Exosomes from bone marrow mesenchymal stem cells are a potential treatment for ischemic stroke
Chang Liu, Tian-Hui Yang, Hong-Dan Li, Gong-Zhe Li, Jia Liang,*, Peng Wang
AbstractExosomes derived from human bone marrow mesenchymal stem cells (MSC-Exo) are characterized by easy expansion and storage, low risk of tumor formation,low immunogenicity, and anti-inflammatory effects.The therapeutic effects of MSC-Exo on ischemic stroke have been widely explored.However, the underlying mechanism remains unclear.In this study, we established a mouse model of ischemic brain injury induced by occlusion of the middle cerebral artery using the thread bolt method and injected MSC-Exo into the tail vein.We found that administration of MSC-Exo reduced the volume of cerebral infarction in the ischemic brain injury mouse model, increased the levels of interleukin-33 (IL-33) and suppression of tumorigenicity 2 receptor (ST2) in the penumbra of cerebral infarction, and improved neurological function.In vitroresults showed that astrocyte-conditioned medium of cells deprived of both oxygen and glucose, to simulate ischemia conditions, combined with MSC-Exo increased the survival rate of primary cortical neurons.However, after transfection by IL-33 siRNA or ST2 siRNA, the survival rate of primary cortical neurons was markedly decreased.These results indicated that MSC-Exo inhibited neuronal death induced by oxygen and glucose deprivation through the IL-33/ST2 signaling pathway in astrocytes.These findings suggest that MSC-Exo may reduce ischemia-induced brain injury through regulating the IL-33/ST2 signaling pathway.Therefore, MSC-Exo may be a potential therapeutic method for ischemic stroke.
Key Words:astrocytes; bone marrow mesenchymal stem cells; brain injury; exosome; IL-33; inflammation; ischemic stroke; neurological function; neuron; ST2 1Liaoning Provincial Key Laboratory of Neurodegenerative Diseases and Department of Neurobiology, Jinzhou Medical University, Jinzhou, Liaoning Province, China; 2Institute of Life Science, Jinzhou Medical University, Jinzhou, Liaoning Province, China; 3College of Pharmacy, Jinzhou Medical University, Jinzhou, Liaoning Province, China*Correspondence to:Jia Liang, PhD, liangjia@jzmu.edu.cn; Peng Wang, PhD, wangpeng@jzmu.edu.cn.https://orcid.org/0000-0001-7311-1260 (Jia Liang); https://orcid.org/0000-0001-9660-0147 (Peng Wang)#Both authors contributed equally to this work.
Introduction
Ischemic stroke, the most common type of stroke, occurs when a thrombus in the cerebral artery blocks cerebral blood flow.Ischemic stroke is one of leading causes of human disability and mortality worldwide.Ischemia/reperfusion-induced inflammatory responses are involved in the mechanisms leading to damage of the cerebral cortex after ischemic stroke.Several studies have shown that anti-inflammatory treatments have beneficial effects on neuronal protection after ischemic stroke (Guo et al., 2022; Yang and Chen,2022).However, most anti-inflammatory drugs have failed in clinical tests.Therefore, novel methods for treating ischemic stroke are urgently required.Cell therapies, such as bone marrow mesenchymal stem cells (MSCs), exert beneficial effects through the secretion of growth and trophic factors and were shown to enhance neurological outcomes after stroke by promoting brain repair.MSC-derived exosomes (MSC-Exo) exhibit several important advantages over cell therapy, such as the ability to be easily expandedin vitroand stored until use, low risk of tumor formation, and reduced immunogenicity.Furthermore, the therapeutic efficacy of MSC-Exo is at least equivalent to parent cell therapy (Doeppner et al., 2015).Studies showed that MSC-Exo exhibit immune activity and anti-inflammatory effects (Zhang et al., 2014; Lo Sicco et al., 2017; Zhou et al., 2022).MSC-Exo are absorbed by neurons, microglia, oligodendrocytes, and endothelial cells in the ischemic brain (Otero-Ortega et al., 2017).MSC-Exo are also ingested by neuronal cell bodies and axons, which causes primary cultured cortical neurons to exhibit enhanced axonal expansion (Zhang et al., 2017).As microvesicles, exosomes easily pass through various biological barriers and facilitate intercellular communication in various processes and diseases, including ischemic stroke.The intercellular communication between the various parts of the neurovascular unit is regulated by exosomes that are produced by nearly all cells.MSC-Exo have shown treatment potential in ischemic cerebral damage(Xiao et al., 2022; Yang and Chen, 2022).
Interleukin (IL)-33, a member of the IL-1 family located in the nucleus,was first identified as an inducer of the type 2 immune response (Cayrol and Girard, 2009).IL-33 interacts with and activates the suppression of tumorigenicity 2 receptor (ST2), negatively regulating the Toll-like receptor IL-1 pathway (Dinarello, 2005).Through its interaction with ST2, IL-33 participates in many inflammatory processes (Ali et al., 2007; Qian and Zhang,2020), and the IL-33/ST2 axis activates the JNK, ERK1/2, nuclear factor-κB and p38 mitogen-activated protein kinase pathways (Mirchandani et al., 2012).A previous report showed that the IL-33/ST2 axis promotes beneficial microglia responses and mitigates cerebral ischemic injury (Yang et al., 2017).Anin vitrostudy reported that IL-33/ST2 signaling potentiated the production of IL-10, which improved neuronal survival in response to oxygen/glucose deprivation (Yang et al., 2017).The IL-33/ST2 axis has been shown to improve the astrocyte response that affords neuronal protection in the ischemic brain(Jiao et al., 2020).Thus, after ischemic stroke, the IL-33/ST2 pathway may be crucial in controlling pathophysiology and inflammatory responses.In this study, we examined the potential therapeutic effects of MSC-Exo in a mouse model of ischemic injury and investigated the underlying molecular mechanism.We hypothesized that MSC-Exo may be an effective approach to increase the efficiency of anti-inflammatory responses for ischemic stroke.
