Mendelian randomization study to investigate the causal relationship between plasma homocysteine and chronic obstructive pulmonary disease
Yanlan Hu ,Ping Tan ,Juntao Wang ,Jun Zeng ,Quan Li ,Shijiao Yan, ,Wenjie Hao,Lanfen He,Xingyue Song,Caihong Zhang,Chuanzhu Lyu,7,8
1 International School of Public Health and One Health, Hainan Medical University, Haikou 571199, China
2 Department of Emergency Medicine, Hunan Provincial People’s Hospital/the First Affiliated Hospital, Hunan Normal University, Changsha 410002, China
3 Emergency Medicine Center, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu 610072, China
4 Emergency Department, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing 100730, China
5 Department of Emergency, Hainan Clinical Research Center for Acute and Critical Diseases, the Second Affiliated Hospital of Hainan Medical University, Haikou 570100, China
6 International School of Nursing, Hainan Medical University, Haikou 570100, China
7 Key Laboratory of Emergency and Trauma of Ministry of Education, Hainan Medical University, Haikou 570100, China
8 Research Unit of Island Emergency Medicine, Chinese Academy of Medical Sciences (No. 2019RU013), Hainan Medical University, Haikou 570100, China
BACKGROUND: Several observational studies have shown an association between homocysteine(Hcy) levels and chronic obstructive pulmonary disease (COPD),but causal relationships are not clear.Our study aimed to explore the causal relationship between plasma Hcy and COPD by two-sample Mendelian randomization (MR).METHODS: A two-sample MR study was performed to infer the causal link.Genetically predicted plasma Hcy was selected as an instrumental variable (IV) from published genome-wide association study (GWAS) meta-analyses.COPD with different etiologies was extracted as outcome variables from other GWAS meta-analyses.The main MR analysis was performed using the inversevariance weighted (IVW) method.Additional analyses were further performed using Cochran's Q-test and MR-Egger regression to evaluate the heterogeneity or horizontal pleiotropy of our findings.RESULTS: MR analysis showed no significant association between plasma Hcy and COPD.The results of the groups were consistent with the sensitivity analysis and repeated analysis,without heterogeneity or horizontal pleiotropy.The IVW results showed COPD hospital admissions (odds ratio [OR] 1.06,95% confidence interval [CI] 0.91-1.24,P=0.42),asthma/COPD (OR 0.97,95% CI 0.89-1.06, P=0.55),COPD-related chronic infection (OR 1.50,95% CI 0.57-3.99,P=0.41),COPD/asthma/interstitial lung disease (ILD)-related pneumonia or pneumonia-derived septicemia (OR 0.93,95% CI 0.86-1.02,P=0.13),and COPD-related respiratory insufficiency (OR 1.00,95% CI 0.7-1.44,P=0.99).CONCLUSION: There is no direct causal relationship between plasma Hcy and COPD in our study.As Hcy is known to have deleterious effects on endothelial function and vascular homeostasis,further studies are needed to investigate whether additional factors mediate the association between Hcy and COPD.
KEYWORDS: Homocysteine;Chronic obstructive pulmonary disease;Mendelian randomization
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease characterized by persistent respiratory symptoms and airflow limitation.[1]It places a substantial burden on patients and society due to the natural progression of COPD,reduced daily activities,exacerbations,and impact on work,which frequently leads to psychological conditions such as anxiety and depression.[2]According to the estimation from the WHO,5% of deaths can be attributed to COPD worldwide.At present,COPD has become a global public health problem;therefore,novel prevention strategies that target modif iable risk factors are urgently needed.
