Risk factors for posttransplant diabetes in patients with hepatocellular carcinoma
Yi-Yun Feng , Ming-Zhi Xu , c,*
a Zhejiang Chinese Medical University, Hangzhou 310053, China
b Department of General Medicine, Zhejiang Cancer Hospital, Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou 310022, China
c Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University, Shulan International Medical College, Hangzhou 310015, China
TotheEditor:
Hepatocellular carcinoma (HCC) is one of the most common cause of cancer death worldwide, and in China, primary HCC ranks 4th for incidence and 2nd for mortality among all cancers [1].Traditionally, the gold standard treatment for HCC is surgical resection, but most patients are not fit due to the advanced disease.In the 1980s, liver transplantation emerged as the treatment of choice for end-stage liver disease and also became an option for HCC patients [2].But elevated blood glucose is a common complication after liver transplantation, affecting approximately 20%-40% of liver recipients [3].Posttransplant diabetes mellitus (PTDM)refers to newly diagnosed diabetes mellitus (DM) after transplantation, regardless of timing or presence but undetected before transplantation [4].In addition to all well-known complications of DM,PTDM is associated with reduced graft function, increased risk of graft failure, acute kidney injury, and increased cardiovascular risk and mortality in liver recipients [ 5 , 6 ].Therefore, identifying highrisk patients and taking steps to limit the development of PTDM may improve the long-term prognosis of patients [7].This study aimed to explore the risk factors influencing the development of PTDM after liver transplantation in HCC patients, to provide interventions for different populations before and after surgical treatment, to prevent postoperative blood glucose elevation and to improve clinical outcomes.
HCC patients who underwent allogeneic liver transplantation at Shulan (Hangzhou) Hospital from January 2017 to June 2021 were retrospectively reviewed.Inclusion criteria were applied as follows:1) those aged ≥18 years and ≤60 years; 2) first-time organ transplant recipients; and 3) patients were followed up regularly postoperatively and for ≥1 year.Exclusion criteria included: 1) those with incomplete preoperative medical history data; 2) transplant recipients who died or were lost to follow-up; 3) multiorgan combined or multiple transplant recipients; 4) organ failures within 1 year of transplantation; and 5) those with preoperative diagnosis of DM.
In this study, the diagnostic criteria of PTDM were in accordance with the China technical code for the diagnosis and treatment of posttransplant diabetes (2019 Edition) [8], and since this study was retrospective and all enrolled patients did not undergo a 75 g oral glucose tolerance test, we used the fasting blood glucose (FBG) level as the diagnostic basis for PTDM.Liver function was graded using Child-Pugh classification [9].All enrolled patients were treated with a hormone free immunosuppressive regimen and were divided into two groups according to the postoperative immunosuppressive regimen: 1) tacrolimus group(tacrolimus + mycophenolate mofetil); 2) tacrolimus + sirolimus group (tacrolimus + sirolimus + mycophenolate mofetil).
The following data were recorded for analysis: 1) general clinical data, including sex, age, body mass index (BMI), family history of DM, hepatitis history, cirrhosis history, liver function grade,etc.; 2) preoperative relevant indicators, including serum biochemistry, abnormal prothrombin, panel reactive antibodies (PRA), human leucocyte antigen (HLA), etc.; 3) intraoperative related indicators, including duration of surgery, surgical approach, etc.; 4) postoperative related indicators, including inflammatory parameters 1 day after surgery, liver function 1 month after surgery, acute rejection, virus infection, immunosuppressive regimens, etc.
SPSS25.0 statistical software (SPSS Inc., Chicago, IL, USA) was used to analyze the data.Normally distributed quantitive data were presented as mean ± standard deviation and compared by Student’st-test.Quantitive data that were not normally distributed were presented as medians (interquartile range, IQR) and were compared by rank sum test.The categorical data were tested by Chi-square test or Fisher’s exact probability method and expressed as percentages.Multivariate logistic regression analysis was used to explore the possible influencing factors of posttransplant diabetes.AP<0.05 was considered statistically different.
