;25th-75th percentiles 6.5-14.2 h).Blood samples from controls were obtained when they were enrolled in the current study.The blood samples were centrifuged within 30 min and promptly stored in a -80 °C refrigerator for subsequent determination.Using a commercially available enzymelinked immunosorbent kit (Shanghai Kexing Trading Co.,Ltd.,Shanghai,China),serum A20 levels were determined following the manufacturer’s instructions.Detection limits ranged from 22 to 800 pg/mL,and coefficients of intra-assay and inter-assay variations were 8% and 10%,respectively.The average values of the two measurements were employed for the final analysis.A batch of samples was melted every six months,and then quantifications were performed by the same technician who was unfamiliar with the clinical information.
Statistical analysis
SPSS 25.0 and MedCalc 9.6.4.0 were applied for statistical analysis,and graphics were made using GraphPad Prism version 9.0.The Kolmogorov-Smirnov test or Shapiro-Wilk test was selected to determine the normal distribution of quantitative data.Quantitative data are reported as the mean±standard deviation if normally distributed and as the median (25th-75th percentile) if non-normally distributed.Qualitative data were expressed in the form of number (percentage).Multiple-group comparisons were performed for nonnormally distributed quantitative data via the Kruskal-Wallis H test.Data were compared between two groups using the Chi-square test,Fisher’s exact test,ttest or Mann-WhitneyUtest as appropriate.Using the Spearman correlation coefficient,bivariate correlations were assessed.Multiple linear regression models were established to determine whether serum A20 levels were correlated with Hunt-Hess scores or modified Fisher scores.To determine the factors independently associated with 90-day clinical outcomes and DCI,we established a binary logistic regression model that included variables withP<0.05 in univariate analysis.Receiver operating characteristic (ROC) curve analysis was performed to calculate the area under the curve.AP-value <0.05 was considered statistically significant.
RESULTS
Participant selection and patient characteristics
In this study,148 aSAH patients were initially evaluated based on the inclusion criteria.We then excluded 36 patients for the reasons shown in supplementary Figure 1.Finally,112 aSAH patients and 112 healthy controls were further analyzed.There were no significant differences in age,sex,smoking or alcohol consumption between patients and controls (allP>0.05;Table 1).
Patients were admitted from 0.5 to 24.0 h after symptom onset (median 9.4 h,25th-75th percentiles 5.6-13.9 h).Hunt-Hess scores and modified Fisher scores ranged from 1 to 5 (median 4,percentiles 25th-75th 3-4) and 1 to 4 (median 3,percentiles 25th-75th 3-4),respectively.MRS scores of 0,1,2,3,4,5 and 6 were found in 9,13,44,13,16,9 and 8 patients,respectively(median 2;25th-75th percentiles 2-4).
A total of 90 intracranial aneurysms were located in the anterior circulation and 22 in the posterior circulation.There were 95 patients with cystic aneurysms and 17 patients with other types of aneurysms.There were 65 patients with aneurysms <10 mm in diameter and 47 patients with aneurysms ≥10 mm in diameter.The number of patients who chose surgical clipping and endovascular embolization to fix the aneurysms was 42 and 70,respectively.Twelve patients had acute hydrocephalus,14 patients underwent external ventricular drainage,and 15 patients and 29 patients suffered from intraventricular hemorrhage or DCI,respectively.There were 23 hypertensive patients and 7 diabetic patients.A total of 46 patients had poor outcomes (MRS scores ≥3).
Changes of serum A20 levels
In the aSAH patients,serum A20 levels ranged from 39.0 to 204.2 pg/mL with a median of 123.7 pg/mL(25th-75th percentiles 108.0-161.9 pg/mL).Among the 112 controls,25 controls had serum A20 levels <22 pg/mL(detection limits of assay).The median serum A20 level in the remaining 87 controls was 25.8 pg/mL (range 22.1-42.8 pg/mL,25th-75th percentiles 23.5 -32.4 pg/mL).Using the Mann-WhitneyUtest,it was clear that serum A20 levels in aSAH patients were significantly higher than those in healthy controls (P<0.001;supplementary Figure 2).
In supplementary Table 1,close correlations were found between serum A20 levels and Hunt-Hess scores (P<0.001),modified Fisher scores (P<0.001),blood glucose levels (P=0.021),and intraventricular hemorrhage (P=0.002).
As shown in Table 2,serum A20 levels were independently correlated with Hunt-Hess scores and modif ied Fisher scores when the above significantly correlated variables were entered in a multiple linear regression model.In addition,the serum A20 levels in patients were significantly elevated with increasing Hunt-Hess scores or modified Fisher scores(P<0.001;supplementary Figure 3).
Correlation of serum A20 levels with 90-day poor outcome
In supplementary Table 2,patients with poor outcomes had a significantly increased percentage ofintraventricular hemorrhage and showed significant elevations in blood glucose levels,serum A20 levels,Hunt-Hess scores,and modif ied Fisher scores compared to patients with good outcomes (allP<0.05).Using multivariate analysis,Hunt-Hess scores,modif ied Fisher scores,and serum A20 levels independently predicted the development of poor outcomes at 90 d after hemorrhagic stroke (allP<0.05;Table 3).