Methods
Animals
Male specific-pathogen-free C57BL/6J mice (n= 143, 2–3 months old,20–25 g) and pregnant C57BL/6J mice (n= 5) were obtained from Vital River Laboratory Animal Technology Company (Beijing, China; license No.SCXK (Jing)2021-0006).The mice were maintained at 22–25°C in 50–60% humidity with 12-hour light and dark cycles; water and food were freely available.Animal protocols followed the National Institutes of Health’s guidelines for the care and use of laboratory animals, and the Jinzhou Medical University Animal Care and Use Committee approved the experimental protocols (approval No.2020101202) on October 12, 2020.All experiments were designed and reported in accordance with the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines (Percie du Sert et al., 2020).Estrogen replacement therapy has been reported to confer beneficial neuroprotective effects (Ma et al., 2021).Therefore, to avoid possible interference of estrogen on experimental results and unnecessary sample waste, only male mice were used in the study.Experimenters were blinded to the groups during surgery and other tests (histology, imaging, and behavior).
Isolation of human bone marrow MSCs
Human bone marrow MSCs (BM-MSCs) were isolated as previously described(Li and Li, 2018).We isolated BM-MSCs from the femoral head from male patients undergoing hip replacement (n= 5, 45–60 years old) using the BM-MSC Isolation Kit (TBD, Tianjin, China).All procedures were approved by the Ethics Committee of the First Affiliated Hospital of Jinzhou Medical University (approval No.KYLL202012) on May 24, 2020.The cells were grown in MesenPRO-RSTM Medium (Gibco, Grand Island, NY, USA, Cat# 12746-012) for 2–3 days.After 7 days, a confluent monolayer of cells was seen.BMMSCs were identified using the human MSC Analysis Kit (BD, Franklin Lake,NJ, USA, Cat# 562245).Animal-component-free medium (MesenCult-ACF Plus Medium, Stemcell, Cambridge, MA, USA, Cat# 05446) was used for BM-MSC culture.
MSC-Exo isolation and characterization
MSC-Exo were isolated and purified from BM-MSCs.BM-MSCs were cultured in MesenCult-ACF Plus Medium; after 24 and 48 hours, 50 mL culture medium was collected.The cells and detritus were separated from the culture media by centrifugation at 800 ×gfor 10 minutes and then at 12,000 ×gfor 20 minutes.Exosomes were isolated from the supernatant by centrifugation at 100,000 ×gfor 2 hours at 4°C in a Type CS150GX II ultracentrifuge (Hitachi,Koki, Co., Ltd, Hitachi, Japan).The pellet (containing MSC-Exo) was suspended once in phosphate-buffered saline (PBS) and then ultracentrifuged at 120,000 ×gfor 2 hours.The pelleted exosomes were then re-suspended in PBS (exosome fraction) and the concentration was determined using a BCA protein assay kit (Beyotime, Zhengzhou, China).
MSC-Exo were characterized by the expressions of exosome-specific markers:tumor susceptibility gene 101 (TSG101), Alix, and CD63 (Willms et al., 2016;Yang et al., 2019).Western blotting of the pellet fraction (exosomes) was performed using anti-CD63 (rabbit, 1:1500, System Biosciences, Palo Alto,CA, USA, Cat# EXOAB-CD63A-1, RRID: AB_2561274), anti-Alix (rabbit, 1:1000,CST, Denver, MA, USA, Cat# 92880, RRID: AB_2800192), anti-Calnexin (rabbit,1:1000, Abcam, Cambridge, UK, Cat# ab22595, RRID: AB_2069006), and anti-TSG101 (rabbit, 1:1000, Abcam, Cat# ab125011, RRID: AB_10974262).MSC-Exo were lysed and the protein concentration was determined with a bicinchoninic acid kit (Beyotime Biotechnology, Shanghai, China).The proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis,and then transferred to polyvinylidene difluoride membranes (Millipore,Temecula, CA, USA).The membranes were then incubated in blocking solution (Solarbio, Beijing, China, Cat# SW3015BSA) at room temperature for 1 hour, and then incubated with primary antibody at 4°C overnight.The membranes were incubated with horseradish peroxidase-labeled anti-rabbit secondary antibody (1:3000, Abcam, Cat# ab205718, RRID: AB_2819160) at room temperature for 1 hour.Finally, target bands were visualized using the NovexTM ECL Chemiluminescent Substrate Reagent Kit (Invitrogen, Carlsbad,CA, USA, Cat# WP20005).
MSC-Exo were also examined using an atomic force microscope (FMNanoview6800, FSM-PRECISION, Suzhou, China) and a transmission electron microscope (JEOL, Tokyo, Japan, JEM-1200EX).We used dynamic light scattering to measure the MSC-surface Exo’s charge and particle size (Zetasizer Nano ZS, Malvern Instruments, Malvern, UK).
