The impact of metabolic dysfunction-associated fatty liver disease on the prognosis of patients with hepatocellular carcinoma after radical resection
Ke-Gong Xiong, Kun-Yu Ke, Li-Fng Chen, Jin-Feng Kong, Ti-Shun Lin,Qing-Bio Lin, Su Lin, Yue-Yong Zhu,c,*
a Department of Hepatology, Hepatology Research Institute, the First Affiliated Hospital of Fujian Medical University, Fuzhou 350001, China
b Department of Hepatology, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou 350001, China
c Fujian Clinical Research Center for Liver and Intestinal Diseases, Fuzhou 350001, China
Keywords:Metabolic dysfunction-associated fatty liver disease Hepatocellular carcinoma Radical resection Prognosis
ABSTRACT Background: Metabolic dysfunction-associated fatty liver disease (MAFLD) is recently proposed an entity by a group of international experts.However, the impact of MAFLD on the prognosis of patients with hepatocellular carcinoma (HCC) is not clear.The aim of this study was to explore the influence of MAFLD for the prognosis of HCC after radical resection.Methods: HCC patients who received radical resection were enrolled.The recurrence-free survival (RFS)and overall survival (OS) were compared between MAFLD and non-MAFLD.Results: A total of 576 HCC patients were included, and among them 114 (19.8%) met the diagnostic criteria of MAFLD.The median RFS was 34.0 months in the MAFLD group and 19.0 months in the non-MAFLD group.The 1-, 3-, and 5-year RFS rates were 64.9%, 49.1% and 36.1% in the MAFLD group, which were higher than those of the non-MAFLD group (59.4%, 35.3% and 26.5%, respectively, P = 0.01).The mean OS was 57.0 months in the MAFLD group and 52.2 months in the non-MAFLD group.There was no statistical difference in OS rate between the MAFLD group and non-MAFLD group.Similar results were found in HBV-related HCC patients in the subgroup analysis.Univariate analysis revealed that MAFLD was a protective factor for RFS in HCC patients after radical resection (P <0.05), and there was no association between MAFLD and OS rate (P >0.05).Multivariate analysis demonstrated that MAFLD was not an independent protective factor for HCC patients with radical resection.Conclusions: MAFLD improves RFS rate in HCC patients with radical resection, but is not an independent protective factor and not associated with OS rate.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the fourth leading cause of cancer-related mortality worldwide [1].The major and the first-line treatment for HCC is radical resection.Of note, within 5 years after curative resection, recurrence of HCC is up to 70% [2].HCC recurrence particularly occurs within 2 years after surgery, which severely impacts patients’ prognosis [3].Nonalcoholic fatty liver disease (NAFLD)is a common chronic liver disease related to insulin resistance and genetic background.The global prevalence of NAFLD is up to 25.2% [4].Moreover, the incidence of HCC related to NAFLD is also increasing [5].HCC with histopathologically confirmed NAFLD was associated with higher surgical morbidity and post-hepatectomy liver failure, but long-term survival was favorable compared with HCC without NAFLD [6].
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a new concept proposed by a group of international experts [7].This new definition emphasizes the essential role of metabolic dysfunction in fatty liver disease [8].To date, little is known about the impact of MAFLD on the prognosis of HCC patients.This retrospective study aimed to compare the prognosis of HCC patients with and without MAFLD after radical resection.
Methods
Patients
Patients who were diagnosed with HCC and underwent radical resection at Mengchao Hepatobiliary Hospital of Fujian Medical University from January 2015 to December 2018 were consecutively included.The inclusion criteria for this study were those with histopathologically confirmed HCC and had received radical resection.The exclusion criteria were as follows: hepatocholangiocarcinoma, other malignancy, transcatheter hepatic arterial chemoembolization or radiofrequency ablation before radical resection, and with lack of complete clinical data.Radical resection was defined as complete macroscopic removal of the tumor with negative histologic resection margin [9].Cirrhosis was defined as pseudolobule formation in histopathology.Fatty liver was diagnosed as more than 5% of liver cells with lipid accumulation in histopathology [10].MAFLD was diagnosed with the evidence of fat infiltration in histopathology, in addition to at least one of the following three conditions: body mass index (BMI)>23 kg/m2,type 2 diabetes (T2D), or metabolic dysregulation, which means presence of at least two of the following metabolic at-risk criteria: hypertension, plasma triglycerides ≥1.70 mmol/L, plasma high-density lipoprotein (HDL)-cholesterol<1.0 mmol/L for men and<1.3 mmol/L for women, prediabetes [i.e., fasting glucose levels 5.6 to 6.9 mmol/L, or glycated hemoglobin A1c (HbA1c)5.7% to 6.4%], plasma high-sensitivity C-reactive protein (hs-CRP)level>2 mg/L [7].This research was approved by the Medical Ethics Committee of Mengchao Hepatobiliary Hospital of Fujian Medical University (2021-035-01).