As shown in Figure 1,using ROC curve analysis,serum A20 levels effectively distinguished patients at risk of poor outcomes (AUC 0.771,95%CI0.682-0.845).The best serum A20 concentration (128.15 pg/mL) was selected using the maximum Youden index,which predicted poor outcomes with medium-high sensitivity (73.9%) and specificity (74.2%) values.Furthermore,in Figure 2,its prognostic ability was similar to those of Hunt-Hess scores and modif ied Fisher scores (allP>0.05).
Relationship between s erum A20 levels and DCI
In supplementary Table 3,the proportion of intraventricular hemorrhage,serum A20 levels,blood glucose levels,Hunt-Hess scores,and modified Fisher scores were higher in patients with DCI than in patients without DCI (allP<0.05).In addition,as shown in Table 4,multivariate logistic regression analysis showed that Hunt-Hess scores,modified Fisher scores,and serum A20 levels were independently associated with DCI risk after aSAH (allP<0.05).
As shown in Figure 3,serum A20 levels effectively differentiated the risk of DCI under the ROC curve (AUC 0.777,95%CI0.688-0.850).Using the Youden method,serum A20 concentrations higher than 160.55 pg/mL distinguished the development of DCI with a sensitivity of 65.5% and a specificity of 89.2%.As shown in Figure 4,its ability to discriminate the occurrence of DCI was equivalent to those of Hunt-Hess scores and modified Fisher scores (allP>0.05).
DISCUSSION
In this study,we conf irmed that elevated serum A20 levels after aSAH were significantly correlated with Hunt-Hess scores and modified Fisher scores and were independently associated with DCI and poor outcomes.In addition,when compared with Hunt-Hess scores and modif ied Fisher scores,serum A20 levels showed a similar ability to predict DCI and poor outcomes at 90 days post-injury.Such results suggest that serum A20 may be a prognostic biomarker of aSAH.
T he tumor necrosis factor receptor-associated factor 6 (TRAF6)/NF-κB signaling pathway is involved in the inflammatory process of ABI diseases,including SAH.[16,17]Mechanistically,A20 could inhibit the TRAF6/NF-κB signaling pathway to down-regulate the expression of some destructive proinflammatory factors,such as interleukin-1beta,interleukin-6 and tumor necrosis factor-alpha,thereby reducing inflammatory responses.[18-20]Specifically,usingin vivoandin vitromodels of SAH,A20 silencing could obviously increase TRAF6,NF-κB,and inflammatory cytokine levels,aggravate neuronal apoptosis,brain edema,and blood-brain barrier damage,and therefore worsen neurological impairments;[6,17,21]nonetheless,A20 overexpression could significantly reverse those effects.Another experimental study showed that supplementation with A20 could significantly reduce mouse neuroinflammation induced by intracerebral hemorrhage through the suppression of TRAF6 polyubiquitination.[13]Similarly,silencing A20 obviously increased neuronal death and neuroinflammation in rats with traumatic brain injury.[14]T hus,A20 may act as an endogenous anti-inf lammatory factor and therefore exert a neuroprotective effect.

Table 2.Multivariate linear regression analysis between elevated serum A20 levels and other variables
Under normal conditions,A20 is predominantly expressed in neurons of the human or animal brain.[13,22,23]After ABI,A20 expression is increased in astrocytes,microglia and neurons of animals with subarachnoid hemorrhage,[18]ischemic stroke,[24]traumatic brain injury[14]and intracerebral hemorrhage.[25,13]The mRNA expression of A20 was increased in the peripheral blood cells of patients with intracerebral hemorrhage.[13]Our study showed similar results: serum A20 levels were significantly higher in patients with aSAH than in healthy controls.In consideration of its neuroprotective effects,it is inferred that an elevation of A20 expression may be a compensatory reaction to ABI.
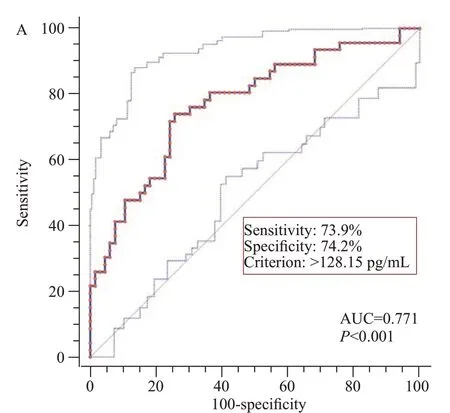
Figure 1.Predictive ability of serum A20 levels for poor outcome at 90 d after aneurysmal subarachnoid hemorrhage under receiveroperating characteristic curve.

Figure 2.Comparison of discriminatory capability with respect to serum A20 levels,Hunt-Hess scores and modified Fisher scores for 90-day poor outcome following aneurysmal subarachnoid hemorrhage under receiver operating characteristic curve.
