Synchronized ventilation during resuscitation in pigs does not necessitate high inspiratory pressures to provide adequate oxygenation
Miriam Renz,Raphael René Cinto Noack,René Rissel,Katja Mohnke,Julian Riedel,Bastian Dunges,Alexander Ziebart,Erik Kristoffer Hartmann,Robert Rummler
Department of Anesthesiology, University Medical Center, Johannes Gutenberg University, Mainz 55131, Germany
The optimal ventilation method for patients suffering from cardiac arrest and receiving cardiopulmonary resuscitation (CPR) remains unclear,and the recent guidelines do not provide detailed information.[1-3]During CPR,changing thoracic pressures due to chest compressions and ventilation pressure inf luence venous return and cardiac output,respectively.[4-6]Novel mechanical ventilation modes that are synchronized to chest compressions may improve blood flow and oxygenation.[4]In this porcine trial,we investigated the feasibility of a specially designed chest compression synchronized ventilation (CCSV) mode with peak pressures limited to 40 mbar (1 mbar=0.1 kPa) and an experimental synchronized ventilation (SV) limited to 20 mbar,respectively,intended to achieve a more lungprotective ventilation pattern during resuscitation.We evaluated whether the different synchronized pressure levels ameliorate hemodynamics,gas exchange and pulmonary function and compared it to intermittent positive pressure ventilation (IPPV).
METHODS
Fifteen German landrace pigs (age 12-16 weeks,weight 29-34 kg) were examined.The general trial setup and execution were described in detail before.[7,8]
After the instrumentation,the animals received a fluid bolus of 30 mL/kg (bodyweight [BW]) balanced electrolyte solution.For ventilation/perfusion (V/Q) ratio measurements,six kinds of chemically inert gases (sulphur hexafluoride,krypton,desflurane,enflurane,diethyl ether,acetone) were given intravenously as described previously.At the baseline healthy (BLH) measurement timepoint,the first multiple inert gas elimination technique (MIGET)measurement (MMIMS-MIGET,Oscillogy LLC,USA)was performed,and arterial and central venous blood gases(radiometer,ABL90flex,Denmark) were taken.Next,ventricular fibrillation was induced.After ECG-confirmed ventricular fibrillation and 4 min of no-flow time and no ventilation,basic life support (BLS) was started with mechanical chest compressions by the LUCAS 2-System(Stryker,USA) with a frequency of 100 compressions/min.Afterwards,the animals were randomized into three intervention groups (n=5 per group) and ventilated with a ventilator (type MEDUMAT Standard²,Weinmann Emergency Medical Technology GmbH+Co.KG,Germany).Ventilator settings are summarized in Table 1.
The first intervention phase of 8 min of chest compressions and ventilation was followed by a second phase with rhythm analyses (RA) and biphasic def ibrillation with 200 J every two minutes and application of 1 mg adrenaline and 15 U vasopressin after the first def ibrillation and repeated doses every second RA.After the third and fifth RA,150 mg of amiodarone was injected.Arterial and central venous blood gas samples and MIGET measurements were taken after 5 min in the first phase,and blood gases were also taken after the third RA in the second phase.If no ROSC was achieved,the trial was terminated after 7 RA.In the case of ROSC,ventilator settings were returned to initial volumecontrolled ventilation.After 6 h,the trial was terminated by euthanizing the animals with a high dose of propofol and potassium chloride.
Postmortem lung tissue samples were taken and investigated as described before.[8]Samples were scored using the diffuse alveolar damage (DAD) score.[8,9]
Statistical analyses were performed using SPSS (IBM SPSS Statistics,Version: 23 V5 R,USA).We performed multiple comparisons of the observed means as a post hoc procedure of analysis of variance (ANOVA).We applied Tukey’s honestly significant difference (HSD) method to adjust the comparisons for multiplicity and report onlyPvalues < 0.05 (statistical significance).
RESULTS
We performed 15 experiments.One animal in the SV 20 mbar group achieved ROSC.The CCSV 40 mbar group showed significantly higher peak (Ppeak,P=0.001) and driving pressures (Pdrive,P=0.001) than SV 20 mbar group (Figure 1)when adjusted for timepoint.Additionally,the mean pressure(Pmean) was significantly lower in the SV 20 mbar (P<0.001)and IPPV (P<0.001) groups than in the CCSV 40 mbar group(Figure 1) when adjusted for timepoint.Minute volumes (MV)were significantly higher in CCSV 40 mbar group compared to SV 20 mbar group (P=0.011) and IPPV (P=0.007),when adjusted for timepoint.The respiratory rate (RR) of CCSV depends on the frequency of chest compressions applied,which were carried out at 100 compressions/min.SV 20 mbar group showed a mean RR of applied ventilation strokes of 56.38±38.61 breaths/min,whereas CCSV 40 mbar group showed 88.99±11.26 breaths/min.
