Hypermethylation of thymosin β4 predicts a poor prognosis for patients with acute-on-chronic hepatitis B liver failure
He Wang , Yan-Ping Yin , Zhen-Li Wang , Yu Qian , Yu-Chen Fan , Hui-Hui Liu ,Kai Wang
a Department of Hepatology, Qilu Hospital of Shandong University, #107 Wenhuaxi Road, Jinan 250012, China
b Department of Hepatology, Qingdao Sixth People’s Hospital, Qingdao 2660 0 0, China
c Department of Gastroenterology, Yantaishan Hospital, Yantai 2640 0 0, China
d Institute of Hepatology, Shandong University, Jinan 250012, China
Keywords:Acute-on-chronic hepatitis B liver failure Acute-on-chronic hepatitis B pre-liver failure Thymosin β4 Methylation Prognosis
ABSTRACT Background: It has been demonstrated that thymosin β4 (Tβ 4) could inflect the severity of acute-onchronic hepatitis B liver failure (ACHBLF), but the relationship between its methylation status and the prognosis of liver failure is not clear.This study aimed to determine Tβ 4 promoter methylation status in patients with ACHBLF and to evaluate its prognostic value.Methods: The study recruited 115 patients with ACHBLF, 80 with acute-on-chronic hepatitis B pre-liver failure (pre-ACHBLF), and 86 with chronic hepatitis B (CHB).In addition, there were 36 healthy controls(HCs) from the Department of Hepatology, Qilu Hospital of Shandong University.The 115 patients with ACHBLF were divided into three subgroups: 33 with early stage ACHBLF (E-ACHBLF), 42 with mid-stage ACHBLF (M-ACHBLF), and 40 with advanced stage ACHBLF (A-ACHBLF).T β4 promoter methylation status in peripheral blood mononuclear cells (PBMCs) was measured by methylation-specific polymerase chain reaction, and mRNA was detected by quantitative real-time polymerase chain reaction.Results: Methylation frequency of Tβ 4 was significantly higher in patients with ACHBLF than in those with pre-ACHBLF, CHB or HCs.However, expression of Tβ 4 mRNA showed the opposite trend.In patients with ACHBLF, Tβ 4 promoter methylation status correlated negatively with mRNA levels.The 3-month mortality of ACHBLF in the methylated group was significantly higher than that in the unmethylated group.Also, Tβ 4 promoter methylation frequency was lower in survivors than in non-survivors.When used to predict the 1-, 2-, and 3-month incidence of ACHBLF, Tβ 4 methylation status was better than the model for end-stage liver disease (MELD) score.The predictive value of Tβ 4 methylation was higher than that of MELD score for the mortality of patients with E-ACHBLF and M-ACHBLF, but not for A-ACHBLF.Conclusions: Tβ 4 methylation might be an important early marker for predicting disease incidence and prognosis in patients with ACHBLF.
Introduction
Acute-on-chronic hepatitis B liver failure (ACHBLF) is a clinical syndrome caused by acute and severe impairment or loss of hepatocyte function in patients that already have chronic liver disease due to hepatitis B virus (HBV) infection [ 1 , 2 ].It is a type of endstage liver disease that progresses rapidly and is characterized by failure of one or more organs; short-term mortality is high [1].The poor prognosis and high mortality of patients with ACHBLF is a challenge for clinicians.In recent years, Chinese experts and scholars have proposed a new clinical type - acute-on-chronic hepatitis B pre-liver failure (pre-ACHBLF), which is the early stage of progression from CHB to liver failure.Hence, early sensitive and accurate prediction scores or biomarkers of prognosis are needed urgently.Early warning and intervention of these patients with pre-ACHBLF are crucial to reduce the morbidity and mortality associated with ACHBLF.The pathogenesis of ACHBLF may contribute to the search for new biomarkers that reflect early prognosis of ACHBLF.As far as we know, the pathogenesis of ACHBLF is complicated and little is known about it.During the course of liver failure, the body releases a large number of inflammatory cytokines[e.g., tumor necrosis factor-α, interleukin-6 (IL-6), IL-8, IL-10, IL-12,and interferon-γ], nitric oxide, reactive oxygen species, and activated immune cells (Kupffer cells), all of which contribute to inflammation and hepatocyte death [3-6].Immune-mediated liver injury and systemic inflammatory responses play a major role in the pathogenesis of HBV infection [3].The genes regulating hepatocyte inflammation may provide new insight into the search for biomarkers of the prognosis of liver failure.
DNA methylation of the mammalian genome is the most intensely studied epigenetic modification because it regulates so many biological processes; methylation is carried out by methyltransferases, which add a methyl group to the C5 position of cytosine to form 5-methyl cytosine (5-methylcytosine, 5mC) [7].A growing body of evidence suggests that DNA methylation is associated with the regulation of imprinted gene expression, X chromosome inactivation, embryonic development, cell differentiation, and tumor suppressor gene silencing in human cancers [8].Recently, it has become a vital method for studying the mechanisms underlying disease.In other words, DNA methylation is associated with many diseases [9-11].Previously, we confirmed that DNA methylation inhibits transcription of several genes and plays an important role in the biological processes that drive liver diseases [12-14].
Thymosinβ4 (Tβ4) is an acidic tripeptide comprising 43 amino acids and has a molecular weight of 4964.5 Da; it is the major G-actin-sequestering molecule in eukaryotic cells [15].Tβ4 plays a key role in wound healing, protecting tissue from damage, promoting tissue regeneration, and promoting epidermal and cardiac wound healing [16-19].A recent study shows that Tβ4 also protects mice from CCl 4 -induced acute hepatocyte damage [20].Moreover, Tβ4 attenuates liver fibrosis by upregulating expression of hepatocyte growth factor (HGF) and downregulating expression of platelet-derived growth factor-β(PDGF-β) [21].A study performed by Shah et al.demonstrated that Tβ4 has antioxidant, antiinflammatory, and antifibrotic potential in mice with alcoholic liver injury [22].Taken together, Tβ4 is involved in many diseases as an anti-inflammatory factor.Previous findings indicated that Tβ4 plays an important role in the prognosis of liver failure [ 23 , 24 ].However, the serum Tβ4 levels were measured only by enzymelinked immunosorbent assay in previous studies, and up to now there has been little relative quantitative assessment of the relationship between the expression of Tβ4 and the prognosis in patients with ACHBLF.Our preliminary findings show that Tβ4 hypermethylation is associated with the severity of ACHBLF and that its methylation status changes with the degree of hepatocyte inflammation in the presence of glucocorticoid therapy.Therefore, it is reasonable to presume that Tβ4 methylation may be an early biomarker that predicts the prognosis in patients with ACHBLF.