Finally, 192 patients were included in the statistical analysis, including 40 with PTDM, 112 with posttransplant impaired FBG, and 40 with completely normoglycemia (FBG<5.6 mmol/L).The detailed information is listed in Table 1.The age was 4 9.53 ± 5.4 9 years in 40 patients (39 males and 1 female) in the PTDM group,45.13 ± 8.31 years in 40 patients (38 males and 2 females) in the normoglycemic group (P= 0.007).The incidence of PTDM in our hospital was 20.83%.
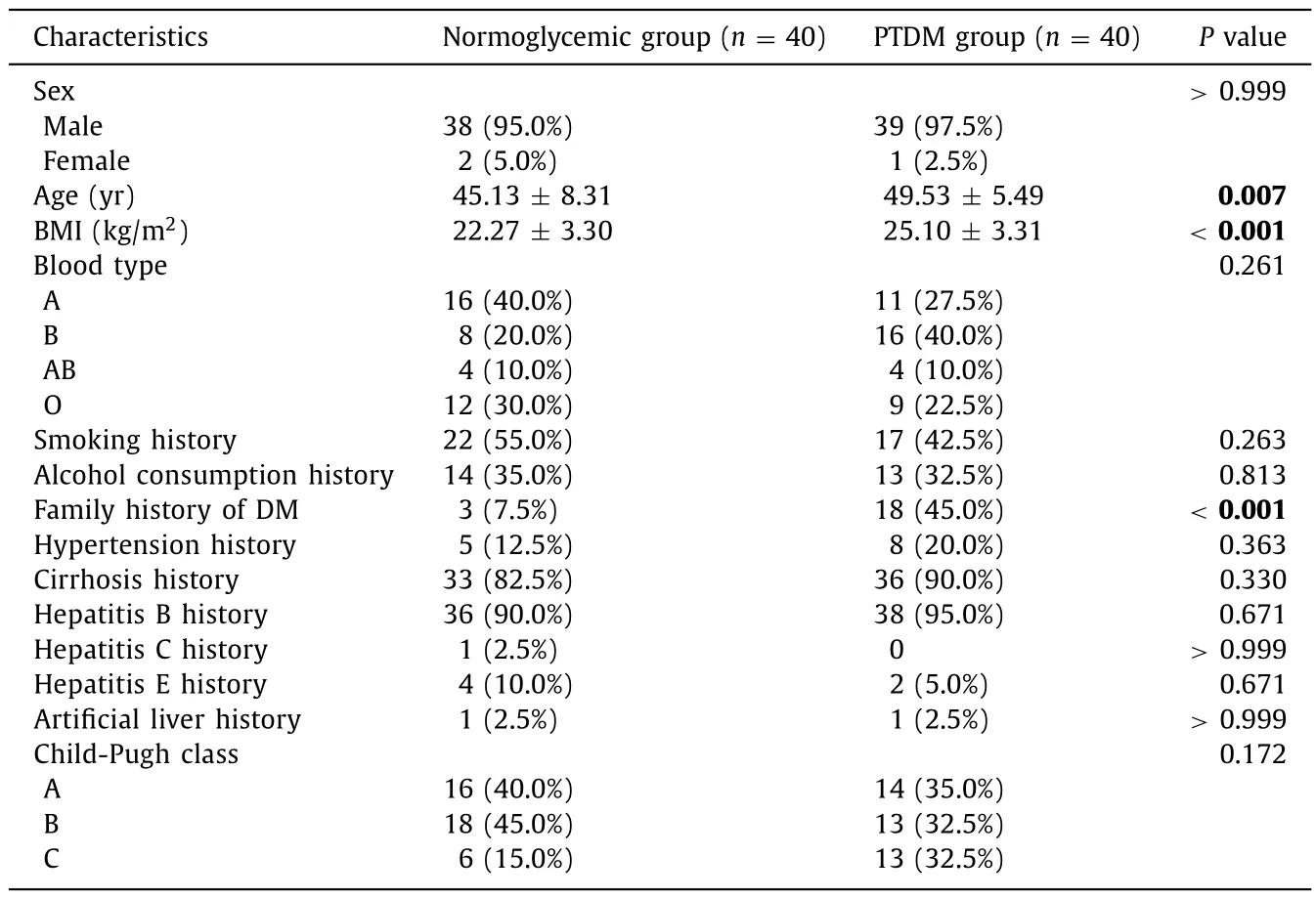
Table 1General clinical data of the two groups.