The mean arterial pressure (MAP) displayed no significant differences (supplementary Figure 1).The central venous pressure (CVP) also showed no significant differences,but SV 20 mbar group showed lower values than IPPV and CCSV 40 mbar groups.
When adjusted for ventilation mode,the end-tidal partial pressure of carbon dioxide (etCO2) was significantly higher in the first phase than in the second phase (P=0.016).SV 20 mbar and IPPV groups showed consistent values of arterial partial pressure of oxygen (PaO2) (supplementary Figure 2).The PaO2of CCSV 40 mbar was higher in BLS with 306.55±254.98 mmHg (1 mmHg=0.133 kPa) and decreased during ALS to 145.73±160.93 mmHg.The arterial partial pressure of carbon dioxide (PaCO2) decreased throughout intervention in IPPV but increased in SV 20 mbar and CCSV 40 mbar.Serum lactate showed significantly higher values in CCSV 40 mbar group than in SV 20 mbar group (P=0.026)when adjusted for timepoint (supplementary Figure 1).MIGET measurements showed a non-significantly higher shunt fraction in IPPV and SV 20 mbar groups than in CCSV 40 mbar group.The percentage of normal V/Q ratio was the highest in SV 20 mbar,whereas CCSV 40 mbargroup showed more low and high V/Q.
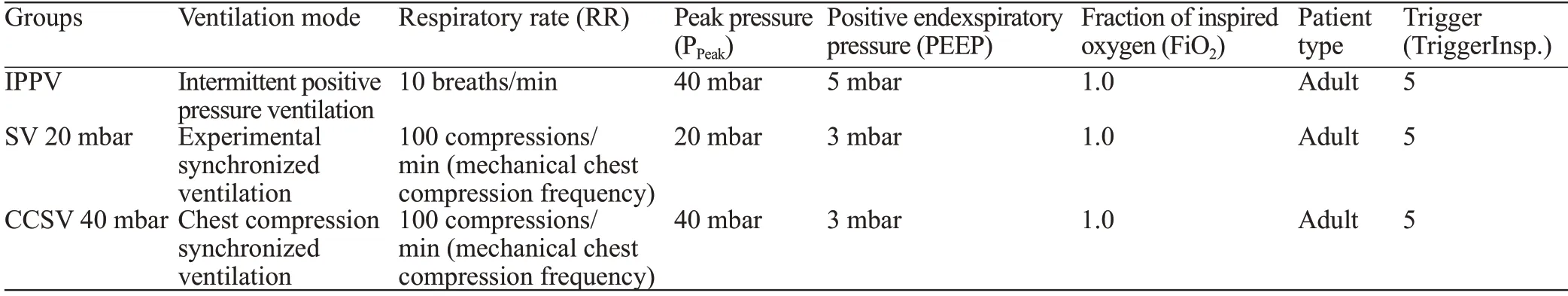
Table 1.Intervention groups and their parameters during resuscitation
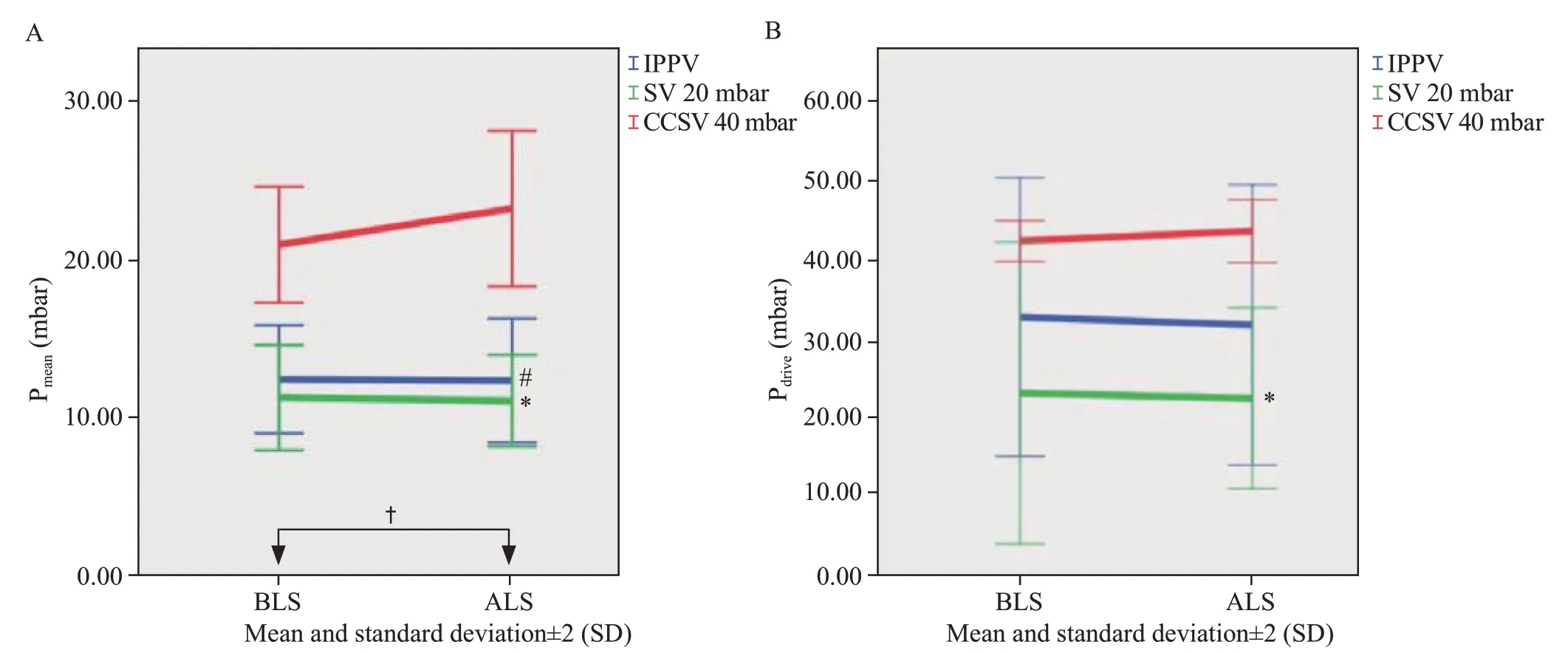
Figure 1.Airway pressures measured in mbar.Data are shown as the mean values and standard deviation (SD).A: mean airway pressure (Pmean);B:driving airway pressure (Pdrive).CCSV: chest compression synchronized ventilation;SV: synchronized ventilation;BLS: basic life support;ALS:advanced life support.Significant differences in Pmean (A): SV 20 mbar vs.CCSV 40 mbar,*P<0.001;IPPV vs.CCSV 40 mbar,#P<0.001 (Tukey);BLS vs.ALS,†P=0.048.Significant differences in Pdrive (B): SV 20 mbar vs.CCSV 40 mbar,*P=0.001 (Tukey).
The evaluation of lung histology using the DAD score showed no significant differences between the CCSV and SV modes.The only significant difference in the DAD score was the higher epithelial destruction in CCSV 40 mbar compared to IPPV (TukeyP=0.036).In the postmortem examination,pneumothorax was found in two IPPV animals and one CCSV 40 mbar animal,and another CCSV 40 mbar animal showed hemopneumothorax.