The present study aimed to investigate the role of Tβ4 promoter methylation status in peripheral blood mononuclear cells(PBMCs) in the prognosis of patients with ACHBLF.
Methods
Patients
From October 2018 to May 2021, 115 patients with ACHBLF,80 with pre-ACHBLF, 86 with chronic hepatitis B (CHB), and 36 healthy controls (HCs) were recruited at the Department of Hepatology, Qilu Hospital of Shandong University.The diagnostic criteria for ACHBLF were based on the consensus recommendation of the Asian Pacific Association for the Study of the Liver (APASL),2014 [25].ACHBLF was diagnosed as follows: a history of serum hepatitis B surface antigen (HBsAg) positivity for>6 months; ascites and/or encephalopathy (as determined by physical examination); serum total bilirubin (TBIL) ≥170μmol/L; and an international normalized ratio (INR) ≥1.5 or prothrombin time activity(PTA)<40%.The criteria for screening patients with pre-ACHBLF were [26]: (1) TBIL, 85.5-170μmol/L; and (2) PTA 40%-50%.Based on the PTA, TBIL, and the number and severity of complications involved, ACHBLF was divided into three stages [26]: the early stage of ACHBLF (E-ACHBLF) was defined as TBIL>170μmol/L, 30%<PTA ≤40%, and no complications and other extrahepatic organ failure; the middle stage of ACHBLF (M-ACHBLF) was defined as increased TBIL, significant bleeding (hemorrhagic spots or ecchymosis), 20%<PTA ≤30% (or INR 1.9-2.6), and one complication and/or failure of one extrahepatic organ; the advanced stage of ACHBLF (A-ACHBLF) was defined as further aggravation of the middle stage of ACHBLF, a tendency to severe bleeding (e.g., ecchymosis at injection site), PTA ≤20% (or INR ≥2.6), and two complications and/or failure of two extrahepatic organs.In accordance with the above criteria, 33 patients were assigned to the EACHBLF group, 42 to the M-ACHBLF group, and 40 to the A-ACHBLF group.Exclusion criteria of ACHBLF or pre-ACHBLF included pregnancy; liver tumor; decompensated cirrhosis; a history of diabetes,cardiac disease, or nephrosis; co-infection with another hepatitis virus; other hepatitis diseases (e.g., alcoholic hepatitis); druginduced hepatitis; Wilson disease; autoimmune hepatitis; or infection by cytomegalovirus, human immune deficiency virus, or Epstein-Barr virus.Enrolled CHB patients were diagnosed according to the American Society for the Study of Liver Diseases (AASLD)practice guidelines (2016) [27], and HBsAg was also positive for more than 6 months prior to trial initiation.The study was approved by the Ethics Committee of Qilu Hospital of Shandong University, and all participants provided written informed consent.
None of the patients received antiviral or immunotherapy during the 6 months prior to the trial, and all were followed up for at least 3 months from the date of diagnosis.Patients with ACHBLF and pre-ACHBLF were treated in accordance with the APASL consensus recommendations and the Diagnostic and Treatment Guidelines for Liver Failure [1].All received standard medical treatment, including bed rest, exogenous supplementation with plasma and albumin, energy supplements and vitamins, maintenance of water-electrolyte and acid-base equilibrium, and antiviral therapy.At the end of the 3-month follow-up, the outcome of patients with ACHBLF was defined as death or survival, whereas the prognosis of patients with pre-ACHBLF was defined as ACHBLF or not.None of the patients received a liver transplant.
Clinical and laboratory parameters
Serum samples were obtained from each patient on the second day after admission.The following serum biochemical markers of liver function were measured by an automatic biochemical analyzer (Cobas c311; Roche Diagnostics, Ltd., Mannheim, Germany):TBIL, alanine aminotransferase (ALT), aspartate aminotransferase(AST), albumin (ALB), cholinesterase (CHE), pre-albumin (preALB),and creatinine (Cr).PTA was evaluated using an ACL TOP 700 (Instrumentation Laboratory, Bedford, MA, USA).Hepatitis B e antigen (HBeAg) was detected by an electrochemiluminescence assay (Roche Diagnostics, Ltd.), and the HBV-DNA load was detected using the ABI 7300 PCR System (Applied Biosystems, Foster City, CA, USA).All of the above parameters were measured at the Department of Laboratory Medicine, Qilu Hospital of Shandong University.The model for end-stage liver disease (MELD)score was calculated using the following formula R = 9.57 × ln[creatinine (mg/dL)]+ 3.78 × ln [bilirubin(mg/dL)]+ 11.2 × ln(INR) + 6.43 × (etiology: 0 if cholestatic or alcoholic, 1 if due to other causes) [28].
PBMC samples
At the time of diagnosis, 5 mL of EDTA-anticoagulated peripheral venous blood was obtained from each participant.PBMCs were isolated by gradient centrifugation on Ficoll-Paque (Pharmacia Diagnostics, Uppsala, Sweden), as directed by the manufacturer.