PKH26 labeling of exosomes
We used PKH26-labeled MSC-Exo to analyze the distribution of MSC-Exo in the brain following ischemic stroke (Huang et al., 2021).MSC-Exo were incubated with PKH26 (1 mM, Sigma-Aldrich, St.Louis, MO, USA) at 37°C for 5 minutes and then held at 4°C for 30 minutes.The mixture was ultracentrifuged at 120,000 ×gfor 2.5 hours.The pellet (PKH26-MSC-Exo) was resuspended in PBS.The head was removed and photographed by anin vivoimaging system(IVIS Spectrum, PerkinElmer, Waltham, MA, USA).
Animal modeling and treatments
Mice were randomly assigned into five groups (n= 5–8/group): sham, middle cerebral artery occlusion (MCAO), MCAO + PBS, MCAO + MSC-Exo, and MCAO+ IL-33 groups.Suture occlusion was used to induce MCAO, as previously described (Hata et al., 1998; Guo et al., 2022).Isoflurane (4–5%, CSPS Co.,Ltd., Beijing, China) was used for anesthesia, followed by maintenance with 1–2% isoflurane inhalation with a mask.For MCAO, a nylon monofilament(180 μm in diameter; Guangzhou Jialing Biotechnology Co., Ltd., Guangzhou,China) was inserted into the common carotid artery by a minor incision and progressed about 9 mm distal from the carotid bifurcation.Reperfusion was made possible after 1 hour by removing the nylon monofilament.In the sham group, mice underwent the same procedure without the nylon monofilament being inserted.A heating pad was used to maintain body temperature at 37.0± 0.5°C.The effectiveness of the cerebral ischemia model was evaluated by watching the mouse circle to the non-ischemic side (left).
For MSC-Exo administration, MSC-Exo was diluted with PBS to 1 mg/mL in 100 μL and administered intravenously 30 minutes after the commencement of MCAO.In the MCAO + PBS group, 100 μL of PBS was infused intravenously.A 100 μg/kg dosage of recombinant IL-33 (Absin Biotechnology Co., Ltd,Shanghai, China) was administered intravenously 30 minutes after MCAO.The PKH26-MSC-Exo mixture was administered intravenously 30 minutes after the commencement of MCAO.
After 24-hour reperfusion, mice were subjected to behavioral tests or anesthetized by intraperitoneal injection of 1% pentobarbital sodium solution(6 mL/kg, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China).
Infarct volume assessment
After administering 25 mL of cold PBS transcardially, the brains were rapidly removed and coronally sectioned into five 1-mm sections.The brain slices were incubated in 0.5% 2,3,5-triphenyltetrazolium chloride (TTC, Cat#298964, Sigma-Aldrich) at 37°C for 10 minutes.ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to measure the stained section on digital images (Schneider et al., 2012).The infarct volume was determined using the following formula: (contralateral hemisphere volume –non-infarct ipsilateral hemisphere volume)/contralateral hemisphere volume× 100% (Jia et al., 2021).
Behavioral tests
Zea-Longa score
At 24 hours after reperfusion, the neurological dysfunction of animals was evaluated using the 5-point Zea-Longa grading criteria as follows (Longa et al., 1989; Xiong et al., 2021): score of 0: no obvious neurological deficit; 1, no extension of right forepaw; 2, circle to the left; 3, lying on the right side; 4,unable to walk autonomously; and 5, death.
Rotarod test
Three days before MCAO, mice were trained to stay on the rotating rod (KW-6C, KewBASIS, Nanjing, China) at speeds between 5 and 50 r/min (Zhang et al., 2022).At 24 hours after reperfusion following MCAO, the mice were again mounted on the revolving rod and the latency to fall was recorded.
Hot plate test
At 24 hours after reperfusion, pain threshold was assessed while mice were kept in a hot plate device (KW-600, KewBASIS) at 55 ± 0.5°C.The measurement was performed twice and the interval was 5 minutes.The response latency to lick a hindpaw was used as the observation index (Bacova et al., 2022).
Immunofluorescence staining
Brain samples were obtained 24 or 48 hours after reperfusion.Sections from peri-infarct penumbra (16-μm-thick) were treated for 1 hour at 25± 3°C with 0.1% Triton X-100.Following a 2-hour incubation in 3% bovine albumin at room temperature, slices were immunostained with anti-glial fibrillary acidic protein (GFAP, mouse, 1:500, Invitrogen, Cat# 14989282, RRID:AB_10598206) or anti-IL-33 (rabbit, 1:300, Abcam, Cat# ab187060, RRID:AB_2894704) overnight at 4°C.Secondary antibody linked to AlexaFluor488(rabbit anti-mouse, 1:10,000, Invitrogen, Cat# A-11059, RRID: AB_2534106)or AlexaFluor568 (goat anti-rabbit, 1:10,000, Invitrogen, Cat# A-11011, RRID:AB_143157) was applied for 1 hour at room temperature.Nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI).Samples were imaged using a fluorescence microscope (Leica, Wetzlar, Germany, Leica6000B).