Data collection
Baseline data were collected from each patient, including age,sex, body weight, BMI, excessive alcohol consumption (male ≥30 g/day, female ≥20 g/day), T2D, hypertension, Child-Pugh grade,Barcelona Clinic Liver Cancer (BCLC) staging, alpha-fetoprotein(AFP) levels, carcinoembryonic antigen (CEA), hepatitis B surface antigen (HBsAg), hepatitis B virus (HBV) DNA, abdominal enhanced computed tomography (CT) or magnetic resonance imaging(MRI), blood transfusion during surgery and pathological features of tumors (histopathology type, size, numbers, capsule, cell differentiation, microvascular invasion, and microsatellite lesions).The following preoperative indicators were also collected: leukocyte count, hemoglobin levels, platelet count, prothrombin time, levels of albumin, total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), plasma triglycerides, plasma HDLcholesterol, fasting glucose, HbA1c, and hs-CRP.
Follow-up and evaluation
Patients were followed up every 3-month in 2 years after radical surgical treatment, every 6-month within 2-5 years and yearly after 5 years.The following examination were performed in each case: biochemistry examination, AFP and abdominal enhanced CT or MRI.The last follow-up was in December 2020.
HCC recurrence was defined as the evidence of new lesions fulfilling the criteria of diagnosis of HCC [9,11].Recurrence-free survival (RFS) was defined as the period from the date of the radical resection to the date of recurrence or last follow-up without recurrence.Overall survival (OS) was defined as the period from the date of radical resection to the date of death or final follow-up.
Statistical analysis
SPSS 22 (SPSS Inc., Chicago, IL, USA) was used to conduct statistical analysis.Continuous variables were expressed by median and interquartile range (IQR), and differences between the MAFLD and non-MAFLD groups were tested by Mann-WhitneyUtest.Categorical variables were tested by Chi-square test.OS and RFS were analyzed using the Kaplan-Meier method and compared using logrank test.Variables that were associated with survival atP <0.05 in univariate Cox regression analysis were included in multivariate Cox regression analysis, and hazard ratio (HR) and 95% confidence interval (95% CI) were calculated.Pvalues<0.05 indicated statistical significance.
Results
Baseline clinicopathological characteristics
Selection of the study population is shown in Fig.1.There were 576 patients in this study, with 472 (81.9%) males and 104 (18.1%)females.Patients were categorized into two groups based on the presence of MAFLD: the MAFLD group (114, 19.8%) and non-MAFLD group (462, 80.2%).There were 532 (92.4%) patients with HBV infection.The median BMI was 22.8 kg/m2.The number of patients with BMI>23 kg/m2was 268 (46.5%), T2D was 89 (15.5%) and metabolic dysregulation was 184 (31.9%), respectively.The median diameter of HCC was 4.0 cm.The majority (509/576, 88.4%) were with solitary tumor (Table 1).
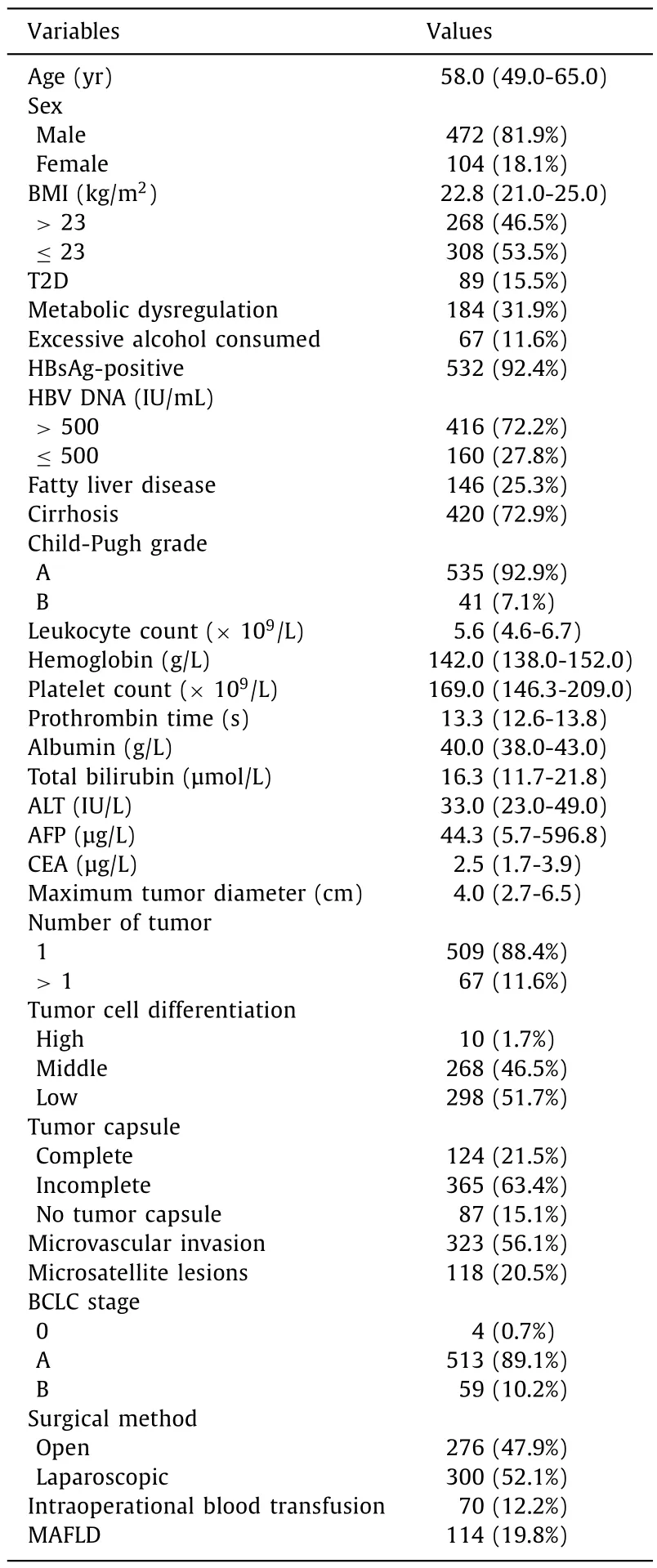
Table 1Characteristics of included HCC patients (n = 576).