Extraction of DNA and RNA, and sodium bisulfite modification
Genomic DNA was extracted from PBMCs using a QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany), and RNA was extracted from PBMCs using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA).The extracted DNA and RNA were stored at -20 °C for later use.Extracted DNA was modified with sodium bisulfite using the EZ DNA Methylation-Gold Kit (Zymo Research, Irvine, CA, USA); in total,20 μL modified DNA was obtained.Bisulfite-modified DNA samples were used immediately as a template for methylation-specific polymerase chain reaction (MSP) or stored at -20 °C for further study.Unmethylated cytokines were converted to uracil derivatives by DNA bisulfite modification, but methylated cytokines were not.
MSP
Primers specific for Tβ4 were as follows [29]: methylated sequence of forward primer, 5’-GTT TTC GGA TGT CGT TTC GAG AC-3’and methylated sequence of reverse primer, 5’-ACG ACG AAC GCA ACT TTA TAA ACG-3’; unmethylated sequence of forward primer,5’-TAG GTT TTT GGA TGT TGT TTT GAG AT-3’ and unmethylated sequence of reverse primer, 5’-AAA TAC AAC AAA CAC AAC TTT ATA AAC A-3’.MSP was performed in a total volume of 25 μL, which included 1 μL modified DNA, 0.5 μL of each primer (10 μmol/L),12.5 μL Premix Taq (Zymo Research Corp., Irvine, CA, USA), and 10.5 μL nuclease-free water.The reaction conditions were as follows: initial denaturation at 95 °C for 10 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s,extension at 72 °C for 60 s, and a final extension at 72 °C for 7 min.PCR-amplified products were electrophoresed on a 2% agarose gel,stained with Gel Red (Biotium, Fremont, CA, USA), and visualized under UV illumination.Each reaction was repeated three times.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Tβ4 mRNA was detected by qRT-PCR.Extracted RNA was reverse-transcribed into complementary DNA (cDNA) using the RevertAidTMFirst Strand cDNA Synthesis Kit (Fermentas, Vilnius,Lithuania); cDNA was then used as a template for PCR.The following primers specific for Tβ4 orβ-actin were designed by Methyl Primer Express: Tβ4-forward, 5-AAA CCC GAT ATG GCT GAG AT-3’, and Tβ4-reverse, 5’-TGC TTC TCC TGT TCA ATC GT-3’;β-actinforward, 5’-ATG GGT CAG AAG GAT TCC TAT GTG T-3’, andβ-actinreverse, 5’-CTT CAT GAG GTA GTC AGT CAG GTC-3’.PCR was performed in a total volume of 20μL, which contained 1 mL cDNA,0.4 mL of each specific primer, 10 mL SYBR Green premix, and 8.2 mL ddH 2 O.
The PCR reaction was performed using SYBRR○ Premix Ex TaqTM (Takara, Shiga, Japan) and a LightCycler (Roche Diagnostics,Mannheim, Germany).The reaction conditions were as follows: initial denaturation at 95 °C for 30 s, denaturation at 95 °C for 5 s,annealing at 60 °C for 30 s, and a final extension at 72 °C for 30 s(45 cycles in total).Each RT-qPCR was repeated three times.The results were calculated using the comparative (2-△△Ct) method.
Statistical analysis
Data were analyzed using SPSS 22.0 (IBM, Inc., Armonk, NY,USA).Quantitative variables were presented as median (interquartile range), and categorical variables were expressed as numbers (%).Differences between groups were analyzed using Mann-WhitneyUtest, Kruskal-Wallis test, or Chi-square test, as appropriate.The relationships between variables were evaluated using Pearson’s or Spearman’s correlation analysis.The diagnostic value of Tβ4 methylation and the MELD score for predicting incidence and mortality of ACHBLF were measured by calculating the area under the receiver operating characteristic (ROC) curve (AUC).Kaplan-Meier method was used to draw survival curves, and significance was analyzed using the log-rank test.All the statistical analyses were two-sided, and aP<0.05 was considered significant.
Results
General characteristics of the participants
From October 2018 to May 2021, 120 patients with ACHBLF were screened.Three patients were excluded due to hepatocellular carcinoma and two due to refusal to participate.Initially, 83 patients with pre-ACHBLF were enrolled, but two died of cardiovascular disease and one was excluded due to pregnancy.During the study period, four patients were excluded from the CHB group due to co-infection with hepatitis C virus (HCV) and human immunodeficiency virus (HIV).Finally, 115 patients with ACHBLF (33 E-ACHBLF, 42 M-ACHBLF, and 40 A-ACHBLF), 80 with pre-ACHBLF,86 with CHB, and 36 HCs were recruited.Table 1 shows the basic demographic and clinical characteristics of the enrolled participants.

Table 1Baseline characteristics of the study participants.
Expression of the T β4 gene in the different groups
As shown in Fig.1 A, Tβ4 promoter methylation frequency increased gradually from HCs through E-ACHBLF, M-ACHBLF and AACHBLF.The frequency was significantly higher for CHB patients(10/86, 11.63%) than for HCs (0/36;P= 0.030).In addition, there were significant difference between CHB and pre-ACHBLF (20/80,25.00%) (P= 0.028), and between pre-ACHBLF and E-ACHBLF(15/33, 45.45%) (P= 0.044).Interestingly, although the Tβ4 promoter methylation frequency of M-ACHBLF (20/42, 47.62%) and AACHBLF (21/40, 52.50%) was higher than that of E-ACHBLF, the difference was not significant (allP>0.05).Moreover, there was no difference in Tβ4 promoter methylation frequency between patients with M-ACHBLF and those with A-ACHBLF (P= 0.825).By contrast, the relative level of Tβ4 mRNA was the highest in HCs,but decreased in the order pre-ACHBLF>E-ACHBLF>M-ACHBLF>A-ACHBLF ( Fig.1 B).The relative level of Tβ4 mRNA in CHB patients was significantly lower than that in HCs (P<0.001), and that in pre-ACHBLF was also lower than that in CHB (P<0.001).There was no significant difference in Tβ4 mRNA levels between pre-ACHBLF and E-ACHBLF (P= 0.155), or between E-ACHBLF and M-ACHBLF (P= 0.359).Moreover, Tβ4 mRNA levels in M-ACHBLF was not significantly higher than those in A-ACHBLF (P= 0.613).A representative gel showing the results of the Tβ4 MSP assay is shown in Fig.2.