Homocysteine (Hcy) is a toxic amino acid and an essential product in the metabolism of methionine,an amino acid bearing a sulfhydryl group.[2]An observational study reported that Hcy was an independent risk factor for COPD.[3]Hcy causes endothelial dysfunction through the induction of inf lammation and oxidative stress,both of which are involved in the pathogenesis of COPD.[4]Wei et al[5]supported that Hcy was positively correlated with the severity of COPD and had predictive value for the occurrence and acute progression of COPD.Zinellu et al[6]supported that plasma Hcy was significantly elevated in COPD patients and was related to COPD severity.However,Sandelowsky et al[7]showed that COPD patients were often comorbid with other diseases,and almost all patients had more than one comorbidity,such as cardiovascular disease (CVD).A Mendelian randomization (MR) study found a causal association between plasma Hcy and CVDs,which indicated that plasma Hcy levels can be affected by various other factors.[8]Evidence from observational studies is limited by study design,residual confounding and detection bias,and based only on observational studies,the evidence of the correlation between exposure and outcome remains challenging.[9]Therefore,the current evidence on the relationship between Hcy and COPD still needs to be rigorously evaluated. MR detects and quantif ies the causal relationship between exposure and disease using genetic variation as an instrumental variable (IV).Compared with observational studies,MR studies using genetic variation can control for unmeasured confounders and reverse causality.[10]Herein,by leveraging large samples of genetic data on COPD and Hcy,[11]we conducted a twosample MR analysis to examine the causal relationship between Hcy and COPD.
METHODS
Data source
The genome-wide association study (GWAS) data for Hcy published by van Meurs et al[11]in 2013 were used as IVs in the present study.The data contained 10 independent cohorts of European ancestry,with a total of 44,147 individuals(supplementary Table 1).The extracted variables were COPD hospital admissions,asthma/COPD,COPD-related chronic (opportunistic) infection,COPD/asthma/interstitial lung disease (ILD)-related pneumonia or pneumonia-derived septicemia,and COPD-related respiratory insufficiency.The GWAS data of outcome came from the IEU Open GWAS database (https://gwas.mrcieu.ac.uk/).We limited our analysis to studies that included individuals of European ancestry to minimize potential bias due to population stratification. All GWAS data are shown in Table 1.
Selection of the tool variables
We extracted single-nucleotide polymorphisms (SNPs)associated with Hcy from published GWAS meta-analyses.The MR had three requirements (supplementary Figure 1).First,IVs were closely related to the exposure factor of Hcy (P<5×10-8).Second,IVs should not be associated with confounding factors.Third,IVs affect the outcomes of COPD only through Hcy and not through other pathways.In addition,we used the PLINK clustering method withR2<0.001 to prune SNPs in linkage disequilibrium to ensure that the selected SNPs were independent.[12]We calculated F-statistics for 18 SNPs to detect the strength of IVs,with F >10 usually recommended in MR analysis,and the F-statistics can be calculated as:
Statistical analysis
In our MR study,a random-effect inverse-variance weighted (IVW) meta-analysis was conducted as the primary analysis to estimate the causal relationship between COPD and plasma Hcy.In addition,we used another four methods to test the reliability and stability of the results,namely,MREgger regression,the weighted median estimator (WME)method,a simple estimator based on mode,and a weighted estimator based on mode.[14]When the effective IVs exceed 50%,the WME method can obtain consistent causaleffect estimation.[15]Cochran’s Q-test was used to evaluate the heterogeneity of MR results,and MR-Egger regression was used to detect horizontal pleiotropy.In the test results,aP-value >0.05 was considered to indicate no heterogeneity or pleiotropy in the included IVs.If theP-value was <0.05,the leave-one-out method was used to conduct sensitivity analysis.All relevant SNPs were removed one by one,and the pooled effect of the remaining SNPs was calculated to evaluate the inf luence of each SNP on the outcome.All statistical analyses were conducted using R (version 4.1.1) with two-sample MR and MR-PRESSO packages.In addition,the statistical analysis results were visualized by plotting the forest plots of Hcy and COPD SNPs using the MR-base platform.The test level wasα=0.05,and aP-value <0.05 was considered significant.