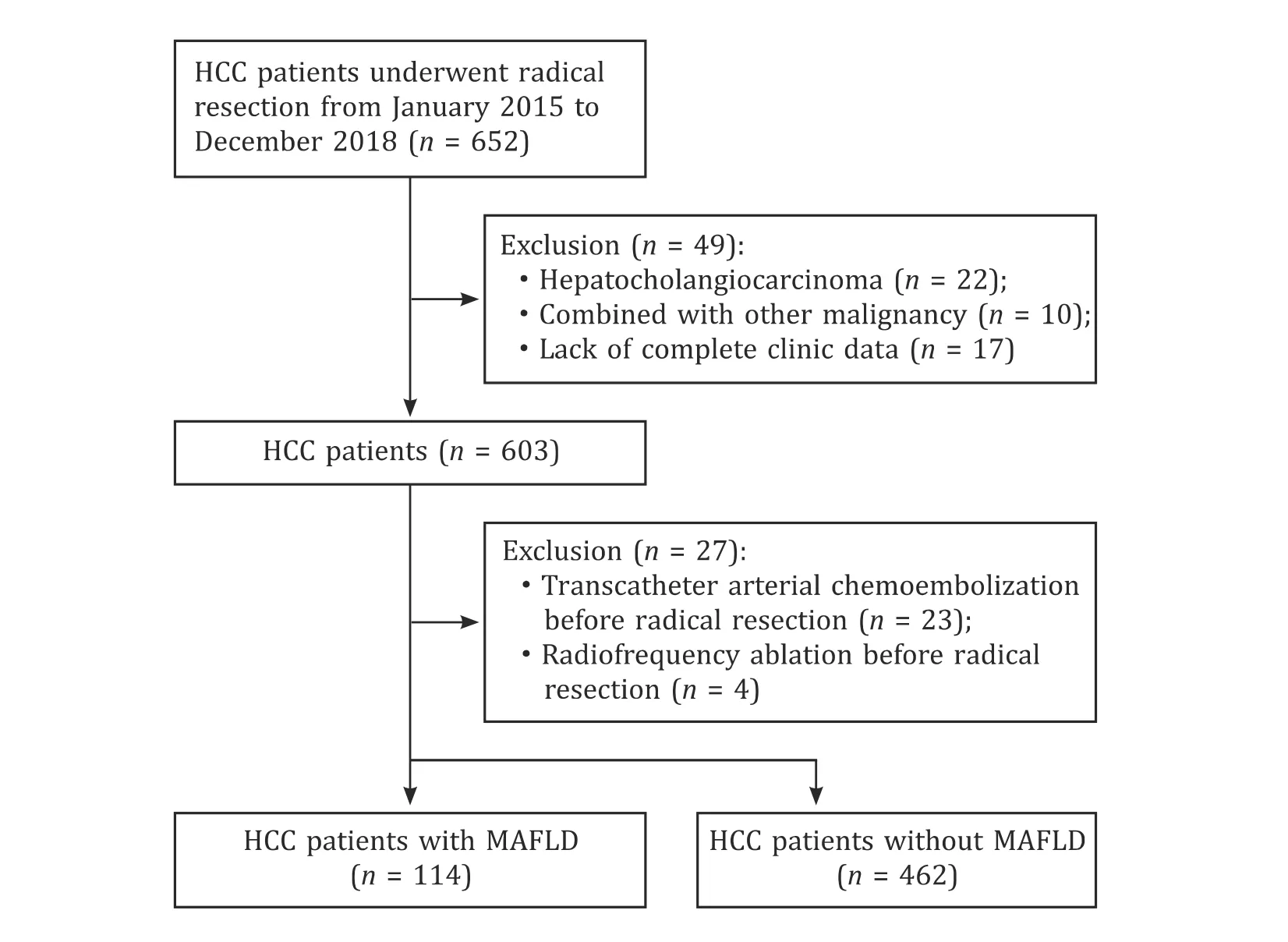
Fig.1.Flow chart for the selection of the study population.HCC: hepatocellular carcinoma; MAFLD: metabolic dysfunction-associated fatty liver disease.