Fig.1.Expression of the T β4 gene in the different groups.A : Methylation of the T β4 promoter in PBMCs from patients with advanced stage ACHBLF (A-ACHBLF) ( n = 40),middle stage ACHBLF (M-ACHBLF) ( n = 42), early stage ACHBLF (E-ACHBLF) ( n = 33), acute-on-chronic hepatitis B pre-liver failure (pre-ACHBLF) ( n = 80), chronic hepatitis B(CHB) ( n = 86), and healthy controls (HCs) ( n = 36); B : expression of T β4 mRNA in PBMCs from patients with A-ACHBLF, M-ACHBLF, E-ACHBLF, pre-ACHBLF, CHB and HCs.*: P < 0.05.PBMCs: peripheral blood mononuclear cells.
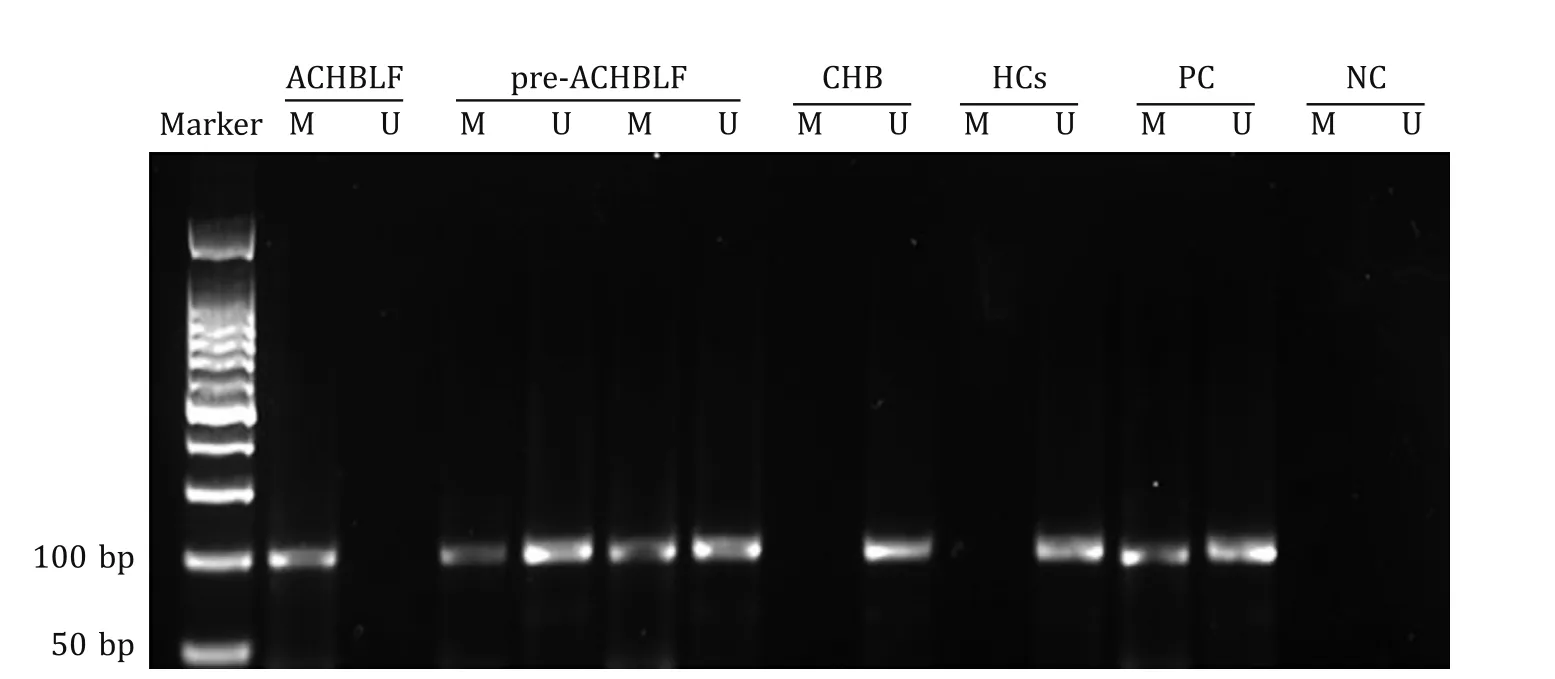
Fig.2.Representative gel showing methylation of the T β4 promoter by MSP.Lane M: methylated sequences; Lane U: unmethylated sequences; PC: positive control; NC: negative control; ACHBLF: acute-on-chronic hepatitis B liver failure; pre-ACHBLF: acute-on-chronic hepatitis B pre-liver failure; CHB: chronic hepatitis B; HCs: healthy controls;MSP: methylation-specific polymerase chain reaction.
Correlation between T β4 promoter methylation and clinicopathological parameters of patients with ACHBLF
The link between Tβ4 promoter methylation and clinicopathological features is shown in Table 2.Tβ4 methylation status was associated significantly with TBIL, PTA, the MELD score, and mortality at the 3-month follow-up.Age, sex, ALT, AST, ALB, preALB,Cr, CHE, HBeAg, and HBV-DNA were not significantly different between the methylated and unmethylated groups.

Table 2Clinicopathological parameters and T β4 promoter methylation status in patients with ACHBLF.
The correlation between Tβ4 promoter methylation and clinicopathological parameters in patients with ACHBLF was further analyzed by Spearman’s correlation analysis.The results indicated that the methylation frequency of Tβ4 promoter correlated positively with TBIL (r= 0.283,P= 0.002) and the MELD score (r= 0.299,P= 0.001), but negatively with PTA (r= -0.241,P= 0.010).There was no correlation between methylation frequency and ALT, AST,ALB, preALB, Cr, CHE, HBeAg, or HBV-DNA.The results of multivariate logistic regression analysis indicated that higher TBIL, PTA, and MELD score were associated with an increased risk of Tβ4 promoter methylation ( Table 3 ).
