Hepatobiliary&Pancreatic Diseases International
General inform ation
Hepatobiliary&Pancreatic Diseases International is a journal published bimonthly in the English language by the First Affiliated Hospital,Zhejiang University School of Medicine,Hangzhou,China.We welcome original research articles,review articles,editorials,and others from any part of the w orld.Manuscripts are review ed by members of the international editorial board and our expert peer review ers,then either accepted for publication or rejected by the chief editor.Manuscripts should be submitted via https://mc03.manuscriptcentral.com/hbpdint.
Most of submissions accepted for publication may undergo revisions recommended by the reviewers,editors or statistical advisers.A decision takes from tw o w eeks to three months,the longer period being due to the multiple review s needed for articles likely to be accepted.Publication varies from tw o to six months after fi nal acceptance.Proofs of edited articles and illustrations are sent to the corresponding author for correction and reply to any queries from editors.A sample copy of the journal containing the articles will be forwarded to the corresponding author within a few days of publication.
Online submissions
Original research articlesincluding randomized trials,intervention studies,studies of screening and diagnostic tests,cohort studies,cost-effectiveness analyses and case-control studies should be prepared in accordance w ith the Uniform Requirements for Manuscripts Submitted to Biomedical Journals(www.icmje.org).Those of randomized controlled trials(RCTs)should follow the CONSORT-statement(www.consort-statement.org).The reporting of other articles should follow the guidelines set by the different initiatives or groups listed in the appendix.The article should be submitted w ith tw o separate fi les,one w ith detailed information about authors and the title,short running title,source of the w ork,in addition to the names,address,telephone and fax number or E-mail address for the corresponding author.Another fi le should show only the title,running title,abstract,main text,tables,fi gures of the submitted article as w ell as a list of references,w hich w ill be sent to review ers.
The authors’names(normally no more than six)and their initials should be in the same form as in their other publications w ith one major qualifi cation such as MD or Ph D,their current appointment and a full postal address.One author should be named for editorial correspondence.
Each submission must be accompanied by a letter of copyright transfer signed by all the authors(see the detailed description in the last section)and other relevant documents including informed consent obtained from research subjects and research approval from the supervising or institutional ethics bodies.Author(s)of each submission should clarify the functions in or contributions to their research and declare,if any,competing interest.A choice of one of the follow ing statements w ill be requested w hen a decision has been made to accept the paper:
1.The author or one or more of the authors have received or w ill receive benefi ts for personal or professional use from a commercial party related directly or indirectly to the subject of this article.
2.No benefi ts in any form have been received or w ill be received from a commercial party related directly or indirectly to the subject of this article.
3.The author or authors do not choose to declare any confl ict of interest related directly or indirectly to the subject of this article.
The statements selected by the author or authors w ill be published w ith the article.The signature of each author w ill be required.No article w ill be published until the completed form has been received at the Hepatobiliary&Pancreatic Diseases International office.
Manuscripts should be prepared with generous margins,and double spacing is essential throughout the text,the references and the captions.Tables,fi gures,captions and a list of references should appear properly in the text.The style should be simple and direct,free from ambiguity and jargon,and w ith minimal use of abbreviations.
Thetitleof the paper should be chosen w ith care:a short one has more impact and may be expanded by a subtitle.
Structured abstractof original articles should include four sections as Background,Methods,Results and Conclusions and have no more than 300 w ords summarizing the most important points in the article,incorporating key w ords suitable for electronic retrieval systems.Background:This section should provide a precise statement of the primary focus of the study and the context in w hich it w as carried out.Methods:This section describes how the study was performed,including details of clinical and/or technical procedures.Results:This section describes the salient results of the study.Conclusions:This section covers conclusions and their clinical application or others,w hile equal emphasis should be given to positive and negative fi ndings of equal scientifi c merit.
Themain textshould be divided under headings.For many research papers the best sequence is Introduction,Methods(Materials or Patients),Results,and Discussion.The text should comment on,but should not repeat,the details given in tables,fi gures or captions.Any acknow ledgements should be made at the end of the main text.
Introduction:This should explain the problem w hich is to be addressed,w ith a defi nition of the hypothesis to be examined if appropriate,out-lining,briefl y,its relevance to the appropriate literature.
Methods(Materials or Patients):The subjects of the study and the methodsemployed in the investigation must be clearly described.For example,the reasons for examining the particular group of patients should be made clear,and reasons for exclusion of individuals from the study must be stated.Any group used as controls must be defi ned accurately.