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
The peri-infarct penumbra was dissected as previously described (Zhao et al., 2014).Total RNA was extracted from brain tissues or astrocytes using TRIzol® reagent (Invitrogen, Cat# 15596018).The ProtoScript® M-MuLV First Strand cDNA Synthesis Kit was used to generate cDNA (New England Biolabs Ltd, Beijing, China, Cat# E6300S).RT-PCR was performed using the Brilliant II SYBR® Green QPCR Master Mix (Agilent Tec., Santa Clara, CA, USA, Cat#600828) following the manufacturer’s instructions.The primer sequences are as follows: Il-33: forward: 5′-TCC TTG CTT GGC AGT ATC CA-3′, reverse: 5′-TGC TCA ATG TGT CAA CAG ACG-3′; St2: forward: 5’-CAT GGC ATG ATA AGG CAC AC-3′, reverse: 5′-GTA GAG CTT GCC ATC GTT CC-3′; β-actin: forward: 5′-ATA TCG CTG CGC TGG TCG TC-3′, reverse: 5′-AGG ATG GCG TGA GGG AGA GC-3′.Experiments were performed in triplicate and gene expression was analyzed using the 2–∆∆CTmethod (Livak and Schmittgen, 2001).
Primary cultures of cortical neurons and astrocytes
Primary cortical neurons were harvested as previously described (Zhang et al., 2020).Primary cortical neurons were isolated from 24-hour postnatal C57BL/6J mice (n= 10).The mice were sacrificed by decapitation after anesthesia by 1% isoflurane inhalation.Cortical parts of the brain were dissected and cut into tiny pieces.The collected cells were grown in neurobasal media (Thermo Fisher, Waltham, MA, USA, Cat# 10888022) with 2% B27 (Gibco) and kept at 37°C with 5% CO2and 95% air.Every 3 days, half of the culture medium was replaced with new medium.
Primary astrocyte cultures were generated from the same C57BL/6J postnatal mice (Wang et al., 2021).Meninges were removed and cortical pieces were dissociated in astrocyte culture medium.Dissociated cells were seeded into cell culture plates.Non-astrocyte cells, such as neurons and microglia, were shaken free from the flasks, and plates were washed with astrocyte culture medium (DMEM containing 10% fetal bovine serum) to remove the floating cells.Cells at first or second passages were used in the experiments.
Small interfering RNA transfection, in vitroischemia conditions, and astrocyte-conditioned media
Small interfering RNA (siRNA) targeting IL-33 or ST2 (GenePharma, Shanghai,China) was transfected into primary astrocytes using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen, Cat# 13778075) following the manufacturer’s instructions.
To simulate ischemia conditionsin vitro, primary astrocytes were subjected to oxygen and glucose deprivation (OGD) 48 hours after transfection.Glucosefree DMEM was added to primary astrocyte culture and cells were cultured at 37°C for 3 hours in an anaerobic chamber filled with 95% N2 and 5% CO2.The astrocytes were then reintroduced to normoxic conditions for 24 hours in regular culture medium.
During OGD/reoxygenation, some wells were treated with PBS, MSC-Exo (100 μg/mL), or recombinant IL-33 (50 ng/mL).Astrocyte-conditioned media (CM)was collected and filtered using a 0.22-μm filter.
Neuron treatment
After 6 days of normal culture condition, primary cortical neurons were grown in 96-well plates in glucose-free DMEM and subjected to OGD.Cells were exposed to anaerobic conditions for 3 hours; the neurons were then maintained in normoxic conditions for 24 hours in a neurobasal medium containing 2% B27.The cultured neurons were also treated with PBS, MSCExo (100 μg/mL), IL-33 (50 ng/mL), or CM for 24 hours.
Cell viability assessment
Neuron viability was assessed using CytoTox 96 nonradioactive cytotoxicity assay lactate dehydrogenase (Promega, Beijing, China, Cat# G1780) and thiazolyl blue tetrazolium bromide (MTT) (0.5 mg/mL; Sigma-Aldrich, Cat#298931) following the manufacturers’instructions.A microplate reader(Thermo Fisher) was used to measure the absorbance at 490 nm (lactate dehydrogenase) or 570 nm (MTT).Cell viability was evaluated following the manufacturer’s instruction.
Statistical analysis
No statistical methods were used to predetermine sample sizes; however,our sample sizes were similar to those reported in a previous publication(Wang et al., 2021).Data are expressed as the mean ± standard deviation(SD).Statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software Inc., La Jolla, CA, USA, www.graphpad.com).Data were evaluated using Student’st-test between two groups or a one-way analysis of variance followed by the Tukey’spost hoctest in more than two groups, depending on whether normality was indicated.If the data, such as neurological scores,were not normally distributed, statistical analysis was performed using the Kruskal-Wallis test.P< 0.05 indicated statistical significance.
Results
MSC-Exo accumulates in ischemic brain tissues in mice with cerebral ischemic injury
MSC-Exo were collected and purified from BM-MSCs.The morphology of MSC-Exo was verified using atomic force microscopy and transmission electron microscopy.As shown in Figure 1A and B, MSC-Exo were characterized by nanometric spheres with a smooth surface and a nanometric diameter.The average size of MSC-Exo was 70 ± 5 nm (Figure 1C).Western blot assay showed that Alix, CD63 and TSG101 (exosomal markers) were expressed in the MSC-Exo (pellet) fraction, but not in the cell culture supernatant, suggesting that MSC-Exo have been isolated and collected by centrifuging culture medium (Figure 1D).Calnexin, which is expressed in endoplasmic reticulum, was not expressed in the MSC-Exo fraction.