DISCUSSION
The optimal ventilation during resuscitation remains unclear,and different ventilation methods during CPR are the subject of current research.[7,8,10]One novel suggested pattern is CCSV,where each chest compression is detected by pressureand flow-sensors of the ventilator triggering a pressurecontrolled ventilation stroke.[4,11]In our trial,Ppeakwas limited to 40 mbar and 20 mbar,respectively,which led to significantly higher airway pressures and MV in CCSV 40 mbar.Additionally,CCSV 40 mbar group showed a non-significant decrease in oxygenation throughout the intervention and higher serum lactate compared to SV 20 mbar group.Additionally,decarboxylation was increasingly restricted in CCSV 40 mbar and SV 20 mbar.Ongoing (hemo-)pneumothoraces were observed in CCSV 40 mbar and IPPV.
The inspiratory pressure limitation to 20 mbar was applied to hypothetically achieve a more lung-protective ventilation pattern,which implies maintaining Ppeakbelow 30 mbar and aiming for a Pdrivelimited to 15 mbar or less.[12,13]The highest Pdriveand Pmeanwere detected in CCSV 40 mbar.Using higher airway pressures could lead to overdistension and cause lung damage and barotrauma.[13-15]Contrarily,accepting the creation of potential auto-PEEP due to higher ventilation frequency or restricted expiration might also lead to improved lung recruitment,similar to airway pressure release ventilation approaches.[16]We could detect these effects as well as a higher potential for structural damage and pulmonary trauma when higher ventilation pressures were applied.We observed no significant differences in lung damage evaluation.However,(hemo)pneumothoraces were seen in CCSV 40 mbar group and IPPV,which could present a higher risk of relevant lung damage using high pressure.However,this might be due to resuscitation efforts alone and cannot exclusively be attributed to ventilation without further investigation.