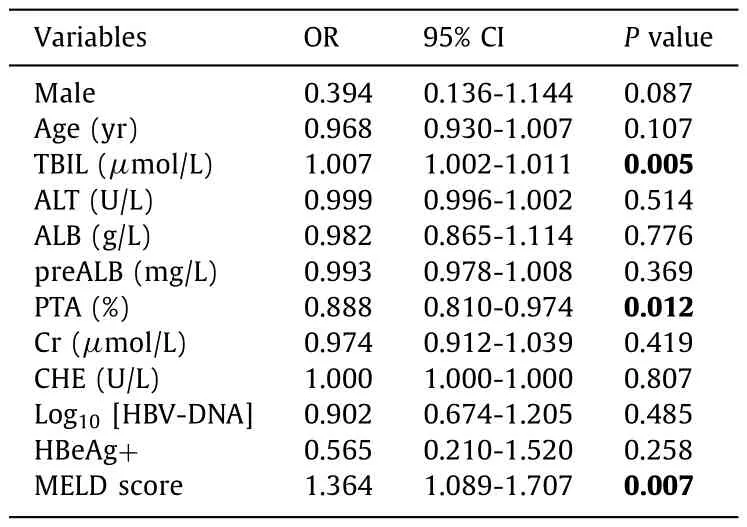
Table 3Multivariate logistic regression analysis of variables in patients with ACHBLF.
Correlation between T β4 mRNA expression and clinicopathological parameters of patients with ACHBLF
Tβ4 mRNA expression was lower in the methylated group than in the unmethylated group (P= 0.007).Spearman’s correlation coefficient indicated that the methylation status of Tβ4 correlated negatively with mRNA levels (r= -0.325,P<0.001).
As shown in Fig.3 , Tβ4 mRNA levels showed a significant correlation with TBIL (r= -0.366,P<0.001), PTA (r= 0.225,P= 0.016), and the MELD score (r= -0.190,P= 0.042).However, there was no correlation between Tβ4 mRNA level and age(r= 0.133,P= 0.157), sex (r= 0.112,P= 0.235), ALT (r= 0.046,P= 0.628), AST (r= -0.031,P= 0.741), ALB (r= -0.035,P= 0.711),preALB (r= -0.010,P= 0.915), Cr (r= -0.102,P= 0.277), CHE(r= 0.148,P= 0.114), HBeAg (r= -0.013,P= 0.891), and HBVDNA (r= 0.027,P= 0.777).
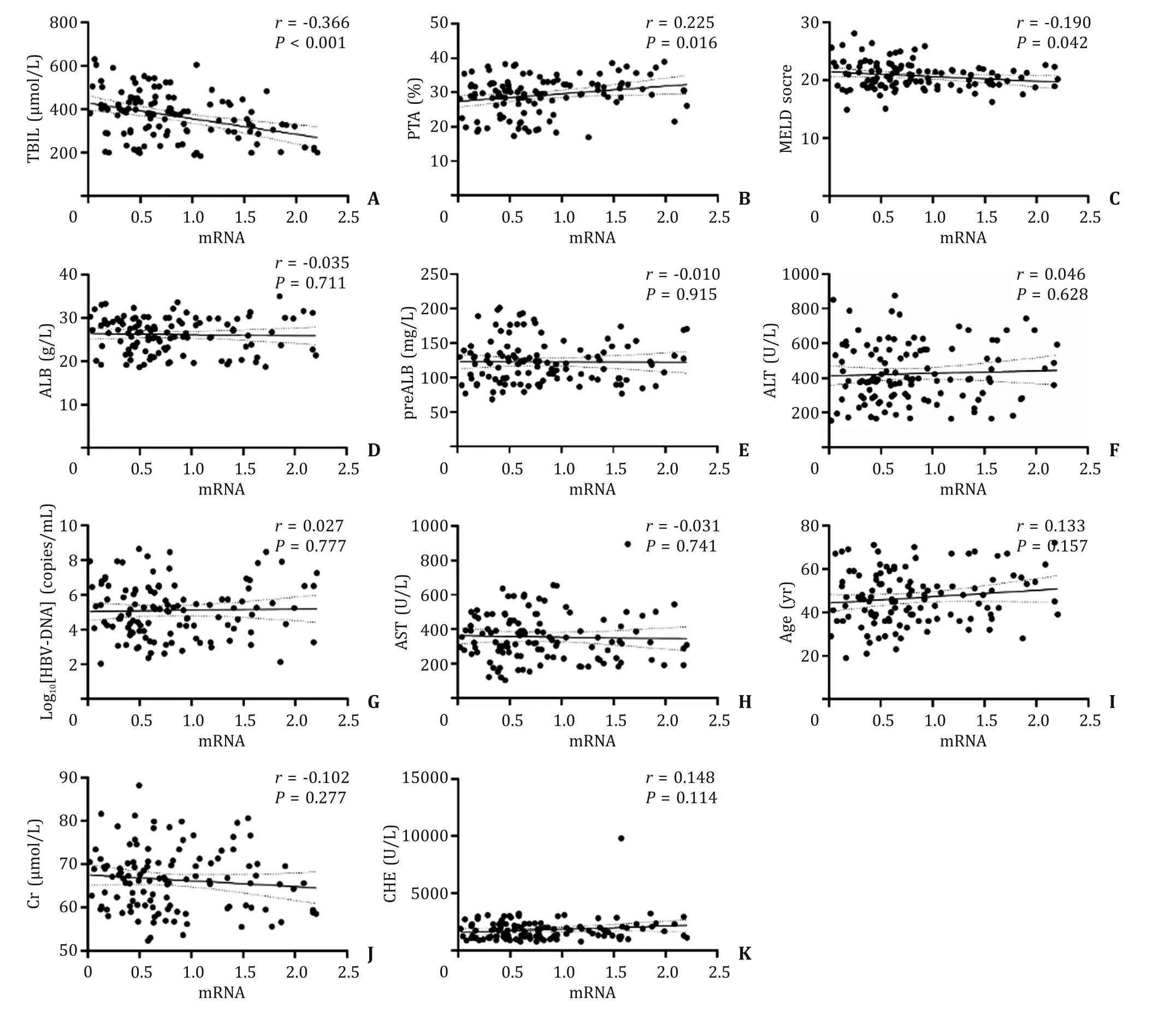
Fig.3.Correlation between T β4 mRNA levels and clinicopathological parameters in 115 patients with acute-on-chronic hepatitis B liver failure (ACHBLF).TBIL: total bilirubin;PTA: prothrombin activity; MELD: model for end-stage liver disease; ALB: albumin; preALB: pre-albumin; ALT: alanine aminotransferase; AST: aspartate aminotransferase;Cr: creatinine; CHE: cholinesterase.