We next labeled MSC-Exo with fluorescence labeling (PKH26) to examine the ability of PKH26-labeled MSC-Exo to penetrate the blood-brain barrier in ischemic injury model animals.PKH26-MSC-Exo fluorescence was observed in both the ischemic hemisphere and contralateral hemisphere, and higher PKH26 fluorescence was seen in the ischemic cerebral hemisphere compared with the contralateral hemisphere (Figure 1E and F).This result indicated that MSC-Exo efficiently crossed the blood-brain barrier.
MSC-Exo exhibits a neuronal protective effect on mice with cerebral ischemic injury

Figure 1|Characterization of MSC-Exo.
The experimental design is depicted in Figure 2A.To investigate the effects of MSC-Exo in ischemia-induced brain damagein vivo, mice underwent MCAO for 1 hour followed by 24 hours of reperfusion.TTC staining of brain demonstrated that MSC-Exo reduced the infarct volume of ischemic mice compared with that of the MCAO + PBS group (Figure 2B).The percentage of infarct volume decreased from approximately 54% in the MCAO + PBS group to approximately 23% in the MCAO + MSC-Exo group (Figure 2C).MSC-Exo treatment improved the neurological outcome compared with the MCAO+ PBS group as assessed by Longa 5-points scores (Figure 2D), rotarod test(Figure 2E), and hot plate test (Figure 2F).These data demonstrate that MSCExo treatment provides neuroprotection in mice with ischemic stroke.
IL-33 is elevated in the brains of mice with cerebral ischemic injury
We next investigated Il-33 mRNA and protein expression in the peri-infarct penumbra of mice using RT-PCR and immunofluorescence labeling.At 12,24, and 48 hours after reperfusion, Il-33 expression was elevated in the peri-infarct penumbra relative to the sham group for 25 hours (Figure 3A).Immunofluorescence labeling also revealed substantial IL-33 expression in the peri-infarct penumbra at 24 and 48 hours after reperfusion (Figure 3B and C).These findings imply that IL-33 may play a crucial role in the response to cerebral ischemic damage.
Up-regulation of IL-33 shows protective effects on cerebral ischemic injury