The clinical features between the MAFLD and non-MAFLD groups were compared (Table 2).The proportions of T2D (24.6%)and metabolic dysregulation (47.4%) in the MAFLD group were higher than those of the non-MALFD group (13.2% and 28.1%, respectively,P <0.05).Median BMI was also higher in the MALFD group (24.6 vs.22.2 kg/m2,P <0.05).No statistical difference was found in regard to other baseline characteristics between the two groups (P >0.05).Same results were found in the comparison between MAFLD and non-MAFLD in HBV infection subgroup.
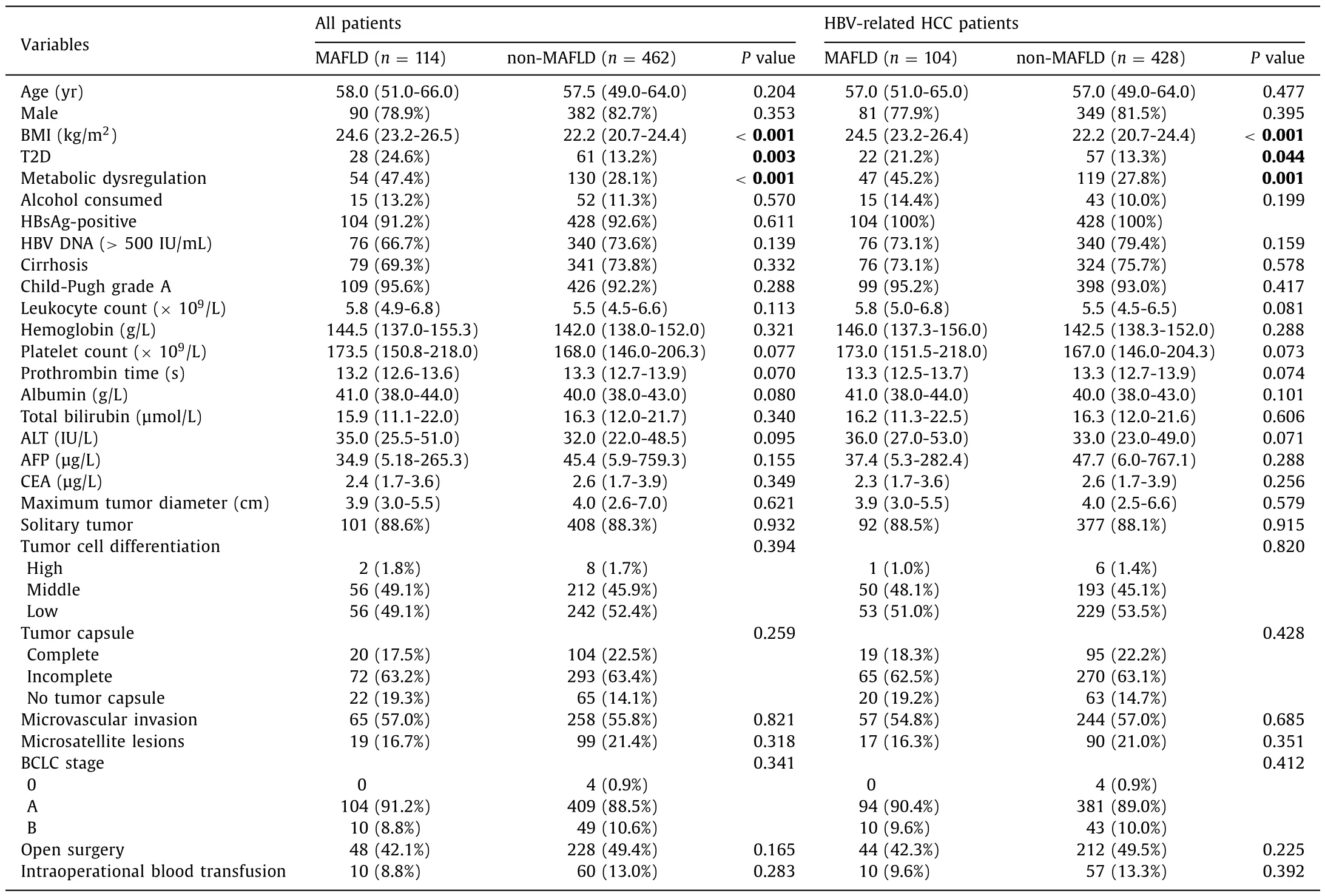
Table 2Clinicopathological characteristics of HCC patients.