Diagnostic value of T β4 methylation for discriminating pre-ACHBLF from CHB
When distinguishing pre-ACHBLF from CHB, Tβ4 methylation showed high specificity; the number of positive cases was 55.The sensitivity was 68.75% (55/80), the specificity was 89.53% (77/86),the positive predictive value (PPV) was 85.94% (55/64), and the negative predictive value (NPV) was 75.49% (77/102).The AUC was 0.803 [95% confidence interval (CI): 0.735-0.861,P<0.0 0 01;Fig.4 A].

Fig.4.A : ROC curve analysis of the diagnostic value of T β4 promoter methylation for discriminating ACHBLF ( n = 80) from CHB patients ( n = 86); B : at 1 month, the AUC value for T β4 methylation was higher than that of the MELD score (AUC, 0.833 vs.0.627, respectively; P = 0.005) for predicting the incidence of ACHBLF; C : at 2 months,the AUC value for T β4 methylation was a better predictor of the incidence of ACHBLF than the MELD score (AUC, 0.848 vs.0.699, respectively; P = 0.037); D : at 3 months,the AUC value for T β4 methylation was a significantly better predictor of the incidence of ACHBLF than the MELD score (AUC, 0.849 vs.0.697, respectively; P = 0.048);E : ROC curve for T β4 methylation and the MELD score for predicting the mortality of patients with E-ACHBLF; AUCs were 0.939 (95% CI: 0.797-0.992) and 0.537 (95%CI: 0.356-0.712), respectively; F : ROC curve for T β4 methylation and the MELD score for predicting the mortality of patients with M-ACHBLF; AUCs were 0.887 (95% CI:0.751-0.964) and 0.672 (95% CI: 0.510-0.808), respectively; G : ROC curve for T β4 methylation and the MELD score for predicting the mortality of patients with A-ACHBLF;AUCs were 0.707 (95% CI: 0.542-0.840) and 0.783 (95% CI: 0.624-0.897), respectively.
Value of T β4 methylation and the MELD score for predicting the incidence of ACHBLF in patients with pre-ACHBLF
As the follow-up time increased from 1 to 2 to 3 months, so did the percentage of pre-ACHBLF that progressed to ACHBLF (36.25%vs.56.25% vs.72.50%, respectively).The value of Tβ4 promoter methylation and the MELD score for predicting the 1-, 2-, and 3-month incidence of ACHBLF in patients with pre-ACHBLF was assessed by ROC curve analysis.At 1 month, the AUC for Tβ4 promoter methylation was 0.833 (95% CI: 0.733-0.907), which was significantly higher than that of the MELD score (0.627; 95% CI: 0.512-0.733).When used to predict the 2-month incidence of ACHBLF,the AUC for Tβ4 promoter methylation and the MELD score were 0.848 (95% CI: 0.750-0.918) and 0.699 (95% CI: 0.587-0.797), respectively.The AUC for Tβ4 promoter methylation at 3- months was 0.849 (SE = 0.054; 95% CI: 0.751-0.919), and for MELD score was 0.697 (95% CI: 0.585-0.795).With respect to the 1-, 2-, and 3-month incidence of ACHBLF, Tβ4 promoter methylation showed a higher predictive value than the MELD score (allP<0.05; Fig.4 BD).
T β4 methylation and the MELD score for predicting the mortality of patients with ACHBLF
The 115 patients with ACHBLF who completed the treatment for at least 90 days were followed up.At the end of the 90-dayfollow-up period, 52 patients with ACHBLF survived and 63 died.As shown in Table 4 , TBIL, PTA, MELD score, and methylation of the Tβ4 promoter were significantly different between survivors and non-survivors.However, age, sex, ALT, ALB, preALB, Cr, CHE,HBeAg, and HBV-DNA were not significantly different between survivors and non-survivors.