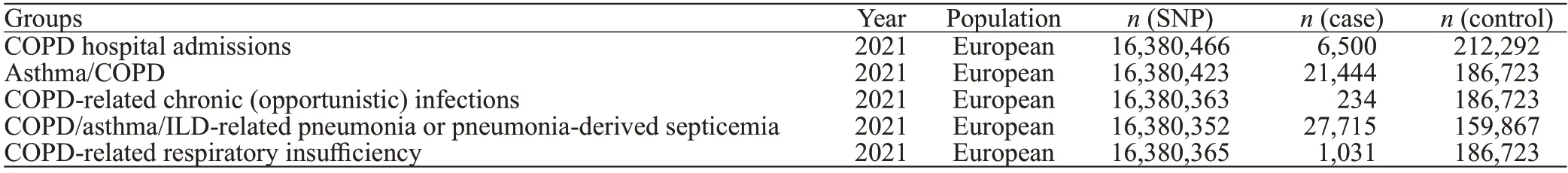
Table 1.Descriptive characteristics of the GWAS meta-analysis used in the outcomes
RESULTS
MR analysis showed no significant association between plasma Hcy and COPD (Figure 1).In this study,the F-statistics for all variables were much greater than 10,indicating that the possibility of weak instrumental bias was quite small.The results of the groups were consistent with the sensitivity analysis and repeated analysis,without heterogeneity or horizontal pleiotropy (supplementary Table 2).
The IVW results showed COPD hospital admissions(odds ratio [OR] 1.06,95% confidence interval [CI] 0.91-1.24,P=0.42),asthma/COPD (OR0.97,95%CI0.89-1.06,P=0.55),COPD-related chronic (opportunistic) infection (OR1.50,95%CI0.57-3.99,P=0.41),COPD/asthma/ILD-related pneumonia or pneumonia-derived septicemia (OR0.93,95%CI0.86-1.02,P=0.13),and COPD-related respiratory insufficiency (OR1.00,95%CI0.70-1.44,P=0.99).In the MR analysis,after we adjusted for all confounding factors,Hcy was still not statistically significant with COPD.
DISCUSSION
In this study,w e found no evidence of a causal relationship between plasma Hcy and COPD.Our MR results were robust,allP-values for heterogeneity and pleiotropy tests were greater than 0.05,and F-statistics for all variables were much greater than 10.A meta-analysis highlighted Hcy as a potential risk factor for COPD.[16]However,Hcy predisposes patients to COPD in a multifactorial manner involving complex pathogenesis.Thus,this might be the cause of how our findings diverge from recent observational research.
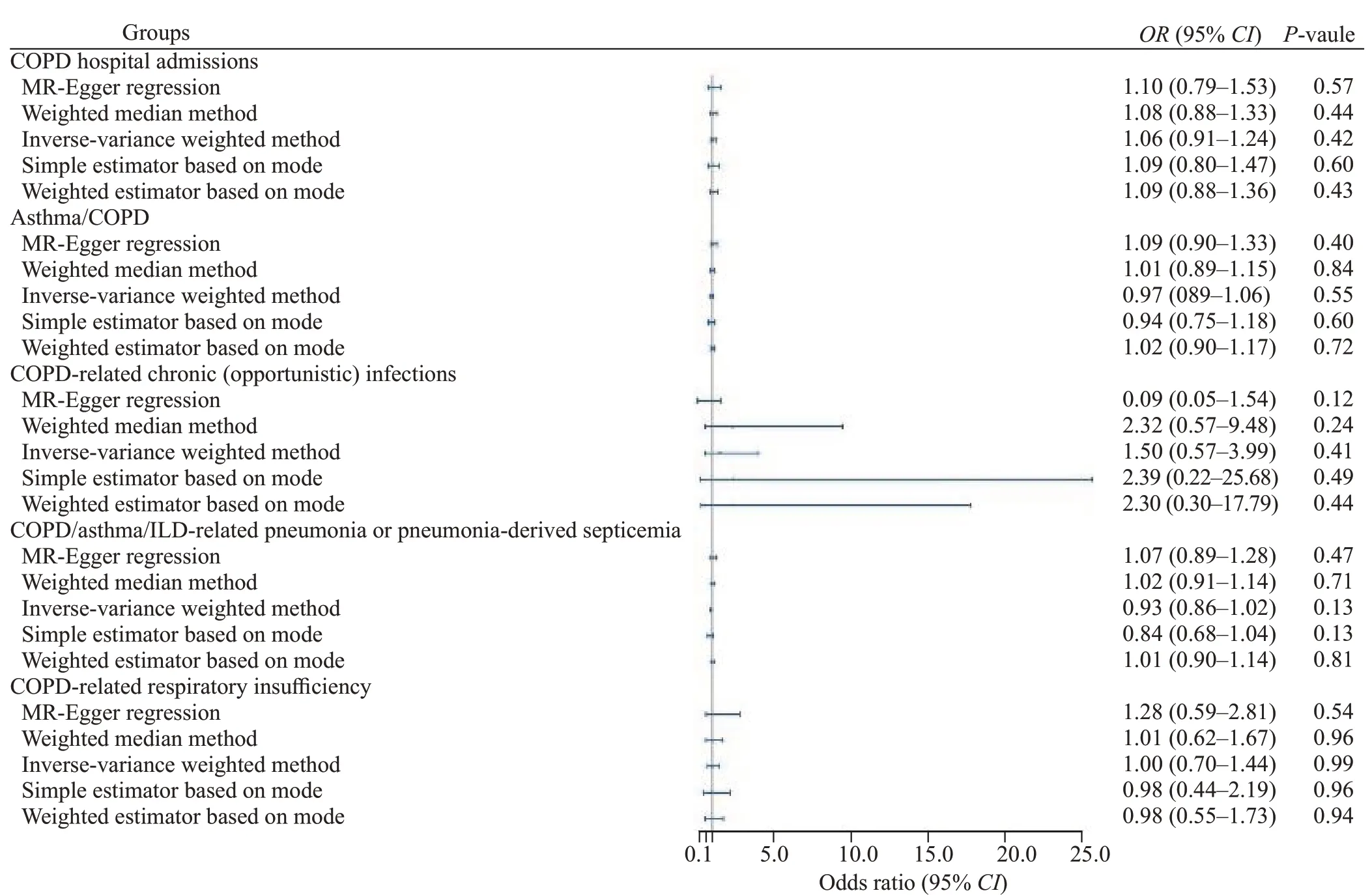
Figure 1.Mendelian randomization analysis of the association between Hcy and COPD.Hcy: homocysteine;COPD: chronic obstructive pulmonary disease;MR: Mendelian randomization;ILD: interstitial lung disease.