Ethical approval of studies and informed consent are required.For all manuscripts reporting data from studies involving human participants or animals,formal review and approval,or formal reviewand waiver,by an appropriate institutional review board or ethics committee is required and should be described in the Methods section.For those investigators w ho do not have formal ethics review committees,the principles outlined in the Declaration of Helsinki should be follow ed.For investigations of humans,state in the Methods section the manner in w hich informed consent w as obtained from the study participants(i.e.,oral or w ritten).Editors may request that authors provide documentation of the formal review and recommendation from the institutional review board or ethics committee responsible for oversight of the study.
Results:Thesemust be clearly expressed in simple language.Tables or similar diagrams can be used but must not duplicate material already described in the text.
Discussion:This section must be succinct,pointing out the relevance of the work described in the article and its contribution to current know ledge.The results must be interpreted clearly,and both strength and weakness expressed.Discussion of pertinent references must be concise and short.
Each of thetablesshould have a short descriptive heading.
Thereferencesin the text should include only those that are important and have been studied fully by the authors.The authorsare responsible for the accuracy and completeness of the cited references and for correct citation of the text.When listing references the names of journals should be abbreviated according to Index Medicus(List all authors and/or editors up to 6;if more than 6,list the fi rst 6 and et al).All references w ill be checked deliberately by the authors;PMIDrootsin the abstract serial number indexed by PubMed(http://www.ncbi.nlm.nih.gov/sites/entrez?db=PubMed)and doi are requested.The references cited should be represented in the text by numbers w ithin square brackets in the order of their appearance.The list of references at the end of the text should be in this numerical order w ith details and punctuation as follows:
Article from journal—more than one author
Cen C,Fang HX,Yu SF,Liu JM,Liu YX,Zhou L,et al.Association betw een ADIPOQ gene polymorphisms and the risk of new-onset diabetes mellitus after liver transplantation.Hepatobiliary Pancreat Dis Int 2017;16:602-609.PMID:29291779 doi:10.1016/S1499-3872(17)60069-9.
Article from journalswith English abstractsonly
Sun JH,Zhang YL,Nie CH,Li J,Zhou TY,Zhou GH,et al.Effect of liver cirrhosis on percutaneous selective portal vein embolization for primary liver cancer[Article in Chinese].Zhonghua Yi Xue Za Zhi 2013;93:3831-3834.PMID:24548443 doi:10.3760/cma.j.issn.0376-2491.2013.48.006.
Monographic series
Davidoff RA.Migraine:manifestations,pathogenesis,and management.Philadelphia,Pa:FA Davis;1995.Contemporary Neurology Series,No.42.
Online journals with volume and page information
Simon JA,Hudes ES.Relationship of ascorbic acid to blood lead levels.JAMA 1999;281:2289-2293.PMID:10386552.Accessed June 11,2009.http://jama.ama-assn.org/cgi/content/full/281/24/2289
Online journals without volume and page information
Li JH,Jia JJ,Shen W,Chen SS,Jiang L,Xie HY,et al.Optimized postconditioning algorithm protects liver graft after liver transplantation in rats.Hepatobiliary Pancreat Dis Int 2018 Feb 2.PMID:29428101 doi:10.1016/j.hbpd.2018.01.006.
Online website
Morse SS.Factors in the emergence of infectious diseases.Emerg Infect Dis[serial online]1995 Jan-Mar[cited 1996 Jun 5];1(1):[24 screens].Available from:w w w.cdc.gov/ncidod/eid/vol1no1/morse.htm
Book—more than one author(list all authors if six or less,otherwise list fi rst three followed by et al)
Baselt RC,Cravey RH.Disposition of toxic drugs and chemicals in man,4th ed.Foster City,CA:Chemical Toxicology Institute;1995.
Chapter from a book
Calne RY.Experimental background.In:Calne RY,ed.Liver transplantation,2nd ed.London:Grune&stratton;1987:3-7.
Book—with editors
Maddrey WC,ed.Transplantation of the liver.New York:Elsevier Science Publishing;1998.
IllustrationsPhotographs,draw ings,diagrams,charts and graphs should meet the editorial demands.The“three-dimensional”bar charts produced by computer programs are not acceptable.Photomicrographs with no inset scale should have the magnifi cation of the print in the caption;stains should be indicated.The lettering on diagrams and graphs should be large enough to be clear after it has been reduced for printing and be consistent in size and style.Color w ill be accepted only w here it is essential.Permission to reproduce any borrowed illustration must be obtained from the author and the publisher,and evidence of this permission must accompany the submitted article.