We next investigated the effects of recombinant IL-33 on ischemia-induced neuronal injury.TTC staining results demonstrated that IL-33 administration in ischemic mice reduced the infarct volume compared with PBS administration(Figure 4A).The infarct volume was decreased from 53 ± 18% in the MCAO +PBS group to 26 ± 12% in the MCAO + IL-33 group (Figure 4B).Neurological function was evaluated using the Longa 5-points scores (Figure 4C), rotarod test (Figure 4D), and hot plate test (Figure 4E).The results showed that IL-33 administration improved neurological function in all tests compared with PBS administration.
MSC-Exo enhances the IL-33 signaling pathway after cerebral ischemic injury
We next investigated the effects of MSC-Exo administration on the IL-33 signaling pathway after ischemic injury.At 24 hours following MCAO, Il-33 and St2 mRNA expression levels were elevated in the peri-infarct penumbra of mice; both Il-33 and St2 expression levels were enhanced by MSC-Exo treatment (Figure 5A and B).We then co-stained brain slices for IL-33 and the astrocyte marker GFAP (Xu et al., 2020).As shown in Figure 5C and D,the immunofluorescence intensity of IL-33 was higher in the MCAO group compared with the sham group, and MSC-Exo treatment further increased IL-33 level compared with levels in the MCAO group.Immunofluorescence staining showed that IL-33 was mainly co-localized with astrocytes (indicated by GFAP staining) (Figure 5D).These data suggest that MSC-Exo modulates the IL-33/ST2 pathway in astrocytes after cerebral ischemic injury.
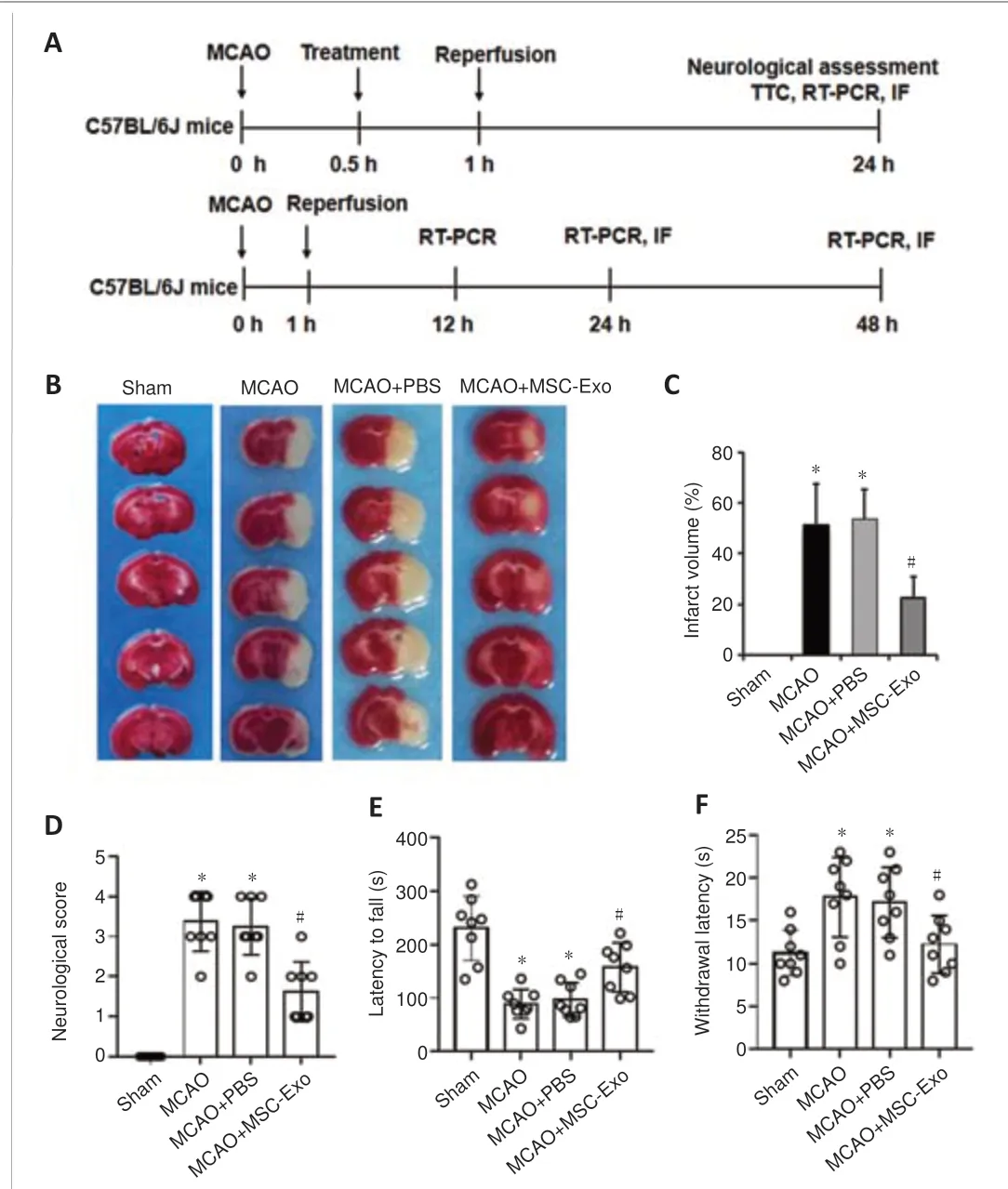
Figure 2|Effects of MSC-Exo on the infarct volume and neurological function of mice with ischemia-induced cerebral injury.
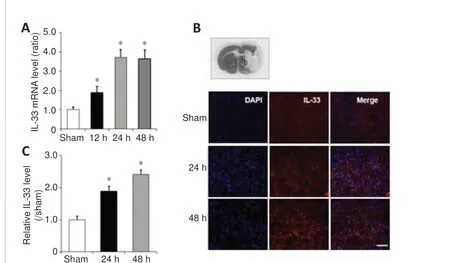
Figure 3| IL-33 expression level in the peri-infarct penumbra of mice with ischemic stroke.
The IL-33/ST2 pathway in astrocytes is essential for the neuroprotective effects induced by MSC-Exo in vitro
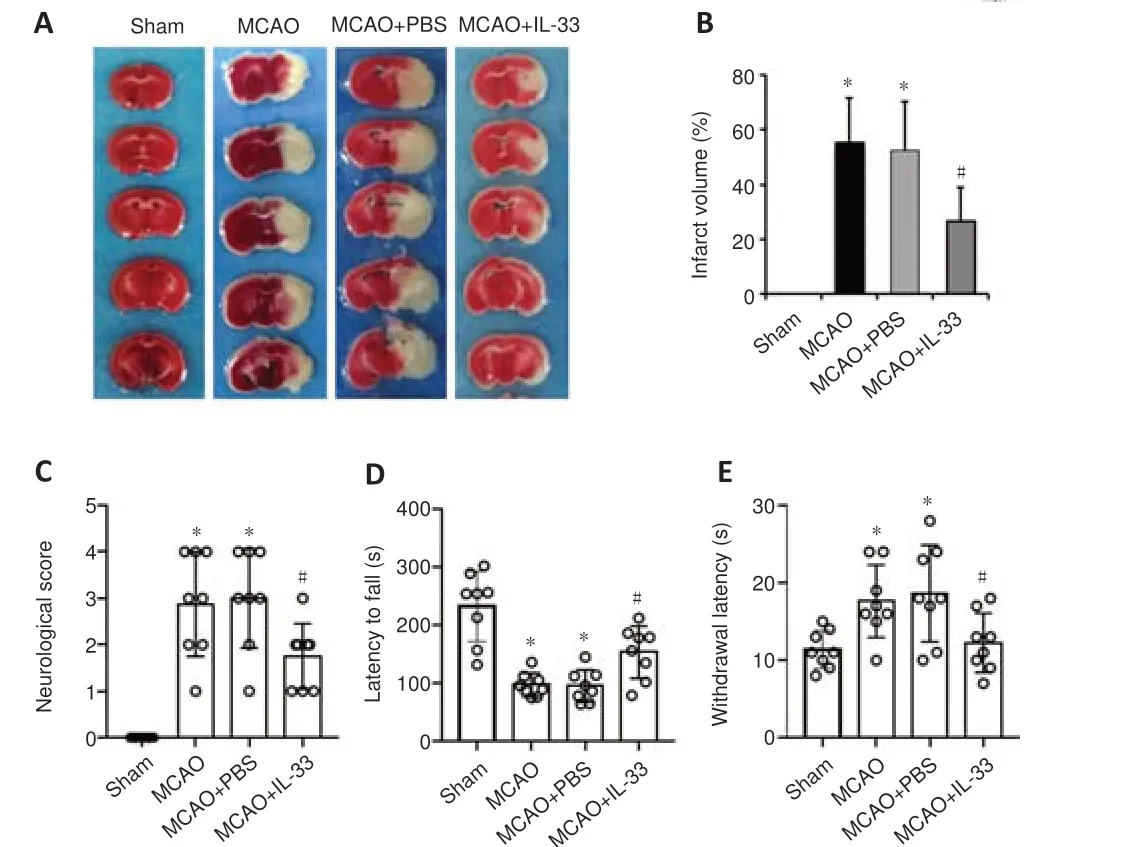
Figure 4|Recombinant IL-33 alleviates the infarct volume and improves neurological functional outcomes in mice with ischemic stroke.