RFS and OS in HCC patients
The median follow-up time was 32 months (IQR: 19-48).The median RFS in the MAFLD group was 34.0 months and 19.0 months in the non-MAFLD group.The 1-, 3-, and 5-year RFS rates were 64.9%, 49.1%, and 36.1% in the MAFLD group, which were higher than those of the non-MAFLD group (59.4%, 35.3%, and 26.5%, respectively,P= 0.01).The mean OS was 57.0 months in the MAFLD group and 52.2 months in the non-MAFLD group, with 1-, 3-, and 5-year OS rates being 88.6%, 74.4%, 72.3%, and 87.3%, 67.8%, 62.7%,respectively (P= 0.199, Fig.2).
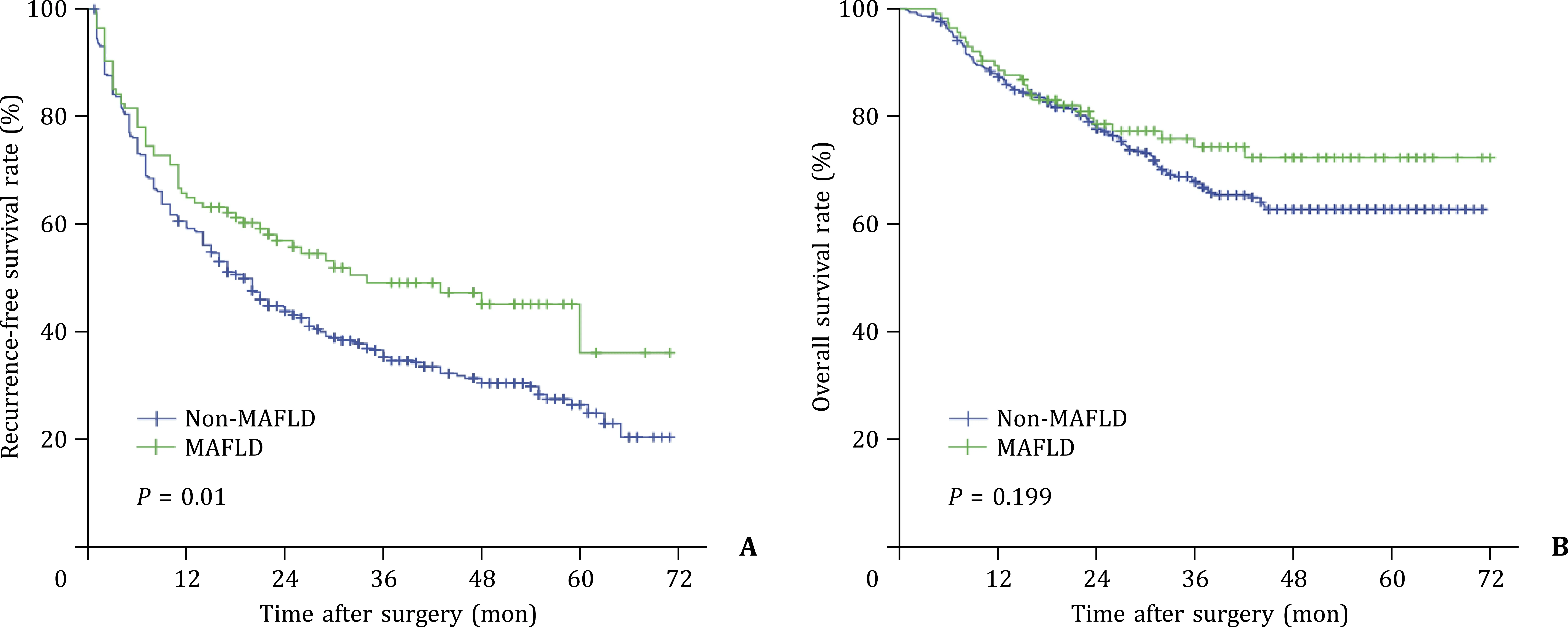
Fig.2.Kaplan-Meier analysis of recurrence-free survival (A) and overall survival (B) for HCC patients after radical resection.HCC: hepatocellular carcinoma; MAFLD:metabolic dysfunction-associated fatty liver disease.
Univariate and multivariate analyses of prognostic factors to RFS and OS in HCC patients
Univariate analysis demonstrated that MAFLD was significantly associated with RFS rate for HCC patients after radical surgical treatment (P= 0.012), while no significant connection was found between MAFLD and OS rate (P= 0.201).Multivariate analysis showed that MAFLD was not an independent protective factor for RFS rate in this cohort (P= 0.139).The independent risk factors for RFS and OS rate were tumor diameter ≥5 cm, number of tumor ≥2, poor tumor cell differentiation, microvascular invasion,microsatellite lesions and intraoperative blood transfusion (allP <0.05) (Table 3).