The BMI in the PTDM group was significantly higher than that in the normoglycemic group (25.10 ± 3.31 vs.22.27 ± 3.30 kg/m2,P<0.001).Family history of DM was also significantly higher in the PTDM group than in the normoglycemic group (45.0% vs.7.5%,P<0.001) ( Table 1 ).Comparison of the patients’ preoperative, intraoperative, and postoperative related indexes revealed that patients in the PTDM group had significantly higher preoperativeFBG, higher median direct bilirubin (DBIL) 1 month after surgery,and higher infection with cytomegalovirus (CMV) after surgery than those in the normoglycemic group ( Table 2 , allP<0.05).

Table 2Pre-, mid-, and post-operative factors in the two groups.
High HLA expression has become a widely accepted marker of type 1 DM and has also shown association with type 2 DM in different ethnic populations [10].Other types of DM have also been shown to have some degree of heritability, such as maturity onset diabetes of the young [11].However, the association of HLA with PTDM is unclear.We therefore looked for a link between these alleles and PTDM.The distribution of HLA-A, -B, and -DR showed no statistically significant difference between the two groups (allP>0.05).
Variables withP<0.2 in the above analysis were included in the multivariate logistic regression analysis.The results indicated that BMI [odds ratio (OR) = 1.263, 95% confidence interval (CI):1.029-1.549,P= 0.026], family history of DM (OR = 11.318, 95% CI:1.327-96.539,P= 0.027), preoperative FBG (OR = 9.094, 95% CI:2.200-37.597,P= 0.002), DBIL 1 month after surgery (OR = 1.137,95% CI: 1.002-1.290,P= 0.046), and postoperative CMV infection(OR = 19.525, 95% CI: 1.575-241.980,P= 0.021) were independent risk factors for the development of PTDM in HCC patients ( Table 3 ).

Table 3Multivariate logistic regression analysis for PTMD.
In conclusion, the present study showed that 20.83% (40/192)patients developed PTDM.Higher BMI, family history of DM, preoperative FBG, DBIL 1 month after surgery, and postoperative CMV infection were independent risk factors for the development of PTDM in HCC patients.The key finding was that DBIL levels at 1 month after surgery might be a risk factor for the development of PTDM after liver transplantation in HCC patients.The association between DBIL and T2DM has been documented [12], and there has been no literature reporting relationship with PTDM.Although we forced the inclusion of HLA related data into the multivariate logistic regression analysis, there was no statistically significant difference between HLA expression and PTDM.
Because the occurrence of PTDM is associated with reduced graft function, increased risk of organ transplant rejection, increased cardiovascular risk, organ transplant mortality, and reduced quality of life, it is essential to identify patients with highrisk factors and manage them to minimize the development of PTDM.
Acknowledgments
None.
CRediT authorship contribution statement
Yi-Yun Feng:Conceptualization, Data curation, Formal analysis,Writing - original draft, Writing - review & editing.Ming-Zhi Xu:Conceptualization, Supervision, Writing - review & editing.
Funding
This study was supported by grants from the National Key R&D Program of China (2021YFA1301100 and 2021YFA1301104).
Ethical approval
This study was approved by the Ethics Committee of Shulan(Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College, China (No.KY2022075).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2023年4期
Hepatobiliary & Pancreatic Diseases International2023年4期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Novel re-intervention device for occluded multiple uncovered self-expandable metal stent (with video)
- Hepatopancreatoduodenectomy for the treatment of extrahepatic cholangiocarcinoma ✩
- Microbiological cultures and antimicrobial prophylaxis in patients undergoing total pancreatectomy with islet cell autotransplantation
- The role of targeting protein for Xklp2 in tumorigenesis of hepatocellular carcinoma
- Isolated IgG4-associated autoimmune hepatitis or the first manifestation of IgG4-related disease?
- Left abdominal mass with carcinosis: Unusual presentation of pancreatic acinar cell carcinoma