Review articlesare welcome especially systematic,critical assessments of the literature and data sources pertaining to clinical topics,emphasizing factors such as cause,diagnosis,prognosis,therapy,or prevention.All articles and data sources review ed should include information about the specifi c type of study or analysis,population,intervention,exposure,and tests or outcomes.All articles or data sources should be selected systematically for inclusion in the review and critically evaluated,and the selection process should be described in the article.Meta-analyses also w ill be considered as review articles.Editors ask potential authors of review articles to check the MOOSEinitiative for meta-analysis of observational studies in epidemiology(See the appendix).
The articles in department ofNew techniquesfocus on the new techniques used,w hich are of interest to clinicians and researchers.Clinical im ages,clear and interesting,are submitted w ith descriptive paragraph of 500-800 w ords.Authors must obtain signed informed consent from the patient.
View pointsmay address virtually any important topic in the fi elds of hepatobiliary and pancreatic diseases,medical,surgical,radiological,pathological,biochemical,physiological and histological aspects,and generally are not linked to a specifi c article.It can be up to 1500 w ords,tw o tables or fi gures,no more than 10 references and 6 authors.
Letters to the editoron matters of recently published articles in the journal are especially w elcome,w hich should not exceed 400 w ords.Letters of general interest,unlinked to items published in the journal,can be up to 1500 w ords long and are to be sent for peer-review ed.Correspondence lettersare not usually peer review ed,but the journal might invite replies from the authors of the original publication,or pass on letters to these authors.Only tw o tables or fi guresarepermitted,and there should be no more than 10 references and six authors.All accepted lettersare edited,and proofs w ill be sent out to authors before publication.
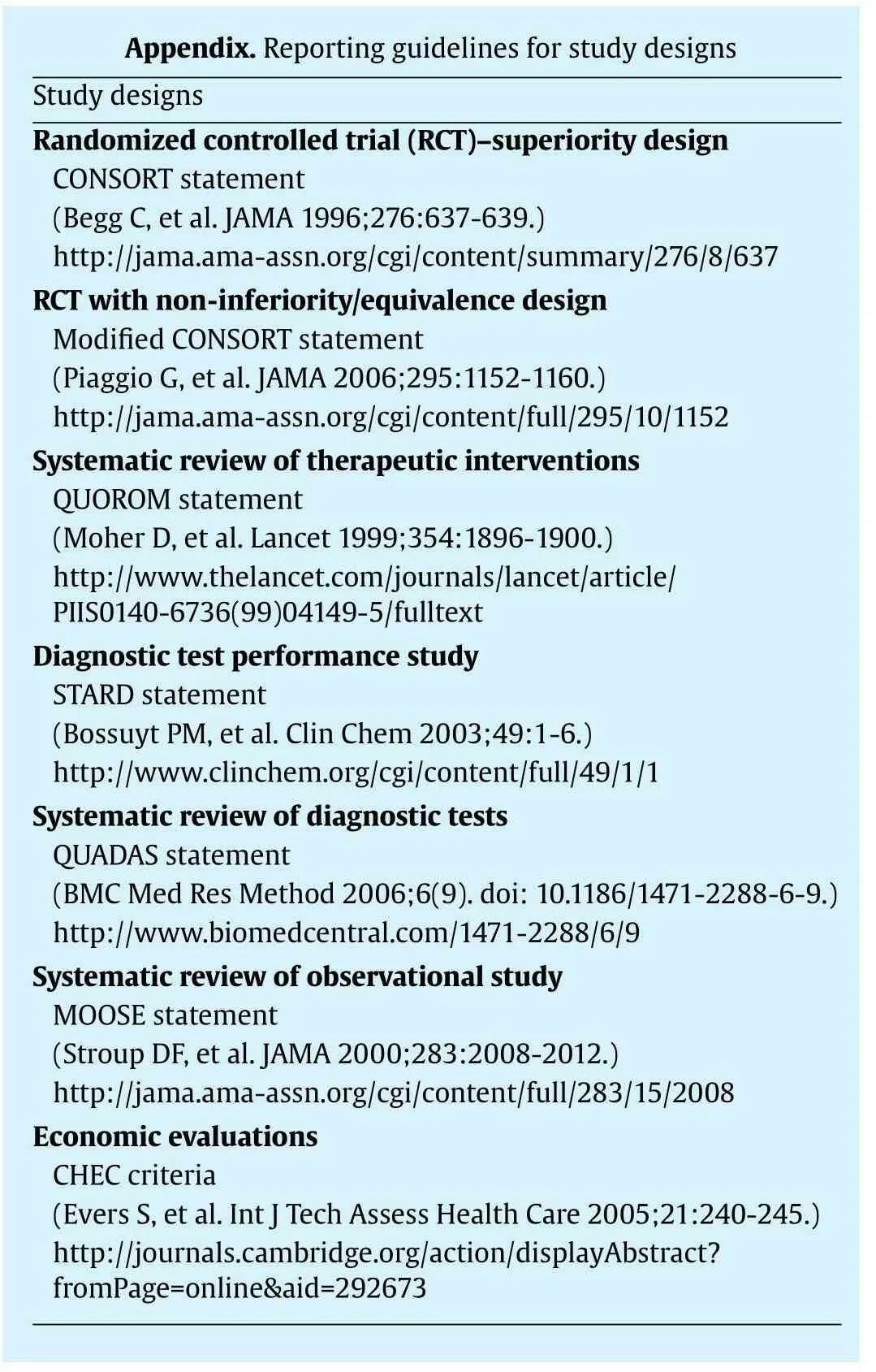
Appendix.Reporting gui delines for study designs Study designs Random ized controlled trial(R CT)-superiority design CONSORTstatement(Begg C,et al.JAMA 1996;276:637-639.)http://jama.ama-assn.org/cgi/c ontent/summary/276/8/637 RCTw ith non-inferiority/equiv alence design Modifi ed CONSORTstatement(Piaggio G,et al.JAMA 2006;29 5:1152-1160.)http://jama.ama-assn.org/cgi/c ontent/full/295/10/1152 System atic review of therapeu tic interventions QUOROM statement(Moher D,et al.Lancet 1999;35 4:1896-1900.)http://w w w.thelancet.com/jou rnals/lancet/article/PIIS0140-6736(99)04149-5/ful ltext Diagnostic test perform ance st udy STARD statement(Bossuyt PM,et al.Clin Chem 2 003;49:1-6.)http://w w w.clinchem.org/cgi/c ontent/full/49/1/1 System atic review of diagnosti c tests QUADASstatement(BMCMed Res Method 2006;6(9).doi:10.1186/1471-2288-6-9.)http://w w w.biomedcentral.co m/1471-2288/6/9 System atic review of observati onal study MOOSEstatement(Stroup DF,et al.JAMA 2000;2 83:2008-2012.)http://jama.ama-assn.org/cgi/c ontent/full/283/15/2008 Economic evaluations CHECcriteria(Evers S,et al.Int JTech Assess Health Care 2005;21:240-245.)http://journals.cambridge.org/action/displayAbstract?from Page=online&aid=292673