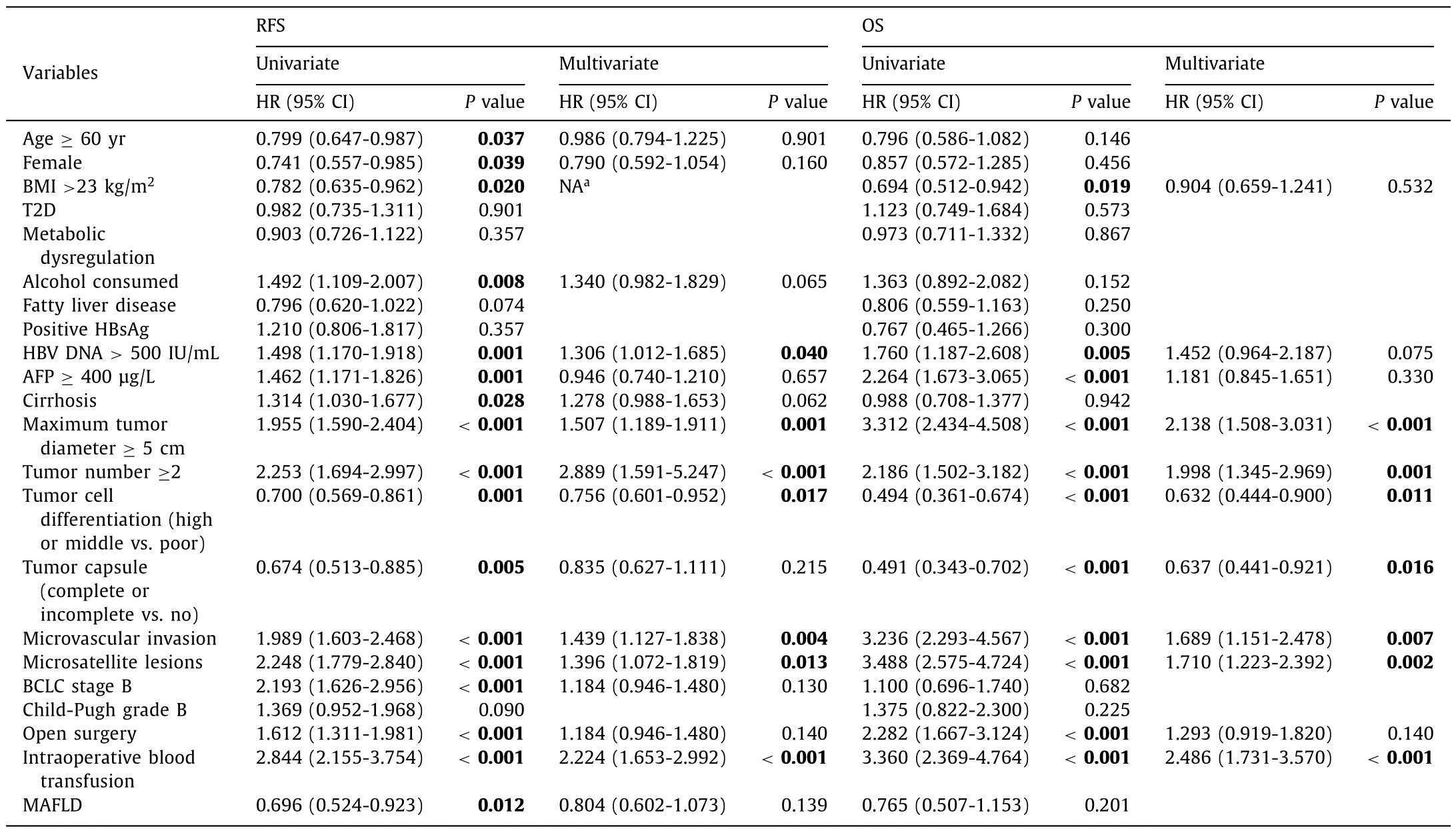
Table 3Univariate and multivariate analyses of prognostic factors for RFS and OS in HCC patients.
RFS and OS in HCC patients with HBV infection (HBV-related HCC patients)
As HBV infection was common in this cohort, we performed a subgroup analysis in patients with HBV infection.The median RFS of patients with HBV-related HCC in the MAFLD group was 30 months and 19 months in the non-MAFLD group.The 1-, 3-, and 5-year RFS rates in the MAFLD group were 63.5%, 46.3% and 33.7%,and 59.0%, 35.6%, and 26.6% in the non-MAFLD group in HBVrelated HCC.The comparison of the RFS between these two groups was statistically significant (P= 0.038).The mean OS were 56.8months and 52.7 months in the MAFLD group and non-MAFLD group in HBV-infected population, and the 1-, 3-, and 5-year OS rates were 89.4%, 73.8%, and 71.7% in the MAFLD group, and 88.0%,68.6%, and 63.8% in the non-MAFLD group.The difference was not significant (P= 0.291) (Fig.3).