The evidence regarding the association between Hcy and COPD is complex in observational studies.Nunomiya et al[3]reported that Hcy levels were significantly associated with forced vital capacity.At the genetic level,we found no statistically significant causal relationship between Hcy and COPD. COPD often coexists with other diseases that may have a significant impact on prognosis.[17]I t has been suggested that almost all COPD patients have ≥1 comorbidity,with half having ≥4.[18]Moreover,one disease increases the severity of other diseases.[19]Common COPD comorbidities include bronchiectasis,CVD,chronic kidney disease,dyslipidemia,diabetes,hypertension,lung cancer,mental disorders,osteoporosis,obstructive sleep apnea syndrome,and skeletal muscle dysfunction.[19]The strongest predictor of death in COPD patients was heart failure,which was associated with a nearly doubled mortality risk.[7]Hyperhomocysteinemia is a well-known risk factor for CVD.[20]A t present,many MR studies have shown that plasma Hcy levels are cause-effect associated with small vessel stroke,[21]cerebral small vessel disease,[22]metabolic syndrome,[23]sleep apnea syndrome,[24]estimated glomerular filtration rate,[25]and immunoglobin A.[26]Therefore,the factors leading to hyperhomocysteinemia are diverse,and multiple factors can modulate Hcy levels;therefore,determining the primary cause of hyperhomocysteinemia in an individual is complex.Previous observational studies of Hcy and COPD were unable to control the inf luence of known and unknown confounding factors on the results,such as comorbidity of COPD,the causal association of plasma Hcy,and multiple comorbidities of COPD at the genetic level.Compared with observational studies,MR studies using genetic variation can control unmeasured confounders and reverse causality.T herefore,this may be one of the reasons why our results differ from the present observational studies.