Notes,new s or announcementsfor conferences,symposia or meetings may be sent for publication at least 3 months before the required date of publication.These are mostly included under Meetings and Courses w ithout payment.Provide title,date(s)and place of the event and contact address,telephone,and E-mail address.Hepatobiliary&Pancreatic Diseases International reserves the right to be selective in publishing these announcements.Those w ishing for a detailed publication may submit as advertisements.For academic information,subsidized rates are available.
Letter of copyright transfer
We require a letter of copyright transfer signed by all named authors,stating that they have taken a signifi cant and active part in the preparation of the article,that they have read and approved the fi nal version,and are w illing to discuss it in detail.This is essential for the review ing process to be completed.Possibly duplicative materials(i.e.,those containing substantially similar content or using the same or similar data)that have been previously published or are being considered elsew here must be provided at the time of manuscript submission.
To conform w ith the Copyright Act of China,this letter must contain the follow ing paragraph:
In consideration of Hepatobiliary&Pancreatic Diseases International review ing and editing my(our)submission,the author(s)undersigned hereby transfer(s),assign(s),or otherw ise conveys all copyright ow nership to Hepatobiliary&Pancreatic Diseases International represent(s)that he(they)ow n(s)all rights in the material submitted.The author(s)further confi rm(s)that the article is original,that it is not under consideration by another journal in any language,and that it has not been previously published,in whole or in part,in another journal in any language.There is no confl ict of interests relevant to the study reported in this article.
For convenience potential author can dow nload the form of the letter from our journal’s w ebsite.This assignment is to take effect only if the work is accepted for publication in Hepatobiliary&Pancreatic Diseases International.
These instructions can also be found on the journal’s website at www.hbpdint.com and may be copied by those intending to submit an article.
 Hepatobiliary & Pancreatic Diseases International2023年4期
Hepatobiliary & Pancreatic Diseases International2023年4期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Novel re-intervention device for occluded multiple uncovered self-expandable metal stent (with video)
- Hepatopancreatoduodenectomy for the treatment of extrahepatic cholangiocarcinoma ✩
- Microbiological cultures and antimicrobial prophylaxis in patients undergoing total pancreatectomy with islet cell autotransplantation
- Risk factors for posttransplant diabetes in patients with hepatocellular carcinoma
- The role of targeting protein for Xklp2 in tumorigenesis of hepatocellular carcinoma
- Isolated IgG4-associated autoimmune hepatitis or the first manifestation of IgG4-related disease?