Univariate and multivariate analyses of prognostic factors for RFS and OS in HBV-related HCC patients
Univariate analysis revealed that the presence of MAFLD were significantly associated with RFS rate for HBV-related HCC afterradical surgical treatment (P= 0.041), but no correlation regarding OS rate (P= 0.292).Multivariate analysis showed that MAFLD was not an independent protective factor for RFS rate after surgery(P= 0.316).The independent risk factors for RFS and OS rates were HBV DNA>500 IU/mL, tumor diameter ≥5 cm, microvascular invasion, microsatellite lesions and intraoperative blood transfusion(allP <0.05) (Table 4).
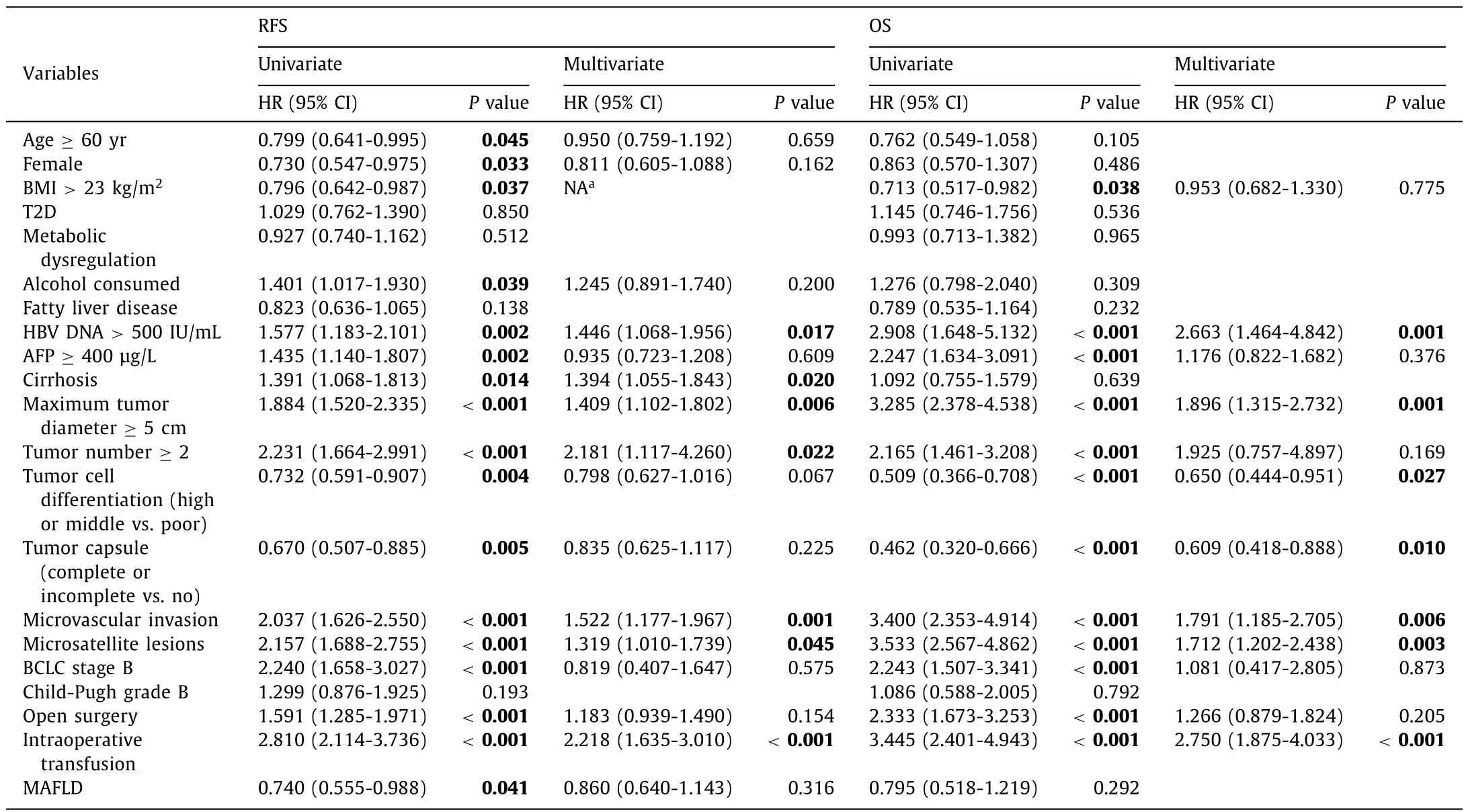
Table 4Univariate and multivariate analyses of prognostic factors to RFS and OS in HBV related HCC patients.
Discussion
This research for the first time explored the impact of MAFLD on the prognosis of HCC patients after radical resection.The results showed that MAFLD may improve the RFS rate, but not the OS rate.However, multivariate analysis found that MAFLD was not an independent protective factor for RFS.Similar result was found in HCC patients with concurrent HBV infection.