Since COPD is a systemic disease,the disease process requires a large amount of energy and is prone to lead to a negative energy balance,resulting in reduced folate and vitamin B content.[27]Folate is an essential intermediate for the methylation of biomolecules in the metabolic process of one carbon unit and regulates epigenetics by affecting the synthesis of nucleic acids.[28]Another member of the vitamin B family,vitamin B12,also plays a major role in one-carbon metabolism.W hen a methyl group is transferred to Hcy from 5-methyl tetrahydrofolate to form methionine,vitamin B12 acts as a cofactor for the enzyme methionine synthase.[29]Folate is trapped as 5-methyltetrahydrofolate in B12 deficiency,which leads to the accumulation of Hcy and decreases the synthesis of methionine.[30]Fimognari et al[31]showed in a case-control study that COPD patients were deficient in B vitamins due to a systemic inflammatory response,which resulted in elevated plasma Hcy.This suggests that Hcy is related to folate and vitamins rather than having a direct association with COPD.Therefore,previous observational studies have found that the increase in plasma Hcy concentration in COPD patients may be due to an increase in energy expenditure under systemic inf lammation,which leads to an increase in plasma Hcy concentration due to insufficient folate and vitamin B affecting methylation.
Limitations
T he results of this study have some limitations.First,the main limitation of MR is bias due to pleiotropy,as genetic variation affects various phenotypes.S econd,it is difficult to rule out all SNPs that might affect COPD risk through mechanisms other than affecting plasma Hcy levels.Although we did not find any evidence of pleiotropy in the MR-Egger regression analysis,this result may have been influenced by the relatively small number of SNPs.Future studies with larger GWAS datasets for Hcy need to be conducted.Third,we lacked data on the severity of COPD.We were unable to assess COPD of different severities separately with plasma Hcy levels.Fourth,MR analyses typically assess the effects of lifetime exposure on outcomes and may be subject to the effects of short-term clinical interventions on exposure.The effect sizes estimated in this study are limited to explaining associations between key exposure periods and outcomes.Finally,to control bias due to population stratification,we reduced this possible bias by restricting the study population to individuals of European ancestry.However,this population bias limits the generalizability of our findings to other populations.
CONCLUSIONS
Our study showed no causal relationship between plasma Hcy levels and COPD.As Hcy is known to have deleterious effects on endothelial function and vascular homeostasis,further studies are needed to investigate whether additional factors mediate the association between Hcy and COPD.
Funding:This study was supported by grants from Hainan Provincial Natural Science Foundation of China (821RC557,2019RC232),National Natural Science Foundation of China (81871611,82160647),Finance Science and Technology Program of Sichuan Province(2022YFS0602),and Hainan Clinical Medical Research Center Project(LCYX202310).
Ethical approval:This research was based on publicly available data.Ethical approval was obtained by the original study authors.
Conf licts of interest:The authors declare no conf licts of interest.
Contributors:YLH and PT contributed equally to this study.CZL,YLH,PT,JTW,JZ,QL,CHZ,and SJY conceived and designed the study.YLH and PT drafted the manuscript,and YLH,JTW,QL,ZJ,CHZ,SJY,and CZL revised the manuscript.XYS,LFH,and WJH participated in data collection.All authors contributed substantially to its critical revision and final approval.
All the supplementary files in this paper are available at http://wjem.com.cn.
 World journal of emergency medicine2023年5期
World journal of emergency medicine2023年5期
- World journal of emergency medicine的其它文章
- Emergency department approach to monkeypox
- The neuro-prognostic value of the ion shift index in cardiac arrest patients following extracorporeal cardiopulmonary resuscitation
- A prospective cohort study on serum A20 as a prognostic biomarker of aneurysmal subarachnoid hemorrhage
- Cardiopulmonary prognosis of prophylactic endotracheal intubation in patients with upper gastrointestinal bleeding undergoing endoscopy
- Effects of mesencephalic astrocyte-derived neurotrophic factor on sepsis-associated acute kidney injury
- Synchronized ventilation during resuscitation in pigs does not necessitate high inspiratory pressures to provide adequate oxygenation