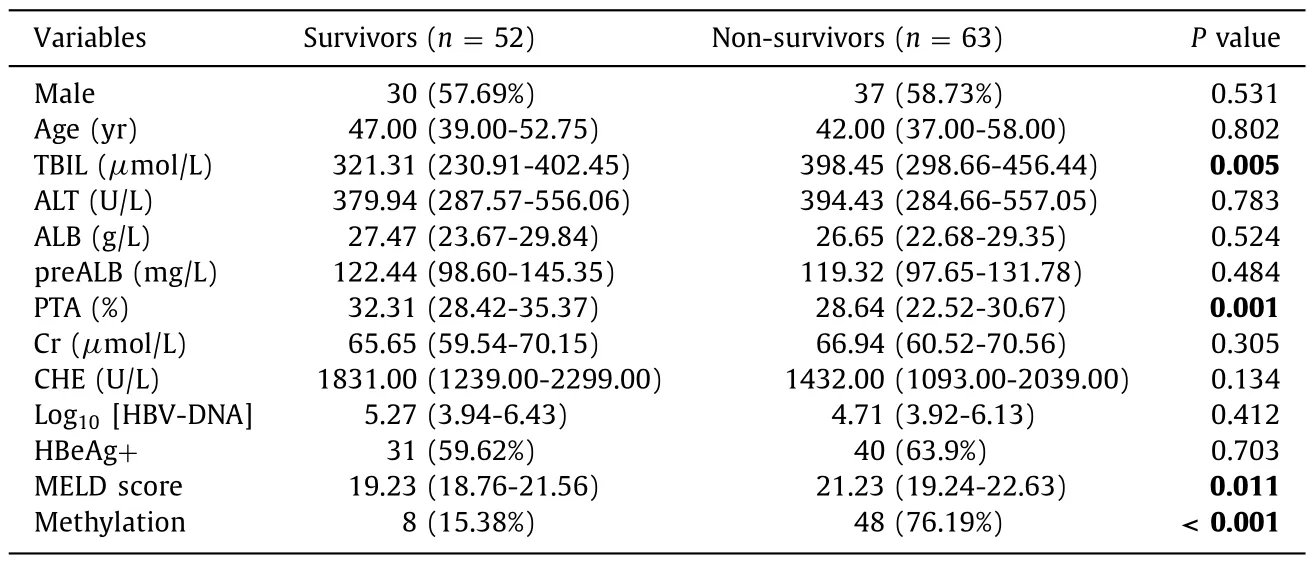
Table 4Clinicopathological parameters of survivors and non-survivors with ACHBLF.
At the end of the 90-day follow-up period, the mortalities of patients with E-ACHBLF, M-ACHBLF, and A-ACHBLF were 45.45%(15/33), 54.76% (23/42), and 62.50% (25/40), respectively.The AUC of Tβ4 promoter methylation for predicting the mortality of EACHBLF patient s was 0.939 (95% CI: 0.797-0.992), which was higher than that for the MELD score (AUC = 0.537; 95% CI:0.356-0.712;P<0.001; Fig.4 E).Moreover, Tβ4 promoter methylation had a sensitivity of 86.67%, a specificity of 88.89%, a PPV of 86.67%, and an NPV of 88.89% for prognosis of E-ACHBLF.As shown in Fig.4 F, the AUC for Tβ4 promoter methylation in MACHBLF patient s (AUC = 0.887; 95% CI: 0.751-0.964) was also higher than that of the MELD score (AUC = 0.672; 95% CI: 0.510-0.808;P= 0.036).Tβ4 promoter methylation had a sensitivity of 82.61%, a specificity of 94.74%, a PPV of 95.00%, and an NPV of 81.82%.Interestingly, the AUC for Tβ4 methylation (AUC = 0.707;95% CI: 0.542-0.840) for predicting the mortality of A-ACHBLF patients was lower than that for the MELD score (AUC = 0.783; 95%CI: 0.624-0.897), but the difference was not significant (P= 0.429;Fig.4 G).Tβ4 methylation showed a sensitivity of 68.00%, a specificity of 73.33%, a PPV of 80.95%, and an NPV of 57.89%.These results suggest that Tβ4 promoter methylation is a better early predictor of ACHBLF prognosis compared with the MELD score.
Discussion
ACHBLF is an acute hepatic insult that occurs underlying chronic liver disease caused by hepatitis B virus infection.Systemic inflammatory responses play an important role in the outcome of liver failure.Recently, some studies reported that Tβ4 might be an effective anti-inflammatory factor [ 22 , 30 , 31 ].Tβ4 is a potent regulator of actin polymerization in living cells and plays an important role in many biological processes [ 32 , 33 ].It also plays a role in the pathogenesis of many liver diseases [34-36].Accumulating evidence suggests that Tβ4 plays anti-inflammatory, immunomodulatory, and anti-oxidative stress roles by regulating expression of cytokines [37-39].In addition, Tβ4 has shown antioxidant, antiinflammatory, and antifibrotic potential in mice with alcoholic liver injury [22].Liang et al.reported that Tβ4 plays a role in the occurrence and development of inflammation in patients with CHB with steatosis by inhibiting oxidative stress [40].DNA methylation impacts normal cell physiology by regulating gene expression [41].DNA methylation of promoter regions leads to gene transcription silencing, down-regulation of expression, and even loss of gene function [ 7 , 42 , 43 ].Long-term CHB infection may be associated with abnormal methylation of the promoter regions of certain genes [ 44 , 45 ].Aberrant methylation of Tβ4 occurs in patients with chondrosarcoma or hepatocellular carcinoma [ 29 , 46 ].Nevertheless,no study has examined whether Tβ4 methylation plays a role in ACHBLF.
Here, we first observed that the frequency of Tβ4 promoter methylation in patients with ACHBLF was higher than that in HCs and in patients with pre-ACHBLF or CHB.Moreover, the result also indicated that the methylation frequency increased gradually from E-ACHBLF to M-ACHBLF to A-ACHBLF.The opposite trend was observed for mRNA expression; indeed, there was a negative correlation between Tβ4 promoter methylation and mRNA expression.These findings were consistent with those reported by Liu et al.[23], who demonstrated that serum Tβ4 levels in ACHBLF were higher than those in HCs.These data indicate that Tβ4 may play an important role in the pathogenesis of ACHBLF.Further analyses revealed that Tβ4 promoter methylation correlated significantly with TBIL, PTA, and the MELD score, which reflects liver function.These results suggest that Tβ4 promoter methylation affects liver function.Indeed, Han et al.reported that serum Tβ4 levels reflect the severity of liver failure [24].Hypermethylation of the Tβ4 promoter might downregulate its expression, thereby reducing its antioxidant capacity.The resulting increase in oxidative stress would further damage liver cells and tissues.Therefore, we consider that Tβ4 methylation reflects the inflammatory status of hepatocytes and might be a biomarker for ACHBLF severity.