In view of the increasing prevalence of MAFLD and the better understanding of its pathogenesis, a new “positive” diagnostic criteria for MAFLD was proposed in 2020 [7,8].The definition of MAFLD emphasizes the contribution of metabolic dysfunction and no longer needs to exclude alcohol intake or other chronic liver disease.Consistently, studies showed that the criteria for diagnosis of MAFLD were superior to the NAFLD criteria for identifying patients with significant hepatic fibrosis [12,13], chronic kidney disease [14], and cardiovascular disease [15].The MAFLD burden is increasing rapidly in the world, especially in the Asia-Pacific region [16,17], and patients with MAFLD complicated with chronic hepatitis B or alcoholic liver disease are very common [18-21].
In this research, 19.8% of HCC patients met the diagnosis criteria of MAFLD.This proportion was similar in HBV-related HCC (19.5%),indicating that the MAFLD is common in HCC patients.The major differences of baseline features between the MAFLD group and the non-MAFLD group in this HCC cohort were the metabolic profiles but not the characteristic of HCC or liver function.The predictive factors for the outcome of HCC in this cohort were HBV DNA, tumor diameter, tumor number, microvascular invasion, microsatellite lesions and intraoperational blood transfusion, which were consistent with previous studies [22-27].
Unexpectedly, we found that the 1-, 3-, and 5-year RFS in the MAFLD group were all significantly higher than those in the non-MAFLD group, in both overall HCC population and the HBV-infected HCC subgroup.Metabolic dysfunction has long been proved to be the risk factor for malignancy.For example, patients with obesity or diabetes are at high risk of HCC [28,29].However, in this cohort, MAFLD seemed to be a favorable factor for RFS in HCC after surgery.Inconsistent with our results, some studies found that the RFS rates of HCC patients were not influenced by NAFLD [30], obesity [31], or diabetes [32,33].The result of a study involving 996 patients who underwent liver resection for HCC shows that, NAFLD increases the surgical morbidity and posthepatectomy liver failure however improves the long-term survival outcomes [6].A plausible explanation for this could be the better nutrient and energy store in MAFLD.MAFLD patients usually had higher BMI, which is an indicator for nutrient status.Lower BMI is associated with malnutrition and sarcopenia [34].HCC patients with low BMI were reported to have significantly lower RFS rate than those with high BMI after surgery [35].The presence of MAFLD as a surrogate of better nutrient may lead to better recovery after surgery.But compared with the classic parameters of HCC recurrence, the impact of MAFLD is weakened and no longer significant.Another possible cause is that MAFLD may affect tumor cell differentiation and microvascular invasion [36,37].Future experimental studies are needed to explore the underlying mechanisms of MAFLD on HCC recurrence.
Furthermore, we also found that there was no statistical difference in regard to OS rate between the MAFLD group and the non-MAFLD group, indicating that MAFLD did not influence OS rate.This was similar to previous conclusion that no impact of NAFLD [30], BMI [38]or diabetes mellitus [32,33]was found on the OS of HCC.
There were some limitations in this research.First of all, the data set was based on single medical center.Secondly, it was a retrospective cohort study.Last but not least, the research was mainly focused on patients with surgical treatment.Therefore, it was unknown if our research result was consistent with patients with other therapy.
In conclusion, MAFLD is common in HCC patients and is associated with RFS of HCC after radical resection.However, it is not an independent protective factor for RFS and not relevant to OS rate.
Acknowledgments
None.
CRediT authorship contribution statement
Ke-Gong Xiong:Data curation, Formal analysis, Investigation,Methodology, Project administration, Supervision, Validation, Writing - original draft, Writing - review & editing.Kun-Yu Ke:Data curation, Formal analysis, Project administration, Resources, Supervision.Li-Fang Chen:Data curation, Investigation.Jin-Feng Kong:Data curation, Funding acquisition, Resources.Tai-Shun Lin:Data curation.Qing-Biao Lin:Data curation.Su Lin:Conceptualization,Supervision, Validation, Writing - original draft, Writing - review & editing.Yue-Yong Zhu:Conceptualization, Formal analysis,Methodology, Project administration, Supervision, Validation, Writing - original draft, Writing - review & editing.
Funding
This study was supported by a grant from the Foundation of Science and Technology Bureau of Fuzhou (2020-WS-73).
Ethical approval
This research was approved by the Medical Ethics Committee of Mengchao Hepatobiliary Hospital of Fujian Medical University(2021-035-01).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2023年4期
Hepatobiliary & Pancreatic Diseases International2023年4期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Novel re-intervention device for occluded multiple uncovered self-expandable metal stent (with video)
- Hepatopancreatoduodenectomy for the treatment of extrahepatic cholangiocarcinoma ✩
- Microbiological cultures and antimicrobial prophylaxis in patients undergoing total pancreatectomy with islet cell autotransplantation
- Risk factors for posttransplant diabetes in patients with hepatocellular carcinoma
- The role of targeting protein for Xklp2 in tumorigenesis of hepatocellular carcinoma
- Isolated IgG4-associated autoimmune hepatitis or the first manifestation of IgG4-related disease?