CCSV aims to enhance intrathoracic volume by ventilating during compression.Previous studies showed a higher MAP when using CCSV with a Ppeakof 45 mbar or higher compared to IPPV.[4,11]Our trial showed no significant differences in MAP,which could be explained by the explicitly lower Ppeak.We observed that there were fewer applied ventilation strokes in SV 20 mbar than in CCSV 40 mbar.This might be due to the technical device setup,which was experimentally designed specifically for this trial and appeared to have issues with adequate synchronization when using low inspiratory pressures.This might have resulted in inadequate pressure-controlled ventilation and could result in minor intrathoracic volume increases.
Gas exchange parameters showed no significant differences between the groups.High,normal,and low V/Q and shunt volume can be measured using the MiMMSMiget technique.[17]Although no significant differences were observed in our trial,SV 20 mbar had more normal V/Q and shunt,while CCSV 40 mbar showed more low and high V/Q.A higher shunt in SV 20 mbar could be due to increased atelectasis,leading to impaired oxygenation.However,oxygenation in CCSV 40 mbar group decreased during ongoing intervention,possibly pointing to an increased generation of atelectasis or subsequent tissue damage.Increased high V/Q suggests potential overinf lation of the lungs,posing a risk to lung tissue,especially during prolonged CPR.It can be assumed that the proportion of shunts in SV 20 mbar group did not increase during the procedure since oxygenation remained constant.The potentially impaired oxygenation in CCSV 40 mbar group during the second intervention phase aligns with the higher lactate levels observed in this group and could be attributed to ongoing impaired V/Q or structural lung damage.At SV 20 mbar,the afore-mentioned impaired RR did not appear to cause a decrease in oxygenation,lactate levels also remained lower,and one animal achieved ROSC.However,decarboxylation was increasingly impaired in SV 20 mbar group during intervention,likely due to the lower RR.
This pilot trial has some limitations,such as the low ROSC rate and relatively small intervention groups.A goal for further studies is to aim for a higher ROSC rate to be able to evaluate the long-term effects of the different ventilation modes.Additionally,technical difficulties arose,such as missing ventilation strokes due to improper trigger adaptation and suboptimal trigger performance.This may result in misaligned ventilation strokes,defeating the purpose of synchronized ventilation as a whole.Further adjustments of hardware,software and general experimental setup are necessary to account for all those effects.
CONCLUSION
This pilot trial of ventilation during CPR with the specially designed SV 20 mbar mode showed constant oxygenation throughout the intervention with lower lactate levels.No differences in hemodynamics were observed.This could indicate that synchronized low-pressure ventilation during CPR is not inferior to synchronized high-pressure ventilation or IPPV.Synchronized low-pressure ventilation should be further examined to determine whether it could be a more lung-protective method for ventilation during CPR.
ACKNOWLEDGEMENTS
This trial will be part of the doctoral thesis at the Johannes Gutenberg-University Mainz,written by Raphael René Cinto Noack.
Lung tissue samples were provided by the tissue bank of the University Medical Center Mainz,Germany,in accordance with the regulations of the tissue biobank and the approval of the ethics committee of the University Medical Center Mainz.Statistical planning and interpretations were performed with the assistance of the Institute of Medical Biometrics and Epidemiology of the Johannes Gutenberg University Mainz,Germany.
We would like to thank D.Dirvonskis for her indispensable commitment in conducting the study.
Funding:This study was partly funded by internal university research funding of the University Medical Center Mainz granted personal to Miriam Renz as well as a personal grant of the German Research Foundation to Robert Ruemmler (RU 2371/1).
Ethical approval:The trial was approved by the State and Institutional Animal Care Committee Rhineland Palatine (approval no.G16-1-042).
Conf licts of interest:The Medumat respirator (type MEDUMAT Standard²,Weinmann Emergency Medical Technology GmbH+Co.KG,Germany) and the LUCAS 2-System (Stryker,Kalamazoo,USA) were provided unconditionally by the manufacturers for animal research purposes only.
Contributors:MR and RR designed the study;RR supervised the experiments;MR wrote the manuscript;MR,RRCN,ReRi,KM,and JR performed animal experiments and analyzed the data;BD performed MIGET analyses;EKH,AZ and RR revised the manuscript and approved the final draft.The author(s) read and approved the final manuscript.
All the supplementary files in this paper are available at http://wjem.com.cn.
 World journal of emergency medicine2023年5期
World journal of emergency medicine2023年5期
- World journal of emergency medicine的其它文章
- Acute hemolytic anemia in a 34-year-old man after inhalation of a volatile nitrite “popper” product
- Severe disseminated intravascular coagulation complicated by acute renal failure during pregnancy
- Key elements and checklist of shared decisionmaking conversation on life-sustaining treatment in emergency: a multispecialty study from China
- Establishment and evaluation of animal models of sepsis-associated encephalopathy
- Open surgical approach for two coincidental splenic artery aneurysms: a case report
- Progressive eschar-like wound and peripheral neurological dysfunction with severe inf lammatory status: infection or unnatural immune response?