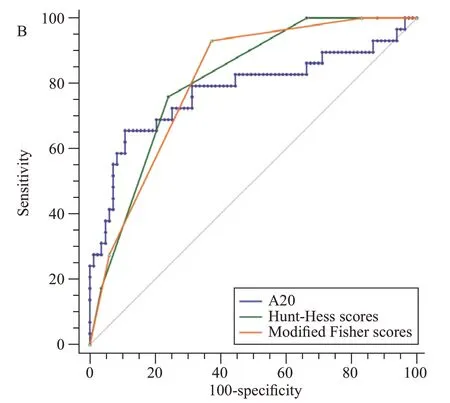
Figure 4.Comparison of discriminatory capability with respect to serum A20 levels,Hunt-Hess scores and modified Fisher scores for delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage under receiver operating characteristic curve.
Compelling evidence has confirmed that A20 can be released from brain tissues after ABI.[14]Thus,A20 may leak into peripheral blood via a disrupted bloodbrain barrier,thereby leading to an enhancement of A20 levels in the peripheral blood.Our study found a significant elevation in serum A20 levels in patients with aSAH compared to healthy controls.Therefore,it is deduced that A20 in serum from aSAH patients may be at least partially derived from injured brain tissues.However,the mRNA expression of A20 was increased in the peripheral blood cells of patients with intracerebral hemorrhage,implying that A20 could be directly derived from peripheral blood cells.A systemic inflammatory response after ABI could be induced,[26]and hence,an increase in A20 mRNA expression by peripheral blood cells may compensate for systemic injury after ABI.Thus,it is presumed that a portion of serum A20 among patients with aSAH in the current study may originate from peripheral blood cells.
It is unclear whether there is a strong association of serum A20 with a poor long-term outcome in patients with aSAH.However,A20 has anti-inflammatory,antioxidative and antiapoptotic effects,therefore displaying neuroprotective functions.[27]Herein,we speculate that serum A20 may be a predictor of poor outcomes 90 d after aSAH.In this study,poor results were defined as MRS scores ≥3.The final results showed that in addition to Hunt-Hess scores and modified Fisher scores,serum A20 levels were independently associated with poor outcome.In addition,serum A20 levels,Hunt-Hess scores and modif ied Fisher scores had similar prognostic abilities under the ROC curve.Our study provides further evidence to support the hypothesis that serum A20 may be a useful biomarker for assessing disease severity after aSAH.
As is known to all,Hunt-Hess scores and modified Fisher scores are commonly used clinically to evaluate the poor outcome of aSAH patients.[2,3]In this study,our results suggest that A20 levels are also a prognostic determinant of aSAH.In addition,serum A20 levels were independently associated with DCI,which occurs after aSAH and leads to a poor outcome in such patients.The results indicated that serum A20 levels were of certain value in predicting the outcome of aSAH compared with Hunt-Hess scores and modified Fisher scores.These results may strongly support the postulation that serum A20 may represent a promising prognostic biomarker for aSAH.However,the current study included only a medium sample of aSAH patients.Perhaps this is a preliminary study regarding the role of serum A20 as a prognostic biomarker of aSAH.In the future,a larger cohort study is needed to verify this conclusion.
CONCLUSIONS
Our study found that disease severity reflected by the Hunt-Hess scores and modified Fisher scores is closely related to serum A20 levels,which independently predict a poor outcome and DCI after aSAH.Therefore,we speculate that A20 may be a potential biomarker of outcome in aSAH.
Funding:This work is financially supported by grants from Key Research and Development Projects of Zhejiang Province(2020C03071) and the Construction Fund of Medical Key Disciplines of Hangzhou (OO20200485,OO20200055).
Ethical approval:This study was approved by the Institutional Review Committee of the Affiliated Hangzhou First People’s Hospital,Zhejiang University School of Medicine (058-01).
Conf licts of interest:The authors have no conf licts of interest.
Contributors:TY,ZYC,and SDZ contributed equally to this work.TY,ZYC,SDZ,XQD,and SYD conceived,designed the study,analyzed the data,and wrote the paper.ZFW,QD,WHY,WH,YKZ,and KYW contributed to data acquisition and analysis.
 World journal of emergency medicine2023年5期
World journal of emergency medicine2023年5期
- World journal of emergency medicine的其它文章
- Emergency department approach to monkeypox
- The neuro-prognostic value of the ion shift index in cardiac arrest patients following extracorporeal cardiopulmonary resuscitation
- Mendelian randomization study to investigate the causal relationship between plasma homocysteine and chronic obstructive pulmonary disease
- Cardiopulmonary prognosis of prophylactic endotracheal intubation in patients with upper gastrointestinal bleeding undergoing endoscopy
- Effects of mesencephalic astrocyte-derived neurotrophic factor on sepsis-associated acute kidney injury
- Synchronized ventilation during resuscitation in pigs does not necessitate high inspiratory pressures to provide adequate oxygenation