Our previous studies showed that for several genes, aberrant promoter methylation predicts poor prognosis in those with ACHBLF [47-49].It is reported that serum Tβ4 is an important predictor of ACHBLF prognosis [23].Here, we found at the 3-month follow-up that the mortality of ACHBLF patients in the Tβ4-methylated group was significantly higher than that of those in the Tβ4 non-methylated group.Moreover, Tβ4 methylation frequency in survivors was lower than that in non-survivors.These results indicate that Tβ4 methylation is associated with the mortality of patients with ACHBLF.Hence, we considered that aberrant Tβ4 methylation might predict the prognosis of patients with ACHBLF.
Considering that pre-ACHBLF represents the early stage of liver failure, early identification, intervention and treatment could reduce the incidence of liver failure and associated mortality.For pre-ACHBLF patients, oxidative stress and epigenetic changes such as DNA methylation of gene promoters could be detected due to chronic inflammatory infection of HBV [50].Here, we found that hypermethylation of the Tβ4 promoter was more common in patients with pre-ACHBLF than in those with CHB.This result also indicates that Tβ4 methylation could discriminate pre-ACHBLF from CHB (specificity, 89.54%).In this respect, our results are similar to those reported by others [ 12, 51], suggesting that hypermethylation of the Tβ4 promoter helps identify patients with pre-ACHBLF.
Currently, the MELD score is used to evaluate disease severity and to predict mortality due to ACHBLF.However, the MELD score has some limitations [ 52 , 53 ].Here, when used to predict the 1-,2-, and 3-month incidence of ACHBLF in patients with pre-ACHBLF,Tβ4 methylation was superior to the MELD score.Therefore, Tβ4 methylation is a potential early marker for ACHBLF.We also found that at the end of the 90-day follow-up period, the mortality of patients increased from E-ACHBLF through to A-ACHBLF.Furthermore, the predictive value of Tβ4 methylation was higher than that of the MELD score in patients with E-ACHBLF and M-ACHBLF,but not for those with A-ACHBLF.Thus, Tβ4 methylation is an early biomarker for predicting the mortality of ACHBLF.In addition, early intervention for pre-ACHBLF patients according to Tβ4 methylation status could reduce the incidence of ACHBLF, and early prognosis,assessment, and treatment of ACHBLF could reduce mortality.
This study has several limitations.First, the specimens were mainly PBMCs.The methylation status and mRNA level of Tβ4 in liver tissues were not measured directly due to the risk of hemorrhage caused by liver biopsy.However, previous studies showed that the expression of Tβ4 in peripheral blood is consistent with that in liver tissue [ 40 , 54 ].Therefore, the expression of Tβ4 in PBMCs from patients with ACHBLF also can reflect that in liver tissue.Of course, the functional tests in cells or animals may better reveal that Tβ4 methylation might predict disease incidence and prognosis in patients with ACHBLF.In future studies, cell-based experiments and animal models should be used to examine Tβ4 expression and promoter methylation.Second, the study was conducted to assess disease severity and mortality among patients with end-stage liver disease based on the MELD score.In addition to the MELD score, clinicians have long used the Child-Turcotte-Pugh (CTP) and MELD-sodium (MELD-Na) scores [53].However,the CTP and MELD-Na are not supported by many statistical data,nor do they undergo rigorous validation in the same way as MELD [52].At present, MELD is recognized as the best predictor of survival for those with liver failure.In future studies, we will examine the effect of combining Tβ4 methylation status with other prognostic models or scores.Finally, it has been reported that Tβ4 has antioxidant, anti-inflammatory, and antifibrotic potential during mouse and rat liver injury [ 22 , 30 , 31 ].Tβ4 upregulated the expression of HGF, downregulated the expression of platelet-derived growth factor-βreceptor mRNAs in hepatic stellate cells, which indicated that Tβ4 has antifibrogenic potential [ 21 , 32 ].Cell-based experiments and animal models should be used to examine Tβ4 expression and promoter methylation in liver failure.
In conclusion, hypermethylation of the Tβ4 promoter occurs in ACHBLF, and it correlates significantly with TBIL, PTA, and the MELD score, suggesting that Tβ4 methylation is a biomarker for severity of ACHBLF.Early identification of pre-ACHBLF by examining Tβ4 methylation status provides a new opportunity for early diagnosis and treatment of pre-ACHBLF.In addition, it could be an early predictor of mortality due to ACHBLF.Early detection and treatment are important measures to reduce the risk of progression to liver failure and to reduce the incidence and mortality of ACHBLF.
Acknowledgments
None.
CRediT authorship contribution statement
He Wang:Investigation, Data curation, Methodology, Formal analysis, Writing - original draft.Yan-Ping Yin:Methodology, Investigation, Data curation.Zhen-Li Wang:Methodology, Writing -original draft.Yu Qian:Data curation, Formal analysis, Methodology.Yu-Chen Fan:Software, Writing - review & editing.Hui-HuiLiu:Data curation, Methodology.Kai Wang:Conceptualization,Project administration, Resources, Supervision, Writing - review &editing.
Funding
This study was supported by grants from the Key Project of the Chinese Ministry of Science and Technology ( 2017ZX102022022 ),the National Natural Science Foundation of China ( 81970522 ), and the Key Research and Development Project of Shandong Province( 2019GSF108023 ).
Ethical approval
The study was approved by the Ethics Committee of Qilu Hospital of Shandong University, and all participants provided written informed consent.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2023年4期
Hepatobiliary & Pancreatic Diseases International2023年4期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- The impact of metabolic dysfunction-associated fatty liver disease on the prognosis of patients with hepatocellular carcinoma after radical resection
- Novel re-intervention device for occluded multiple uncovered self-expandable metal stent (with video)
- Laparoscopic liver resection for hepatocellular carcinoma complicated with significant portal hypertension: A propensity score-matched survival analysis
- Clinical analysis of Wernicke encephalopathy after liver transplantation
- Hepatobiliary&Pancreatic Diseases International
- Wnt/beta-catenin signaling and its modulators in nonalcoholic fatty liver diseases