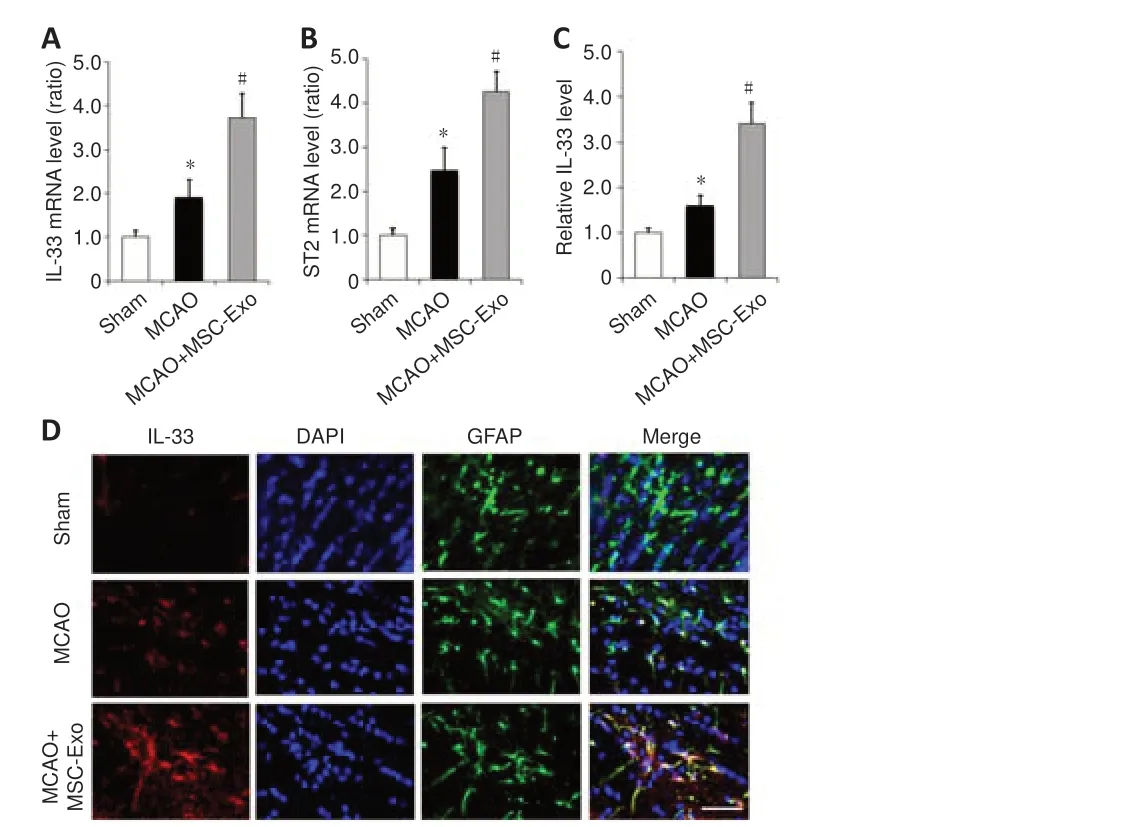
Figure 5|MSC-Exo regulates the IL-33/ST2 pathway in the peri-infarct penumbra of mice.
The above data showed MSC-Exo treatment may alleviate cerebral ischemic injury in mice.We next investigated whether the neuroprotective effects of MSC-Exo treatment were associated with IL-33/ST2 signaling in astrocytes.MTT assay showed that the viability of primary cortical neurons was considerably diminished following 3-hour OGD/24-hour reoxygenation; treatment with IL-33 or MSC-Exo had no protective effects (Figure 6A).We next examined whether astrocyte IL-33/ST2 activation protects neurons from ischemia-induced cell deathin vitrousing siRNA against IL-33 and ST2 (Figure 6B and C).CM derived from OGD astrocytes was introduced to cultures of primary cortical neurons that had been subjected to OGD.Neuronal survival was higher in the OGD + IL-33-CM group compared with the OGD + PBS group, and ST2 siRNA abolished the protective effect of IL-33-CM on neuronal survival, as measured by MTT and lactate dehydrogenase (LDH) assays (Figure 6D and E).These data suggest that IL-33/ST2 pathway in astrocytes is vital for neuronal survival after 3-hour OGD/24-hour reoxygenation.To further explore whether MSC-Exo-CM exert protective effects on neuronal survival through IL-33/ST2, siRNA against IL-33 or ST2 was transfected into astrocytes before OGD exposure.Inhibition of IL-33 or ST2 abolished the protective effect of MSC-Exo-CM on neuronal survival(Figure 6F and G).These results indicate that MSC-Exo mitigates the neuronal death caused by OGD via IL-33/ST2 in astrocytes.
Figure 6| IL-33/ST2 is essential for MSC-Exo’s neuroprotective effectsin vitro.
Discussion
In this study, we found that MSC-Exo therapy after cerebral ischemic injury induced by 1-hour MCAO followed by 24-hour reperfusion remarkably improved outcomes in mice.The primary and most novel discovery of this work is that MSC-Exo activate the IL-33/ST2 pathway in astrocytes, thereby protecting mice from neuronal death after ischemic injury.
Compared with other cell types, cultured MSCs secrete an abundance of exosomes (Yeo et al., 2013).Previous studies have shown that MSCExo provide therapeutic benefits after stroke by modulating the cerebral microenvironment (Xin et al., 2013; Doeppner et al., 2015).We used PHK26-labeled MSC-Exo andin vivoimaging showed that PHK26 fluorescence was detected in both the non-ischemic hemisphere and ischemic hemisphere, and the fluorescence intensity was stronger in the ischemic hemisphere compared with the non-ischemic hemisphere.We previously found that that exosomes derived from cells have distributions in the brain in various animal models(Wang et al., 2019; Zheng et al., 2019).These data indicate that PKH26-labeled MSC-Exo could efficiently target the ischemic region in mice after ischemic stroke.
The IL-33 cytokine is a member of the IL-1 family (Kurowska-Stolarska et al.,2011).IL-33 is rapidly released from injured central nervous system cells and plays a role in the induction of immune responses in lesion sites (Foster et al., 2015).Activating the IL-33/ST2 pathway after cerebral ischemia has protective effects against ischemic injury (Yang et al., 2017; Jiao et al., 2020).In this study, we found that IL-33 was increased in ischemic hemisphere after reperfusion.This result suggested that IL-33 may play a role in the response after cerebral ischemic stroke.Our data confirmed MSC-Exo administration significantly increased the IL-33/ST2 signaling pathway at 24 hours after ischemia in mice.Our findings suggest that MSC-Exo may represent an important therapeutic approach to reduce neuronal damage through the IL-33/ST2 pathway in the early stage of ischemic injury.
Following loss of oxygen and nutrient delivery, rapid cell loss occurs within the ischemic core, which triggers an immune response (Iadecola and Anrather,2011).We investigated the potential molecular mechanisms underlying the protection of neurons caused by MSC-Exo in ischemic stroke model mice.In models of spinal cord injury, intraperitoneal injection of IL-33 reducedtissue loss and ameliorated white matter demyelination (Pomeshchik et al.,2015).Secreted IL-33 interacts with the ST2 receptor, and IL-33/ST2 signaling promotes the synthesis of cytokines (Griesenauer and Paczesny, 2017).Several studies showed that central nervous system cells quickly release IL-33 after injury, which activates the immune system in the vicinity of the lesion(Gadani et al., 2015; Pomeshchik et al., 2015).Our research revealed that the peri-infarct penumbra had high IL-33 levels.Additionally, after ischemia injury, increased IL-33 expression was primarily restricted to astrocytes.In vitrostudies showed that astrocytes triggered by IL-33 released a number of neurotrophic factors that are essential for improving neuronal survival under OGD (Jiao et al., 2020).These results led us to investigate the role of IL-33/ST2 signaling in the MSC-Exo-induced response.In the current study, we focused on the IL-33/ST2 pathway in astrocytes, which regulates inflammatory responses.ST2 or IL-33 inhibition in astrocytes abolished the neuroprotective effect of MSC-Exo, suggesting that MSC-Exo may protect against OGD-induced neuronal death by IL-33/ST2 signaling in astrocytes.
Our results provide some promising data on the neuroprotective effect and mechanism of MSC-Exo treatment in cerebral ischemic stroke.However, this study still has some limitations.First, we only investigated the effects of MSCExo treatment on neuronal protection in an animal ischemic stroke model.The exact components in MSC-Exo that rescues the injured brain tissue require further investigation.Second, in this study, we focused on the IL-33/ST2 pathway.However, we cannot exclude the possibility that MSC-Exo trigger other pathways to prevent cerebral injury, and this will be investigated in a subsequent study.Third, this study explored administration within 30 minutes after injury induction.For clinical application, it may be worth addressing the value of treatment at later time points in terms of real-world applications.
In conclusion, our results indicated that MSC-Exo treatment reduced infarct volume and improved neurological function in the acute stage of ischemic stroke.By stimulating the IL-33/ST2 pathway in astrocytes, MSC-Exo may aid in the development of neuroprotection against ischemia injury.These data indicate that MSC-Exo treatment could be a viable neuroprotective treatment for ischemic stroke.
Author contributions:Investigation, validation, data analysis, and manuscript draft: CL and THY; methodology, validation and data curation: HDL and GZL;conceptualization, writing original draft.Supervision and reviewing: JL and PW.All authors approved the final version of this paper.
Conflicts of interest:The authors declare no conflict of interest.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Creed Stary, Stanford University, USA.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain

