MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
Bridget Martinez, Philip V.Peplow
AbstractEpilepsy is a common and serious neurological disease that causes recurrent seizures.The brain damage caused by seizures can lead to depression, anxiety, cognitive impairment, or disability.In almost all cases chronic seizures are difficult to cure.MicroRNAs are widely expressed in the central nervous system and play important roles in the pathogenesis of several neurological disorders,including epilepsy.A variety of animals (mostly mice and rats) have been used to induce experimental epilepsy using different protocols and miRNA profiling performed.Most of the recent studies reviewed had performed miRNA profiling in hippocampal tissues and a large number of microRNAs were dysregulated when compared to controls.Most notably, miR-132-3p, -146a-5p, -10a-5p, -21a-3p,-27a-3p, -142a-5p, -212-3p, -431-5p, and -155 were upregulated in both the mouse and rat studies.Overexpression of miR-137 and miR-219 decreased seizure severity in a mouse epileptic model, and suppression of miR-451, -10a-5p, -21a-5p, -27a-5p, -142a-5p, -431-5p, -155, and -134 had a positive influence on seizure behavior.In the rat studies, overexpression of miR-139-5p decreased neuronal damage in drug-resistant rats and inhibition of miR-129-2-3p, -27a-3p, -155, -134, -181a, and -146a had a positive effect on seizure behavior and/or reduced the loss of neuronal cells.Further studies are warranted using adult female and immature male and female animals.It would also be helpful to test the ability of specific agomirs and antagomirs to control seizure activity in a subhuman primate model of epilepsy such as adult marmosets injected intraperitoneally with pilocarpine or cynomolgus monkeys given intrahippocampal injections of kainic acid.
Key Words:epilepsy; experimental models; microRNA; mouse; rat; seizures; therapeutic targets
Introduction
Epilepsy is a common and serious neurological disease that causes recurrent seizures and affects at least 65 million people worldwide, with developing countries accounting for many of the cases (Thurman et al., 2011; Naderali et al., 2018).The recurrent seizures cause multiple physiological and pathological changes, such as loss of neurons, germination of mossy fibers,and dendritic damage (Tomari et al., 2017).Brain damage caused by seizures can lead to depression, anxiety, cognitive impairment, or disability (Maes et al., 2009).Chronic seizures are difficult to cure in almost all cases, especially in patients with temporal lobe epilepsy (TLE) (Eadie, 2012).
TLE, characterized by seizures arising from or involving the hippocampus, is the most common focal epilepsy syndrome in adults (Schuele and Lüders,2008).It is frequently refractory to pharmacotherapy.Diagnosis of TLE relies on clinical examination, patient history, electroencephalography (EEG)recordings, and brain imaging which require a range of technical expertise and are resource-intensive (Sidhu et al., 2018; Amin and Benbadis, 2019; Galovic et al., 2019).Misdiagnosis or delay in diagnosis often occurs (Amin and Benbadis, 2019; Mathias and Bensalem-Owen, 2019).This includes patients who exhibit non-epileptic attack disorder (psychogenic non-epileptic seizures)which can result in patients being treated with ineffective antiepileptic drugs instead of receiving appropriate psychiatric care (Dickson et al., 2017;Goldstein et al., 2019).It is also not yet possible to identify patients at risk of developing TLE following potential epilepsy-inciting events such as traumatic brain injury, stroke, or status epilepticus (SE) (Garner et al., 2019; Engel and Pitkänen, 2020; Klein and Tyrlikova, 2020; Lӧscher, 2020).There is a major need for biomarkers of epilepsy, which might aid clinical diagnosis, prognosis,and inform treatment decisions.This is particularly relevant in resourcelimited countries (Engel et al., 2013; Caraballo and Fejerman, 2015).
Epilepsy can occur at any age, and prolonged treatment may impose various psychological and physical burdens on patients and an enormous economic burden on families and societies (Ivanova et al., 2010; Allers et al., 2015;Jost et al., 2017).The exact mechanism regulating epilepsy remains unclear.No new antiepileptic drugs or treatments have been developed that can target the primary pathogenesis of epilepsy, and approximately one-third of patients with epilepsy develop refractory epilepsy (Beleza, 2009; Kwan et al., 2010).Neonates and infants have the highest risk of developing seizures as it is during this period of rapid growth and synaptogenesis that seizure susceptibility is greatest due to the natural excitability of the brain at this age.Intractable epilepsy is a common condition among children.It is thought that abnormalities in synaptic transmission and plasticity alter neuronal excitability,resulting in an abnormal excitatory/inhibitory balance in the central nervous system and ultimately leading to the development of epilepsy (Khazipov,2016; Fukata and Fukata, 2017).
MicroRNAs (miRNAs) are a class of endogenous small (18–24) nucleotides.They are non-coding RNAs, which regulate post-transcriptional gene expression by directing mRNA destabilization, translational repression, or a combination of both (Hauser, 1995; Bartel, 2009).MiRNAs are widely expressed in the central nervous system and not only participate in regulating the differentiation, development, and maturation of the central nervous system but are also intimately involved in regulating synaptic plasticity (Aksoy-Aksel et al., 2014; Li et al., 2018).They play key roles in the pathogenesis of a variety of neurological disorders, including epilepsy (Jimenez-Mateos et al.,2012; Tan et al., 2013; Li et al., 2014; Rajman et al., 2017).Analysis of brain samples from patients with TLE suggests that large-scale dysregulation of gene expression accompanies the development of recurrent seizures.The dysregulation influences entire networks of genes that regulate pathways including gliosis, inflammation, neuronal function, and synaptic structure(Budanov et al., 2004; Johnson et al., 2015).Modulation of specific miRNA alterations may regulate neuronal responsiveness, regulate neuronal excitability, and alleviate motor disorders associated with seizures by controlling the expression of several ion channels, transporters, and genes linked to epilepsy, neurotransmitter-driven neuronal excitability, and motor activity (Tan et al., 2013; Zheng et al., 2014).
A variety of animals (mostly mice and rats) have been used to induce experimental epilepsy using different protocols and microRNA profiling performed.In vivouse of oligonucleotide microRNA inhibitors (antagomirs)has demonstrated functional roles for several microRNAs in seizure control and epileptogenesis (van Rooij and Kauppinen, 2014; Henshall, 2017).
Animal Models of Epilepsy
Many animal models have become available for use in searching for the molecular mechanisms of epileptogenesis (Grone and Baraban, 2015).Kainic acid-induced model: Hippocampal pyramidal cells are highly sensitive to damage induced by kainic acid (KA) (Nadler et al., 1978), which is a cyclic analog of L-glutamate and an agonist of ionotropic glutamate receptors.The use of this drug was proposed as a model of TLE on discovering that intraamygdala injections of KA in rodents induce behavioral seizures and produce neuropathological lesions in the hippocampus similar to those occurring in TLE patients (Ben-Ari et al., 1979).The initial SE produced by KA was followed days later by the occurrence of spontaneous seizures (Calvalheiro et al., 1982;Riban et al., 2002).An intrahippocampal kainate mouse model of mesial TLE has been described (Klein et al., 2015; Duveau et al., 2016).More widespread and bilateral neuronal damage, particularly in limbic regions, occurs when KA is injected systemically (Lévesque et al., 2016).
Pilocarpine-induced model: This is the most frequently used model of post-SE TLE.Systemic intraperitoneal (i.p.) administration of pilocarpine (PILO),a cholinergic muscarinic agonist, in rodents was followed by a sequence of automatisms and motor limbic seizures evolving into SE (Turski et al., 1983).The brains of these animals showed widespread damage in the olfactory cortex, amygdala, thalamus, neocortex, hippocampus, and substantia nigra(Turski et al., 1989).Pilocarpine-treated animals showed spontaneous seizures approximately 2 weeks after the initial SE, establishing this as a model of TLE (Calvalheiro et al., 1991), reproducing both the typical histopathological alterations and spontaneous chronic seizures observed in patients with TLE.Electrical stimulation models: In addition to chemical induction of SE,sustained electrical stimulation of the hippocampus or amygdala results in the development of sustained repetitive seizures following a seizure-free latent period (Mazarati et al., 2006).Individual epileptic rats differed markedly in their response to phenobarbital, an antiepileptic drug (Brandt et al., 2004).Most of the phenobarbital non-responders also did not respond to phenytoin,a second antiepileptic drug (Bethmann et al., 2007), so this model fulfills the minimum criteria of a model of drug-resistant epilepsy (Stables et al., 2003).Perforant pathway stimulation model: Perforant pathway stimulation (PPS)in rodents causes hippocampal granule cell discharges and seizures, which when allowed to persist for several hours lead to neuronal cell loss (Sloviter and Damiano, 1981) and permanent epilepsy (Norwood et al., 2010).PPS can replicate fundamental characteristics of human TLE, such as hippocampal sclerosis and temporal-onset seizures (Norwood et al., 2010; Norwood et al., 2011).PPS is more reliable (e.g., absolute control over insult duration, no non-responders, no mortality), albeit significantly more resource intensive,than the more commonly used chemoconvulsant-based animal models.The latter approach, although unquestionably useful for studying seizure-induced brain injury, has significant inter-animal variability, low response rates,uncontrollable neurodegeneration, as well as considerable morbidity and mortality (Cavalheiro et al., 2006; Dudek et al., 2006).
Kindling model: Kindling is a process in which subconvulsant seizure stimuli cause a sustained reduction in seizure threshold and the development of generalized seizures (Karler et al., 1989).A chemical kindling method uses pentylenetetrazole (PTZ) and administration of PTZ 20 mg/kg every 48 hours in rats caused myoclonic jerks, and ultimately generalized tonic-clonic seizures(Mason and Cooper, 1972).Administration of PTZ 50 mg/kg every 24 and 48 hours in mice achieved kindling in 80% of animals after 15 injections (Karler et al., 1989).Rechallenging the PTZ kindled mice with a subconvulsant PTZ dose after 2 weeks of a stimulus-free period induced the same intensity of generalized seizures observed during the kindling maximum stage (Karler et al., 1989).
MicroRNAs in Mouse and Rat Models of Epilepsy
A PubMed search was performed for original research articles published from January 2012 to May 2022 on possible microRNA biomarkers in brain and blood samples and as therapeutic targets in mouse and rat models of epilepsy.The steps involved in the review and its contents are shown (Figure 1).A total of 19 articles were found for this review, of which 5 had been performed in mice, 11 in rats, and 3 in both mice and rats.In the mouse studies where gender and age were reported, the animals were males 6–7/8 weeks of age and indicated as being adults (Jackson et al., 2017).In the rat studies, where gender and age were indicated, the animals were mostly males with ages ranging from 4–13 weeks, with the majority being 6/7–8/9 weeks of age.The younger rats were 4–6 weeks of age and could be considered to be juvenile and approaching adulthood, while the older rats were 9–13 weeks of age.
Mouse Model Studies
Weng et al.(2020) treated C57BL/6 mice, 6–8 weeks of age, with KA (20 mg/kg,intracerebroventricularly (i.c.v.) to the right ventricle).KA-treated and vehicletreated mice (control) were euthanized on days 5, 10, 20, and 30 after treatments, and the behavior was evaluated on days 5, 10, 15, 20, 25, and 30 after treatments.KA-treated mice exhibited generalized seizures reaching Racine stage 4 (Racine, 1972) till day 30, while there were no obvious behavior changes in the control group.Using RT-PCR, KA induced increased expression of miR-451 in the hippocampal neurons.The increase in miR-451 declined after day 20.MiR-451 antagomir, given i.c.v.to the right ventricle at 6 hours after KA treatment, induced a significantly decreased mean seizure score compared with miRNA negative control from days 5 to 30.MiR-451 knockout attenuated KA-induced seizures.The number of disorganized cells in the hippocampus was increased markedly when treated with KA, while miR-451 knockout showed a significant decrease in the extent of disorganized cells.A loss of neurons (Nissl body, Nissl staining) was observed when being treated with KA, while miR-451 knockout reduced the loss of the neurons and improved the pathological changes in the hippocampus.
In a study by Venø et al.(2020), male C57BL/6 mice, 6–8 weeks of age, were treated with KA (intra-amygdala injection) and euthanized at 1 hour, 24 hours,48 hours, 72 hours, day of the first spontaneous seizure (typically 3–5 days after SE) or at 4 weeks (chronic epilepsy).Epilepsy was also induced in adult male NMRI mice using the PILO method and euthanized at the same time points.Ago2 immunoprecipitation followed by small RNA sequencing was performed on hippocampal samples.Disease stage-specific differences for individual miRNAs were detected, including shortly after the epileptogenic insult, on the day of the first spontaneous seizure, and chronic epilepsy.Within the chronic epilepsy phase, the period most relevant to how a miRNA-based therapeutic might be used clinically (i.e., treating patients with pre-existing, refractory epilepsy (Henshall et al., 2016)), 8 up- and 1 downregulated miRNAs were common to both models.This included miR-132-3p and mR-146a-5p for which there are already significant functional data linking them to epilepsy (Jimenez-Mateos et al., 2011; Huang et al.,2014; Iori et al., 2017; Tao et al., 2017), and 6 miRNAs (miR-10a-5p, -21a-3p, -27a-3p, -142a-5p, -212-3p, and -431-5p) for which there are limited or no functional data linking to epilepsy.Custom locked nucleic acid-modified oligonucleotide miRNA inhibitors (antagomirs) were used to assess seizure responses afterin vivoknockdown of miRNAs.MiR-132-3p and miR-146a-5p were excluded to prioritize miRNAs not previously linked to epilepsy and excluded miR-21a-3p because it is not fully conserved in humans.Instead, the fully conserved miR-21a-5p was selected, which also satisfies basal expression criteria and upregulation (at least 15%) in both models.Mice received an i.c.v.injection of one of 6 targeting antagomirs, a scrambled antagomir (Scr), or PBS vehicle 24 hours before induction of SE by an intra-amygdala microinjection of KA.Seizure severity was significantly reduced during SE in mice preinjected with antagomirs against miR-10a-5p, -21a-5p, and -142a-5p.Seizure burden,determined by measuring only ictal epileptiform activity, was significantly reduced by the same antagomirs and also significant for anti-miR-431-5p.Analysis of brains from the mice killed 24 hours after SE revealed significant neuroprotection for 5 of the 6 antagomirs (those targeting miR-10a-5p, -21a-5p, -27a-5p, -142a-5p, and -431-5p) relative to controls.A single antagomir mixture (“combi-antimiR”) was obtained by combining the three most effective antagomirs (targeting miR-10a-5p, -21a-5p, and -142a-5p).When preinjected with combi-antimiR, mice displayed reduced seizure severity during SE in the intra-amygdala KA model.Additional mice were subjected to KA intra-amygdala treatment and then monitored over the next 2 weeks for spontaneous recurrent seizures (SRSs).After establishing equivalent baseline rates of SRSs, mice were randomly assigned to scramble or combi-antimiR post-treatments.Combi-antimiR reduced both the occurrence and severity of seizures when administered as a post-treatment in epileptic mice, 2 weeks after SE.
Brennan et al.(2020) used male C57BL/6JO-1aHsd mice, 6–8 weeks of age,treated with KA (0.3 μg in 0.2 μL, intra-amygdala injection) to induce SE,followed after 40 minutes by i.p.lorazepam (8 mg/kg) to reduce morbidity and mortality.Blood samples were obtained at baseline (acute phase), 48 hours (mid-epileptogenesis), and 4 weeks (chronic epilepsy) post-KA or PBS injection.Also, adult male NMRI mice were treated with PILO (300 mg/kg, i.p.).The development of SE was recorded and blood samples were collected at baseline (acute phase), 48 hours (mid-epileptogenesis), and 4 weeks (chronic epilepsy) and from PBS time-matched controls.By OpenArray profiling,differential expression analysis of miRNAs revealed substantial changes in blood plasma miRNA composition at each stage of the disease and in both mouse models.A single miRNA whose expression was only dysregulated in the epileptogenesis stage of the disease could not be found.Most miRNAs whose expression was altered during epileptogenesis remained altered in chronically epileptic mice.Five miRNAs in plasma were identified with epilepsy biomarker potential: miR-93-5p (elevated in epileptogenesis and epilepsy), miR-574-3p(reduced in epileptogenesis and epilepsy), miR-182-5p (present in epilepsy only), miR-142-5p (elevated in epilepsy and implicated in the pathogenesis of epilepsy (Venø et al., 2020)), and miR-199a-3p (present in epilepsy only).Using Taqman individual RT-PCR assays, miR-93-5p, -574-3p, and -182-5p all showed the same expression in both mouse models with miR-93-5p and-182-5p upregulated in epilepsy and miR-574-3p downregulated in epilepsy.MiR-199a-3p was upregulated in the KA model at all time points but was not significantly different in the PILO model, and miR-142-5p failed to validate using the individual Taqman assays.SE and subsequent epileptogenesis were induced in male C57BL/6JO-1aHsd mice, 6–8 weeks of age, by intra-amygdala KA, and then received antagomir oligonucleotides targeting miR-134 (Ant-134, 120 pM, i.c.v.) or a scrambled oligonucleotide, 1 hour post-SE to inhibit epileptogenesis and reduce the development of chronic epilepsy.Blood was taken both pre-SE and 2 weeks post-SE.RT-PCR analysis revealed that plasma levels of miR-93-5p and miR-182-5p were no longer elevated in KA mice that received Ant-134.Furthermore, Ant-134 prevented the reduction of miR-574-3p levels normally seen in this model.Ant-134 had no observable effects on miR-199a-3p or miR-142-5p.
Male C57BL/6 mice, ~8 weeks of age, were administered KA (15 mg/kg,i.p.) in a study by Fu et al.(2019).By qRT-PCR, the levels of miR-155 were significantly increased in both the cortex and hippocampus in mice injected i.p.with KA compared to those in mice injected with vehicle.No significant changes occurred in the levels of miR-338, -200b, or -23a.The mice in the KA group developing acute seizure (≥ stage 4, Racine scale) were subjected to i.c.v.injection to right ventricle of antagomir NC or miR-155 antagomir.MiR-155 antagomir treatment decreased KA-induced seizure from grade 4–5 to grade 2–3 according to the Racine scale.In addition, miR-155 antagomir treatment markedly improved abnormal EEG recordings induced by KA, and ameliorated neurodegeneration induced by KA.
Wang et al.(2018) injected C57BL/6 mice, 6–8 weeks of age, with atropine sulfate (0.2 mL/100 g) and 30 minutes later with PILO (320 mg/kg, i.p.).Seizure behaviors were monitored according to Racine’s standard criteria(Racine, 1972).At 30 minutes after the injection, PILO was repeatedly administered (at 1/3 of the initial dose) every 15 minutes until the mice exhibited seizure activity exceeding stage 4.Each mouse received no more than 3 PILO injections.After 1 hour of SE, atropine sulfate (0.2 mL/100 g,i.p.) and diazepam (0.2 mL/100 g, i.p.) were administered to terminate SE.Beginning one day after SE induction and for the next 30 days, the chronic epilepsy model was confirmed by the observation of at least one SRS.All mice were euthanized 1 month after the PILO injections.Only mice with observed SRSs during the chronic period were included in the epilepsy group and mice that did not exhibit SRSs were used as controls.Adult male C57BL/6 mice were injected with a subconvulsive dose of PTZ (25 mg/kg)at 2 pm daily.Immediately after each injection behaviors were observed for 60 minutes.Only mice with at least five consecutive seizures scoring 4 or 5 were considered fully kindled and designated to the epilepsy group.Mice that were not kindled after 25 injections were used as controls.All mice were euthanized 24 hours after the last PTZ injection.By qRT-PCR, in the PILOinduced epilepsy mouse model, significantly lower levels of miR-137 were detected in both the hippocampus and temporal neocortex of the SRS group than in the non-SRS group.In the PTZ-kindled epilepsy model, significantly lower levels of miR-137 were observed in both the hippocampus and temporal neocortex of the fully kindled epileptic mice than in the non-kindled group.SE was induced in adult male C57BL/6 mice via i.p.PILO injections and miR-137 agomir, agomir scrambled NC, miR-137 antagomir, or antagomir NC was slowly injected into the bilateral hippocampus.Mice in the control group were administered an intrahippocampal injection of saline.The time until the first SRS, referred to as the latency to SRS, was recorded.In the 4thweek after SE, the total number of SRSs in each group was recorded and used to assess the degree of seizures.Only convulsive stage 4 and 5 seizures were recorded.Adult male C57BL/6 mice were also randomly divided into a control group, an agomir group, an antagomir group, an agomir NC group, and an antagomir NC group to assess the epileptic behaviors of the PTZ-kindled mice.Saline,miR-137 agomir, agomir scrambled NC, miR-137 antagomir or antagomir scrambled NC was injected into the hippocampus of the mice.72 hours later,a subconvulsive dose of PTZ was injected into the mice for kindling, and the latency until full kindling and the highest class of seizure was recorded daily.In the PILO-induced epilepsy mouse model, the duration of the latency to the first SRS was different in each observation group.The latency to the first SRS in the agomir group (19.3 ± 1.1 days) was significantly delayed compared with the latencies of the control group (15.3 ± 0.8 days) and the agomir NC group(13.3 ± 1.3 days).In addition, the latency to the first SRS in the antagomir group (11.6 ± 0.9 days) was decreased compared with the latencies of the control group and the antagomir NC group (15.4 ± 1.2 days): the differences among each group were statistically significant.A significantly greater number of SRSs was observed in the control group (5.3 ± 1.1) and in the agomir NC group (4.8 ± 1.1) than in the agomir group (1.3 ± 0.5).In addition, the number of SRSs in the antagonir group (8.6 ± 0.5) was significantly increased compared with the number of SRSs in the antagomir NC group (4.8 ± 0.5) and the control group.In the PTZ-kindled epileptic mice, the latency to full kindling in the agomir group (17.2 ± 1.0 days) was prolonged compared with the latency to full kindling in the control group (13.8 ± 0.7 days) and the agomir NC group (14.0 ± 0.6 days): these differences were statistically significant.The latency to full kindling in the antagomir group (9.8 ± 0.7 days) was significantly reduced compared with the latency to full kindling in the control group and the antagomir NC group (12.8 ± 0.9 days).The agomir group displayed a lower class of seizures than the control group, and the difference between the two groups was statistically significant from the 5thto 12thdays.The antagomir group displayed a higher class of seizures than the control group, and the difference between the two groups was statistically significant from the 4thto 9thdays.
Male C57BL/6J mice, 6–8 weeks of age, were injected i.p.with different doses of PTZ (60, 80, or 100 mg/kg) by Reschke et al.(2017).Lorazepam (8 mg/kg, i.p.) was administered after the first tonic-clonic seizure to reduce morbidity and mortality.Mice were followed for 30 minutes or 4 hours after PTZ administration for the appearance of seizures by EEG and behavioral methods.A dose of 80 mg/kg produced generalized tonic-clonic seizures in all mice and with a more consistent and shorter latency than with the 60 mg/kg dose.A dose of 100 mg/kg also caused rapid-onset tonic-clonic seizures in all mice but was associated with high mortality.A dose of 80 mg/kg was used for subsequent experiments.By RT-PCR, the levels of mature miR-134 were significantly increased in the hippocampus, but not in the cortex, 30 minutes after PTZ-induced generalized tonic-clonic seizures in mice.A second cohort of mice received i.c.v.injection of 0.12 nmol/2 μL 3′-cholesterol-tagged locked nucleic acid oligonucleotide targeting miR-134 (Ant-134) or its respective control, a non-targeting scrambled version of the antagomir (Scr; 0.12 nmol/2 μL).At 24 hours later, a baseline EEG recording was obtained.Mice were then injected with PTZ (80 mg/kg, i.p.) and lorazepam (8 mg/kg, i.p.)was administered after the first tonic-clonic seizure to reduce morbidity and mortality.These mice were followed for 30 minutes after PTZ administration for the appearance of seizures.MiR-134 expression in the hippocampus was lower in Ant-134 pre-treated mice compared to Scr-treated mice after PTZinduced seizures.The time to the first PTZ-induced tonic-clonic seizure was significantly longer in mice injected with Ant-134 compared to Scr-injected animals.The severity of PTZ-induced seizures was also significantly lower in Ant-134 compared to Scr-injected mice.Analysis of EEG-recorded seizure activity showed that convulsion duration, Racine scores, and EEG total power were lower in Ant-134 mice compared to the control group after PTZ.
Zheng et al.(2016) treated ICR and C57BL/6 mice, 6–8 weeks of age, with KA(40 μg/kg, i.c.v.to the right ventricle).Unilateral i.c.v.injection of KA into ICR mouse brains produced generalized tonic-clonic seizures reaching grade 4–5 on the Racine scale.EEG recordings 24 hours after injection identified highamplitude, high-frequency discharges in KA-injected mice compared with vehicle-injected mice.By qRT-PCR, KA treatments resulted in a significant decrease of miR-219 levels in the hippocampus on days 1, 3, 7, and 10 after treatments.The reduction of miR-219 levels recovered as time passed and there was no significant difference in miR-219 levels between KA-treated and control mice on day 14 after treatments.MiR-219 levels in the cortex were lower in KA-treated mice than in controls but the differences were not statistically significant.The reduction of miR-219 levels was also verified in the hippocampus in seizure induced by PILO.In C57BL/6 mice, a significant decrease of miR-219 levels in the hippocampus and a trend of reduction though not statistically significant of miR-219 levels in the cortex were found upon KA treatments.In some experiments, 6 hours after i.c.v.injection of KA,mice were subjected to i.c.v.injection of miR-219 agomir, miR-219 antagomir,or scrambled control miRNA at 10 nmol/kg.Mice were euthanized on days 1,3, 7, 10, and 14 after treatments, and cortical and hippocampal tissues were removed.Mice injected with the miR-219 antagomir exhibited staring eyes,beard trembling, and repeated washing face action, accompanied by heads and facial tics, and gradually developed into an involuntary movement of the arms.Such seizures reached grades 2–3 on the Racine scale.Consistently,these mice showed abnormal EEG recordings in the form of high-amplitude and high-frequency discharges.Injection of miR-219 agomir significantly alleviated the reduction of hippocampal miR-219 levels upon KA treatments,markedly reduced seizure severity (the onset and frequency of convulsions),and alleviated abnormal EEG recording in the form of high-amplitude and high-frequency discharges in KA-treated mice.
In a study by Jimenez-Mateos (2012), male C57BL/6 mice, 6–7 weeks of age,were fitted with a cannula and electrode assembly and injected with KA or PBS into the basolateral amygdala nucleus.After 40 minutes, all mice received lorazepam (6 mg/kg, i.p.).Mice were recorded for up to 1 hour thereafter.Nonharmful seizures were induced by a single injection i.p.of KA (15 mg/kg).The duration of high-amplitude high-frequency discharges, also termed type 4 seizures (Shinoda et al., 2004), was calculated between the time of KA injection and the time of lorazepam administration.PCR showed that at 24 hours after SE a significant increase was found in mature miR-134 levels in the ipsilateral CA3 and CA1 subfields.The levels of mature miR-134 were not changed in the undamaged contralateral CA3 subfield.Nonharmful,nonconvulsive seizures induced by a low dose of systemic KA, a model of epileptic preconditioning (Jimenez-Mateos et al., 2010) in which SE does not develop, did not alter miR-134 levels compared to vehicle controls in the CA3 and CA1 subfields.Recurrent spontaneous seizures occur 3–4 days after SE in the model used (Mouri et al., 2008; Jimenez-Mateos et al., 2010), and within 3 weeks the mice show pathological hallmarks of temporal lobe epilepsy,including neuron loss and astrogliosis.MiR-134 levels were significantly elevated in the CA3 subfield 3 weeks after SE and 1 and 3 weeks after SE in the CA1 subfield compared to controls.For i.c.v.injections, additional mice were fitted with a cannula ipsilateral to the side of the KA injection.Mice received a 1μl infusion of either Scr or Ant-134 locked nucleic acid -modified and 3’-cholesterol-modified oligonucleotides in artificial CSF.In mice injected i.c.v.with Ant-134, knockdown of miR-134 was first evident 12 hours after injection of 0.12 nmol Ant-134, and by 24 hours after injection, the levels of miR-134 in the hippocampus of these mice were significantly reduced by> 95%.Increasing the amount of Ant-134 injected to 1nmol seemed to produce an off-target knockdown of miRNAs.The miR-134 levels began to recover by 7 days after Ant-134 injection, although they remained significantly lower than the miR-134 levels in the mice injected with Scr at 1 month after injection.They were no longer different from the levels in mice injected with Scr by 2 months after injection.No evidence of hippocampal neuronal death was found in the brains from mice injected with antagomirs.Seizures evoked by intra-amygdala microinjection of KA were compared in mice 24 hours after injection with either Scr or Ant-134.There was no difference in basal EEG measures between the two groups of mice.Scr-injected mice experienced typical SE, comprising episodes of high-amplitude, high-frequency discharges(Engel et al., 2010).Duration of the high-amplitude, high-frequency discharges, which are associated with damage-causing pathological activity(Shinoda et al., 2004), and total EEG power were significantly lower in Ant-134-injected mice compared to Scr-injected mice.In agreement with the normal course of epilepsy in this model (Mouri et al., 2008), mice injected with Scr after SE experienced the first spontaneous seizures on the 3rdday,and all mice were epileptic by the 4thday after SE.The median epileptic seizure count during 14 days of monitoring of Scr-injected mice was 25,with 200 epileptic seizures recorded in total for all the Scr-injected mice over this period.In contrast, only 60% of mice injected with Ant-134 had a spontaneous seizure by the 11thday after SE.Ant-134-injected mice had a median epileptic seizure count of 2 during the 14-day recording period, with just 16 epileptic seizures recorded in total for these mice during the 2-week recording period, compared to the Scr-injected group.The durations of the individual seizures were similar between the groups.
Rat Model Studies
In a study by Wang et al.(2021), male Sprague-Dawley rats, 6–8 weeks of age, were i.c.v.injected in the right lateral ventricle with KA (40 μg/kg).The beginning of SE was the onset of continuous generalized seizure activity(stage 4–5 on the Racine scale) without regaining normal behavior between seizures.Rats with a seizure score of 4–5 on the Racine scale were included in the study.All the rats were monitored by EEG for up to 90 minutes after the successful modeling.Rats in the control group were injected with the same amount of normal saline in the right ventricle.Spontaneous recurrent seizures were monitored during the study period (6 hours, 1 day, 3 days,7 days, and 14 days after KA treatment).Rats that had been i.c.v.injected with KA into the brain exhibited generalized tonic-clonic seizures after KA treatment.EEG recordings at 24 hours after KA treatment identified highamplitude and high-frequency discharges in rats.KA-induced seizure induced neuronal death in the hippocampus.PCR showed that KA treatment resulted in an increase of miR-129-2-3p level and a decrease ofGABRA1mRNA in the hippocampus on days 1, 3, 7, and 14 after treatment.There was no significant difference in miR-129-2-3p level between KA-treated and control rats after 14 days.In another cohort of rats, miR-129-2-3p antagomir (Ant-129) and miR-129-2-3p antagomir negative control (Ant-129 NC) were injected at concentrations of 200 nmol/mL (1 nmol/5 μL per rat, infusion rate 0.5 μL/min)into the lateral ventricle of anesthetized rats.Rats were subjected to KA treatment 24 hours after antagomir injection.At 72 hours later, miR-129-2-3p level was significantly decreased in the Ant-129 group compared to the Ant-129 NC group.After Ant-129 injection into the rat brain, the severity of KA-induced seizures in rats was significantly reduced and also abnormal EEG recordings associated with high amplitude and frequency.
Male Sprague-Dawley rats, 6–7 weeks of age, were subjected to electrical amygdala kindling by Wang et al.(2020).Under anesthesia, an EEG electrode was stereotaxically implanted in the amygdala.With penicillin treatment(i.p.) in the first 3 days, the rats were allowed to recover for 7 days, followed by after discharge threshold (ADT) test.The parameters of stimulation were 16 Hz frequency, biphasic square pulses of 1 ms duration applied for 10 seconds.The intensity was delivered through a constant current stimulator producing square wave pulses and adjusted every 1 minute until a minimum AD of 3 seconds duration was produced.Stimulation intensities began at 20 μA.If no AD was induced at this level, additional 20 μA intensity could be supplemented in the next trial until an AD was generated.The lowest intensity of stimulation for producing an AD was designated as the ADT.The amygdala was stimulated with 400 μA intensity at 7-minute intervals, and the rat was considered kindled when stage 4–5 seizures on the Racine scale were observed.In addition, 24 hours later after 10 consecutive stage 5 seizures were observed, the ADT of the amygdala-kindled rat was detected.At the end of the 24 hours post-ADT test, the antiepileptic response to phenobarbital was evaluated in the kindled rats by i.p.administration of phenobarbital 30 mg/kg.In each rat, three trials with phenobarbital administration were performed at intervals of 1 week.Drug-resistant rats were defined as those not showing any increase or an average increase of no more than 20% in ADT upon repeated application of phenobarbital, while drug-responsive rats were those showing at least 20% ADT increase in all three trials with phenobarbital.RT-PCR and Western blot analysis were used to determine the expression of miR-139-5p and multidrug resistance-associated protein 1 (MRP1) in the brain tissues from kindled rats after drug administration.MiR-139-5p expression was significantly decreased, while MRP1 mRNA and protein expression were significantly increased in the drug-responsive and drug-resistant rats compared to the control rats.The drug-resistant rats had a significantly lower miR-139-5p expression and a significantly higher MRP1 expression level than the drug-responsive rats in the brain tissues.The drug-resistant rats were injected with a series of recombinant lentiviruses carrying miRNA agomir, gene-specific short hairpin RNA (shRNA), or overexpression plasmids.After the drug-resistant epileptic rats were induced, the rats were instantly anesthetized with 1% pentobarbital sodium and injected with 4 μL lentiviruses at 0.5 μL/min into the rat hippocampus.After 7 days of injection, the rats were euthanized.Compared with normal rats, TUNEL-positive cells were significantly increased in drug-resistant rats injected with NC agomir, sh-NC,and miR-139-5p agomir + overexpression of MRP1.Apoptotic cell number was significantly reduced in the brain tissues induced by overexpression of miR-139-5p or downregulation of MRP1.Nissl staining was performed to observe neuronal damage.The upregulation of miR-139-5p and overexpression of MIRP1 together could trigger significant damage in hippocampal neurons involving disordered cell arrangement, incomplete cell structure, cytoplasmic condensation, karyopyknosis, and reduction of Nissl bodies in the cytoplasm.The above neuronal damage could be markedly ameliorated by miR-139-5p upregulation or MRP1 inhibition.Compared to normal rats, drug-resistant rats injected with NC agomir, sh-NC, and miR-139-5p agomir + overexpression of MRP1 displayed notably reduced surviving neurons, whereas overexpression of miR-139-5p or downregulation of MRP1 contributed to enhanced surviving neurons.There was no significant difference in ADT before/after kindling acquisition and ADT before/after drug administration among drug-resistant rats injected with NC agomir, sh-NC, and miR-139-5p agomir + overexpression of MRP1; the ADT after drug administration in the rats with overexpression of miR-139-5p or downregulation of MRP1 was significantly higher than that before administration.As a consequence, miR-139-5p restoration or MRP1 depletion could reduce drug resistance of refractory epilepsy.
Venø et al.(2020) induced epilepsy in male Sprague-Dawley rats, 10–11 weeks and 7–9 weeks of age, by PPS.Rats were euthanized at 1 hour,24 hours, 72 hours, 10 days, and 16 days after PPS (epileptogenesis),within 1 day of the first spontaneous seizure (early epilepsy), or 1 month after the first spontaneous seizure (chronic epilepsy).Control rats were euthanized on day 17 after surgery (corresponding to day 10 after PPS).Ago2 immunoprecipitation was performed followed by small RNA sequencing on hippocampal samples.Induction of epilepsy led to significant changes in the expression of individual miRNAs, including shortly after the epileptogenic insult, on the day of the first spontaneous seizure and chronic epilepsy.Within the chronic epilepsy phase, 8 up- and 1 down-regulated miRNAs were common to all three models.This included miR-132-3p and mR-146a-5p for which there are already significant functional data linking them to epilepsy(Jimenez-Mateos et al., 2011; Huang et al., 2014; Iori et al., 2017; Tao et al.,2017), and six miRNAs (miR-10a-5p, -21a-3p, -27a-3p, -142a-5p, -212-3p, and-431-5p) for which there are limited or no functional data linking to epilepsy.In a study by Fan et al.(2020), female Wistar rats, 8–10 weeks of age, were randomly divided into four groups (n= 8/group): normal group, epilepsy group, epilepsy group + LV-miR-negative control group (hippocampi transfected with miR-NC, final concentration 50 μM), and epilepsy + LV-miR-15a group (hippocampi transfected with miR-15a mimics, final concentration 50 μM).Transfected LV-miR-NC or LV-miR-15a was subcutaneously injected into the hippocampi of anesthetized rats.Rats in the epilepsy, epilepsy + LVmiR-NC, and epilepsy + LV-miR-15a groups were injected i.p.with lithium chloride (127 mg/kg).Then 18 hours later, PILO (127 mg/kg) was repeatedly injected i.p.every 30 minutes until the rats had seizures with tonic-clonic(head and face clonic, limb clonic) using EEG recordings and observation.Rats in the normal group were injected i.p.with an equivalent volume of saline.The epileptic seizure was terminated after an epileptic state that lasted for 1 hour.After 24 hours, the rats were euthanized.By RT-qPCR, miR-15a expression levels were significantly downregulated in the epilepsy group compared with the normal group, and miR-15a expression was significantly enhanced in the epilepsy + LV-miR-15a group compared with the epilepsy group.MiR-15a expression levels were significantly increased in the epilepsy+ LV-miR-15a group compared with the epilepsy + LV-miR-NC group.The expression levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor alpha(TNF-α), which are important proinflammatory factors (Ng et al., 2018), were significantly upregulated in the epilepsy group vs.normal group, suggesting that there may be a strong inflammatory response in the epilepsy model.The inflammatory factors were significantly decreased in the epilepsy + LV-miR-15a group compared with epilepsy group, suggesting that LV-miR-15a could inhibit an inflammatory response in the epilepsy model.Compared with the normal group, the levels of cell apoptosis were also significantly increased in the epilepsy group and were significantly reduced in the epilepsy + LVmiR-15a group compared to epilepsy group.In the epilepsy + LV-miR-15a group, the levels of cell apoptosis were significantly decreased and mRNA expression levels of IL-1β, IL-6, and TNF-α were significantly downregulated compared with the epilepsy + LV-miR-NC group.These findings imply that the upregulated expression levels of miR-15a may decrease levels of cell apoptosis and inflammation in epilepsy tissues.
Brennan et al.(2020) fitted male Sprague-Dawley rats, 9–13 weeks of age,with bipolar stimulating electrodes positioned in the angular bundles of the PPS pathway, and custom unipolar recording electrodes were lowered into the dorsal dentate gyrus.Electrode locations were determined by optimizing the potentials evoked by low-frequency PPS.The PPS protocol utilized a paradigm designed to evoke and maintain hippocampal seizure activity throughout the stimulation, but not convulsive SE, which consisted of continuous, bilateral 2 Hz paired-pulse stimuli, with a 40 ms interpulse interval, plus a 10 seconds train of 20 Hz single-pulse stimuli delivered once per minute, generated by an S88 stimulator.All pulses (0.1 ms duration) were delivered at 20–24 V with associated current typically between 15 and 30 μA.Stimulation for 30 minutes (on two consecutive days) required only isofluorane to terminate seizures.An 8-hour stimulation on the third day did not induce SE and,therefore, did not require pharmacological termination.Blood samples were taken at baseline, 24 hours (epileptogenesis), 72 hours (epileptogenesis),10 days (epileptogenesis), and the day of the first spontaneous seizure(chronic epilepsy).Blood from rats was collected into tubes coated with K2 EDTA (0.5 M), centrifuged (1300 ×g, 10 minutes, 4°C), and plasma removed.By OpenArray profiling, 5 miRNAs in plasma were identified with epilepsy biomarker potential: miR-93-5p (elevated in epileptogenesis and epilepsy),miR-574-3p (reduced in epileptogenesis and epilepsy), miR-182-5p (present in epilepsy only), miR-142-5p (elevated in epilepsy and implicated in the pathogenesis of epilepsy (Venø, 2020)), and miR-199a-3p (present in epilepsy only).Using Taqman individual RT-PCR assays, miR-93-5p, miR-574-3p, and miR-182-5p all showed the same expression profile with miR-93-5p and miR-182-5p upregulated in epilepsy, and miR-574-3p downregulated in epilepsy.MiR-199a-3p was not significantly different in the PILO model, and miR-142-5p failed to validate using the individual Taqman assays.
Male Sprague-Dawley rats, 4–6 weeks of age, were randomly divided into four groups (n= 8/group) by Lu et al.(2019): (1) the control group received injection i.p.of saline 40 μL/day only; (2) the model group received injection i.p.of KA 8.5 mg/kg/day only; (3) the model + inhibitor control group received injection i.p.of 40 μL/day inhibitor control, 15 minutes prior to the injection of KA; (4) the model + miR-27a-3p inhibitor group received the same treatment used in the model + inhibitor control group but miR-27a-3p inhibitor 40 μL/day was injected instead of inhibitor control.The animal experiment lasted 7 days.In the experiment, the severity of convulsions was assessed by the Racine scale, and only included animals rated 4–5.The animals were euthanized within 5 hours of the occurrence of the last spontaneous seizure.By RT-PCR, the expression level of miR-27a-3p in the hippocampus of the epileptic rat model was significantly higher compared to the control group.The seizure score and duration of the epileptic model group were significantly higher relative to the control group.However, the seizure score, number of seizures in 90 minutes, and duration of seizures of epileptic rats were significantly reduced by miR-27a-3p inhibitor treatment.MiR-27a-3p inhibitor significantly reduced neuronal apoptosis in the hippocampus of epileptic rats.The inflammatory factors IL-1β, IL-6, and TNF-α in the hippocampus tissues of epileptic model group were significantly increased compared with control group.MiR-27a-3p inhibitor significantly reduced the levels of IL-1β, IL-6,and TNF-α in the hippocampus tissues of epileptic rats compared to epileptic model group.
Liu et al.(2019) treated male Wistar Han rats, 7 weeks of age, with subcutaneous injections of KA (5 mg/kg) for 10 minutes and subcutaneous injections of KA (2.5 mg/kg) for 45 minutes.Using PCR, miR-141 expression levels in brains were significantly increased in epilepsy rats compared to normal rats.Behavioral seizure responses and seizure duration of epileptic rats were significantly higher than those of normal rats.
Li et al.(2018a) injected male Sprague-Dawley rats, 7 weeks of age, with KA (0.8 μg, 1 μg/μL in saline) in the CA3 region of the left hippocampus.SE was defined as the onset of continuous generalized (score 4–5) seizure activity lasting no less than 40 minutes, and only those animals that reached SE were included in the study.The KA-induced SE was not terminated pharmacologically.Approximately 71% of the rats had SRSs.Rats with seizures were assigned to 5 groups at week 7 (chronic phase) post-SE : (1) IL-1β control group (n= 10, rats were injected with 1 μL 0.9% saline); (2) IL-1β group (n= 10, rats were injected with 1 μL 50 ng/μL IL-1β, 3 electrode-implanted rats); (3) miR-146a group (n= 10, rats were injected with 1 nmol miR-146a agomir, 3 electrode-implanted rats); (4) miR-146a sponge group (n= 10, rats were injected with 1 nmol miR-146a antagomir): (5) miR-146a control group(n= 10, rats were injected with 1 nmol miR-146a control).At 48 hours after injection, the rats were euthanized and hippocampal tissues were collected.By RT-PCR, miR-146a expression was significantly upregulated in the agomir group compared with the antagomir or control group.However, miR-146a expression was significantly suppressed in the antagomir group compared with the control group.IL-1β expression was significantly upregulated in the miR-146a agomir group compared with the control group or miR-146a antagomir group and was significantly lower in the miR-146a antagomir group than in the control group.To determine the ability of inflammation to regulate the expression of miR-146a in the hippocampus of chronic epileptic rats, the rats were injected with IL-1β at week 7 (chronic phase) post-SE.Hippocampi were collected 48 hours after the injection.Using RT-PCR, miR-146a expression was significantly upregulated in the IL-1β group compared with the negative control group.
Li et al.(2018b) divided male Sprague-Dawley rats, 7 weeks of age, into two groups: experimental group (n= 50) and control group (n= 25).Rats in the experimental group were injected with KA (0.8 μg, 1 μg/μL in saline) in the CA3 region of the left hippocampus.Only those animals that reached SE were included in the study.Based on epilepsy development phases,the experimental and control groups of rats were each randomly divided into 4 groups: (1) acute control group, AC (control rats, 2 hours after saline administration,n= 6); (2) acute seizure group, AS (SE rats, 2 hours after KA administration,n= 8); (3) latent control group 1, LC1 (control rats, 7 days after saline administration,n= 6); (4) latent seizure group 1, LS1 (SE rats, 7 days after KA administration,n= 8); (5) latent control group 2, LC2 (control rats 21 days after saline administration,n= 6); (6) latent seizure group 2, LS2 (SE rats,21 days after KA administration,n= 8); (7) chronic control group, CC (control rats, 60 days after saline administration,n= 6); (8) chronic seizure group, CS(SE rats, 60 days after KA administration,n= 8).Rats were euthanized at 2 hours, 7, 21, and 60 days post-SE or saline administration.All 50 rats in the experimental group successfully developed SE, and 4 rats died in the first 24 hours.The acute seizure and acute control groups were euthanized 2 hours after SE.The modified Racine scale was used to observe SRS.Racine Class 1 and 2 seizures were typically described as non-convulsive seizures, and they were difficult to observe, while Racine Class 3, 4, and 5 seizures were characterized as convulsive motor seizures.Only rats with this performance were used for further research.14 rats failed to develop SRS or died during the process.The latent seizure and latent control groups were euthanized 7 days and 21 days post-SE without any behavioral changes or seizures.SRS was observed occurring mainly 5 weeks after SE.Chronic seizures occurred at 8 weeks, with a frequency of 2–10 seizures/day.By RT-PCR, there was significant upregulation of miR-155 expression in the hippocampus in the epileptic groups compared with control groups during the latent and chronic phases of epilepsy development.Expression of miR-155 was lower in the latent phase 2 (LS2 group) compared with that in the latent phase 1 (LS1 group) and showed the lowest expression in the chronic phase (CS group).There was no difference between the AS group and AC group which suggested that miR-155 expression was not regulated in the acute phase.Using RT-PCR, there was significant upregulation of TNF-α expression in the rat hippocampal tissues in the epileptic groups during three different phases of epilepsy development compared with the control groups.The expression of TNF-α was highest in the acute phase (2 hours post-SE) in the seizure and control groups.TNF-α expression was significantly upregulated in the CS group compared to the LS2 group.There was no significant difference between the CC group and LC2 group.In summary, TNF-α expression during epilepsy development was gradually decreased in the latent phase post-SE but rose again in the chronic phase in the rat hippocampal tissues.A miR-155 antagomir or an antagomir control was dissolved in saline at 1 nmol/10 μL.18 rats were randomly divided into two groups: latent antagomir group (LS1 antagomir,n= 9) and latent antagomir control group (LS1 control,n= 9).At 1 hour after SE, rats received antagomir or antagomir control (1 nmol for each rat).At 7 days after SE onset,rats were euthanized and hippocampal tissues were removed.Using qRT-PCR,there was no significant difference in miR-155 expression between the LS1 control group and LS1 group, which suggested that the miR-155 antagomir control had no effect on miR-155 expression.The miR-155 expression in the LS1 antagomir group was significantly downregulated compared to that in the LS1 control group or LS1 group, which suggested that the antagonist was effective.There was no significant difference in TNF-α expression between the LS1 control group and LS1 group, which suggested that the miR-155 antagomir control had no effect on TNF-α expression.The expression of TNF-α in the LS1 antagomir group was significantly downregulated compared with the LS1 control group or LS1 group, which suggested that the miR-155 antagomir can inhibit TNF-α expression.
Male Sprague-Dawley rats, ~7–8 weeks of age, were divided by Huang et al.(2018) into 8 groups: (A) Sham group, rats treated with equal amount of saline instead of pilocarpine; (B) PILO group, rats underwent PILO-induced epilepsy; (C) Scr group, epilepsy model rats pre-treated with non-targeting antagonist; (D) Ant-155 group, epilepsy model rats pre-treated with miR-155 antagonist; (E) Lentiviral vector-green fluorescent protein (LV-GFP) group,epilepsy model rats preadministered with LV-GFP; (F) LV-Sesn3-shRNA group,epilepsy model rats preadministered with LV-Sesn3-shRNA; (G) (Ant-155)+(LVGFP) group, epilepsy model rats already treated with Ant-155 subsequently administered with LV-GFP; (H) (Ant-155)+(LV-Sesn3-shRNA) group, epilepsy model rats already treated with Ant-155 subsequently administered with LVSesn3-shRNA.Lithium chloride (125 mg/kg, i.p.) was injected 18–20 hours prior to PILO administration (50 mg/kg, i.p.) to induce SE.Pre-treatment with atropine (1 mg/kg, i.p.) 30 minutes prior to pilocarpine administration was used to repress peripheral cholinergic effects.Convulsion severity was assessed by Racine sale, and only those models with a score of 4–5 were included in the study.Choral hydrate (10%, 3 mL/kg, i.p.) was then injected 1.5 hours after PILO administration to terminate the seizure.The sham control rats were injected with an equal volume of saline instead of PILO.The level of rno-miR-155 in hippocampal CA3 and CA1 of rat epilepsy models was investigated at 0, 1, 14, 30, and 60 days post-SE.By RT-PCR, miR-155 was elevated in CA3 at all later four time points compared to sham controls,while it was not significantly increased in CA1.There was a gradual increase in expression of miR-155 that peaked at day 14 post-SE in the CA3 region.To confirm that inflammatory markers associated with miR-155 were present in the hippocampus, the expression of TNF-α in the CA3 region was measured.The expression of TNF-α mRNA was elevated at all four time points compared to its level in sham controls.Ant-155 and Scr were dissolved in artificial CSF at 200 nmol/mL (1 nmol/5 μL per rat, infusion rate 0.5 μL/min) and injected into the left lateral ventricle.The rno-miR-155 level was measured in CA3 at 0, 1, 14, 30, and 60 days post-SE.Significant knockdown of miR-155 was first seen 1 day post-SE after injection of 1 nmol Ant-155.The miR-155 level began to recover by 14 days post-SE.At 30 days it was still lower but had recovered by 60 days post-SE.In all Ant-155 groups, a steady decrease in miR-155 was observed in continuous points of post-SE.The expression of TNF-α mRNA was decreased at the later three time points compared to its level in matched Scr groups.To detect the effect of silencing miR-155 on acute seizure activity,the latency of the first generalized tonic-clonic seizure (GTCS) frequency,Racine scores of 5, and duration time of GTCS were analyzed during the 2.5 hours after PILO administration.The latency to the onset of GTCS was longer and the frequency was decreased in the Ant-155 group.Ant-155 rats displayed milder convulsive behavior, presented as Racine scores of below 5 and decreased duration time of GTCS.Whether Ant-155 had altered the underlying pathology of the hippocampal CA1/CA3 region at 14 days post-SE was determined.In Ant-155 rats, the apoptotic index in CA3 was lower and Western blot analysis showed significant upregulation of anti-apoptotic protein Bcl-xL.In addition, expression of the known pro-apoptotic proteins cleaved caspase-3 and acetylated p53 were similarly reduced, and neuron counts were increased.
Reschke et al.(2017) performed PPS in male Sprague-Dawley rats, 10–11 weeks of age, fitted with bipolar stimulating electrodes positioned in the angular bundles of the perforant pathway, and custom unipolar recording electrodes were lowered into the dorsal dentate gyrus.The PPS protocol utilized a paradigm designed to evoke and maintain hippocampal seizure activity throughout the stimulation, but not convulsive status epilepticus,which consisted of continuous, bilateral 2 Hz paired-pulse stimuli, with a 40 ms interpulse interval, plus a 10 seconds train of 20 Hz single-pulse stimuli delivered once per minute, generated by an S88 stimulator.All pulses (0.1 ms duration) were delivered at 20–24 V, as this voltage reliably evokes granule cell discharging without tissue-damaging hydrolysis.The current associated with these voltages was typically between 15 and 30 μA.Stimulation evoked population spikes with amplitude of 5–10 mV.Stimulation for 30 minutes(on two consecutive days) required only isofluorane to terminate seizures.An 8-hour stimulation on the third day did not induce SE and, therefore, did not require pharmacological termination.All animals survived the 8-hour stimulation.Rats were randomly divided into three cohorts to assess: (1)miR-134 expression after PPS (24 hours, 4 days, 14 days); (2) the effect of Ant-134 on PPS-induced SE, in which rats were pre-treated with Ant-134 or Scr (0.36 nmol/6 μL, i.c.v.) 24 hours before 8-hour stimulation; (3) the effect of Ant-134 on epileptogenesis, in which rats were injected with Ant-134 or Scr (0.36 nmol/6 μL, i.c.v.) immediately after 8-hour stimulation.By TaqMan miRNA assays, the levels of mature miR-134 were not significantly modified in the hippocampus of rats at two different time points“early”and“late”.In rats pre-treated with Ant-134 or Scr 24 hours before the 8-hour stimulation protocol, the severity of evoked seizures in the PPS model was similar.To determine whether silencing miR-134 after SE in the model would alter the emergence of SRSs, rats underwent 8-hour stimulation of the PPS pathway to induce epilepsy and i.c.v.injection of Anat-134 or Scr was given at the end of stimulation.Rats were then monitored with video-EEG until a first spontaneous seizure was recorded, typically 10–25 days after PPS, and they were then followed for a further 8 weeks.Epilepsy developed in all Scrinjected rats over the expected course with the first spontaneous seizure appearing 1–4 weeks after SE.Scr-injected rats averaged ~20 spontaneous seizures recorded during the 8-week recording time.In contrast, no spontaneous seizures were detected over the 8-week period in 6 of 7 rats injected with Ant-134 after SE.Epilepsy occurred in only a single rat treated with Ant-134.
In a study by Ren et al.(2016) with male Sprague-Dawley rats, 4–6 weeks of age, lithium chloride (125 mg/kg) was injected 18–20 hours before the administration of PILO (20 mg/kg, i.p.).The Racine scale was used to evaluate the severity of convulsions.SE (identified with a Racine scale score of 4 or 5) was defined as the beginning of a set of continuous generalized seizure activity lasting no less than 40 minutes.PILO administration (10 mg/kg, i.p.)was repeated every 30 minutes for those animals who scored less than 4 on the Racine scale or otherwise showed no seizure activity.The maximum amount for this injection was set at 60 mg/kg.All subjects in the SE group received chloral hydrate (10%, 3 mL/kg) to terminate epileptic attacks.The control group received saline.Rats were identified as having experienced epilepsy (3 months post-SE) through frequent spontaneous multiple seizures scoring 4 or 5 on the Racine scale.All animals were euthanized within a period of 5 hours from their last spontaneous seizure.Rats were euthanized at 24 hours, 7 days, 14 days, and 3 months after SE was induced.Five miRNAs with potential relevance to epilepsy were selected by a literature search for brain-enriched miRNA or glioblastoma-related miRNA: miR-132, miR-146a,miR-181a, miR-34a, and miR-124.By qRT-PCR, the expression levels of these miRNAs were all significantly upregulated in the hippocampus of rat epilepsy model (fold change 1.75 ± 0.09 for miR-132, 2.34 ± 0.14 for miR-146a, 2.75 ±0.23 for miR-181a, 1.78 ± 0.21 for miR-34a, 2.46 ± 0.06 for miR-124) (n= 10).MiR-181a had significantly increased level in the hippocampus of post-SE rats at 1 day (4.47 ± 0.35), 7 days (4.85 ± 0.53), and 2 weeks post-SE (5.66 ± 0.64),followed by a decrease at 3 months post-SE (2.75 ± 0.31) (n= 10).Despite this decrease, the level of miR-181a¬ was still considerably higher than that of the control sample.MiR-181a antagomir significantly reduced the expression of miR-181a at 7 days post-SE in the rat hippocampus (2.77 ± 0.36) compared to the miR-181a antagomir control group (4.76 ± 0.54) (n= 10).By qRT-PCR,significant upregulation in the expression of activated caspase-3 (3.28 ± 0.38)and caspase-9 (3.44 ± 0.40) was found at 7 days post-SE in rat hippocampus(n= 10).The expression was significantly decreased in miR-181a antagomirtreated group (2.25 ± 0.28) compared to 7 days post-SE group and the antagomir control group (n= 10).By TUNEL staining, the portion of the brain affected by neuronal death increased in the post-SE rat hippocampus.MiR-181a antagomir decreased the seizure-induced neuronal death in the hippocampus of post-SE rats in the regions CA1 and CA3 compared to the antagomir control group at 7 days post-SE.
He et al.(2016) performed electrical stimulation of the left dentate gyrus in male Sprague-Dawley rats, 8–12 weeks of age.A bipolar stimulation electrode (distance between tips 500 μm) was implanted in the angular bundle.Several weeks after electrode implantation, rats underwent tetanic stimulations (50 Hz) of the hippocampus in the form of trains of pulses every 13 seconds.Each train had a duration of 10 seconds and consisted of biphasic pulses (pulse duration 0.5 ms, maximal intensity 500 μA).Stimulation was stopped when the rats displayed sustained forelimb clonus and salivation for minutes, which usually occurred within 1 hour.Stimulation never lasted longer than 90 minutes.Immediately after termination of the stimulation,periodic epileptiform discharges occurred at a frequency of 1–2 Hz and lasted for several hours (SE).During this period, rats had frequent seizures as observed by both their behavior and EEG.The end of SE could be clearly defined by the disappearance of 1–2 Hz periodic epileptiform discharges.The chronic epileptic group (4 weeks after SE) was monitored during and shortly after SE, and during 3 to 5 days before death to determine the frequency of spontaneous seizures.Sham-operated control rats did not receive electrical stimulation.Chronic epileptic rats had frequent seizures (range 5–12).Thetime between the last spontaneous seizure and the time the animals were killed was < 5 hours.Rats were killed 1 week and 4 weeks after the induction of SE.By qRT-PCR, miR-146a expression in rat dentate gyrus region was significantly increased at 1 week (latent phase) and 4 weeks (chronic phase)post-SE compared with control non-SE values.To determine if miR-146a has a regulating effect on seizures in epilepsy model rats, rats were treated by i.c.v.injection of antagomir-146a.It was found that the latency of the first seizures was increased and the frequency and duration of seizures were decreased in the miR-146a antagomir group compared with the control and antagomir-NC groups.
In a study by Alsharafi et al.(2016), male Sprague-Dawley rats, 6–8 weeks of age, were injected i.p.with PILO 20 mg/kg to induce a generalized SE, with a dose of 10 mg/kg given i.p.every 30 minutes until Racine stage 4–5, tonicclonic seizure was elicited.Control animals (n= 15) received an equivalent volume of saline instead of PILO.Lithium chloride 125 mg/kg was injected i.p.18 hours prior to PILO.Methylscopolamine 1 mg/kg was administered i.p.15 minutes before pilocarpine treatment to prevent peripheral cholinergic effects.Of the 63 animals, 55 rats developed SE consisting of generalized motor seizures, which were terminated within 60 minutes after their onset by chloral hydrate (10%, 3 mg/kg, i.p.).The epilepsy rats (60 days after SE) were identified by spontaneous seizures with a frequency of 5–12 seizures that scored 4–5 on the Racine scale.Rats were euthanized at 1, 7, and 60 days after SE.By RT-PCR, miR-139-5p expression was significantly downregulated in rat hippocampus during the acute (day 1) and chronic phases (day 60) of epilepsy compared to non-seizure controls, with markedly lower miR-139-5p expression level at 60 days after SE.There was no significant difference in miR-139-5p levels between PILO-treated and control rats at day 7 (latent phase) after treatments.
Those miRNAs found to have altered expression in the hippocampus,temporal neocortex, or blood plasma in mouse and rat models of epilepsy are summarized in Tables 1 and 2.The effects of overexpression or suppression of specific miRNAs on seizures and alterations in brain tissues or blood plasma are summarized in Tables 3 and 4.
Discussion
Epilepsy is a debilitating disease, which commonly begins in childhood or older adulthood.The symptoms can be reduced in a large proportion of patients by the use of antiepileptic drugs.However, about 30% of patients are resistant to such treatments, and there is an urgent need for new therapies.Dysregulation of microRNAs has been proposed to contribute to neurological injuries and neurodegenerative diseases, including epilepsy (Eacker et al.,2009; Dogini et al 2013; Henshall, 2013), and targeting those microRNAs may provide an effective treatment strategy.Individual microRNAs often have multiple targets, increasing the scope for influencing several pathways or enhanced regulation of single pathways by multiple microRNAs and could be an advantage in treating epilepsy.
Most of the studies reviewed had performed microRNA profiling in hippocampal tissues.For some microRNAs, similar findings were obtained in two separate studies, or in two different models, of mouse or rat epilepsy.For instance, in the mouse studies (Table 1), upregulation of miR-134 occurred in male mice, 6–8 weeks, treated by PTZ (Reschke et al., 2017) and in male mice, 6–7 weeks, treated by KA intra-amygdala injection (Jimenez-Msteos et al., 2012).The same eight hippocampal microRNAs (miR-132-3p,-146a-5p, -10a-5p, -21a-3p, -27a-3p, -142a-5p, -212-3p, and -431-5p) were upregulated in male mice, 6–8 weeks, receiving KA intra-amygdala injection and in adult male mice treated by PILO (Venø et al., 2020).Also, miR-137 was downregulated in male mice, 6–8 weeks, treated by PILO method and in adult male mice treated by PTZ (Wang et al., 2018).Similarly, in the rat studies (Table 2), upregulation of miR-132-3p and miR-146a-5p occurred in male rats, 7–11 weeks, treated by PPS method (Veno et al., 2020) and there was upregulation of miR-132 and miR-146a in male rats, 4–6 weeks, treated by PILO (Ren et al.,2016).MiR-146a was also upregulated in male rats, 8–12 weeks, receiving electrical stimulation (He et al., 2016).Upregulation of miR-27a-3p was found in male rats, 7–11 weeks, treated by PPS method (Venø et al., 2020) and in male rats, 4–6 weeks, treated by KA i.p.injection (Lu et al., 2019).MiR-155 was upregulated in male rats, 7 weeks, with KA injected into CA3 region of the left hippocampus (Li et al., 2018b) and in male rats, 7–8 weeks, treated by PILO (Huang et al., 2018).Alterations in microRNAs found in both the mouse and rat studies (Tables 1 and 2) were the eight upregulated microRNAs (miR-132-3p, -146a-5p, -10a-5p, -21a-3p, -27a-3p, -142a-5p, -212-3p, and -431-5p) (Venø et al., 2020) and upregulated miR-155 (Huang et al., 2018; Li et al.,2018b; Fu et al., 2019).

Table 1 |Alterations of miRNA expression in blood plasma and brain tissues in mouse models of epilepsy
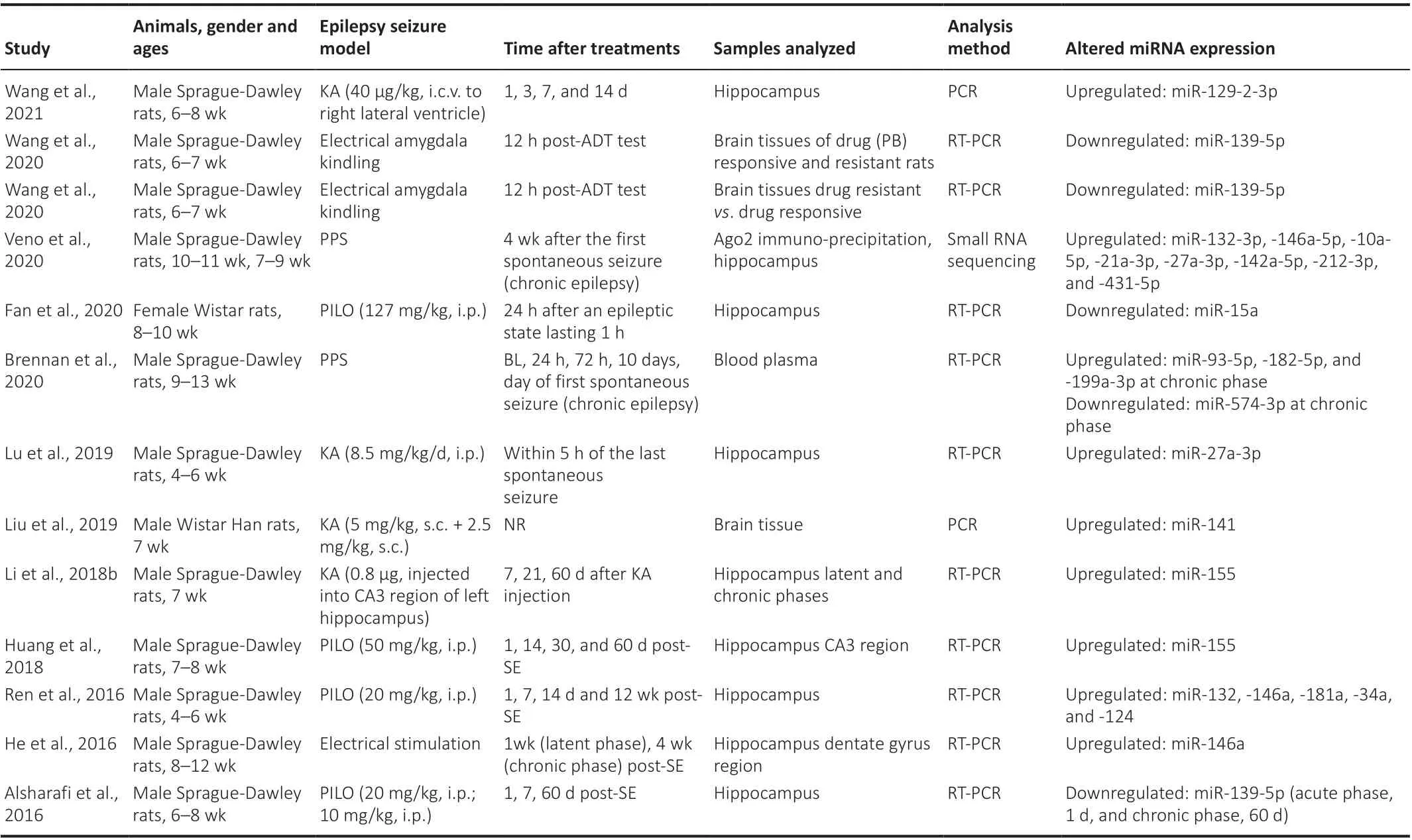
Table 2 |Alterations of miRNA expression in blood plasma and brain tissues in rat models of epilepsy
Modulation of microRNA expression using agomirs to enhance expression or antagomirs to inhibit expression was performed in some of the studies.In the mouse studies (Table 3), overexpression of miR-137 increased the latency to the first spontaneous recurrent seizure in mice treated by PILO or PTZ method and decreased induced seizures in mice treated by PILO (Wang et al.,2018).Overexpression of miR-219 delayed the onset of the first spontaneous recurrent seizure, decreased seizure severity, and alleviated abnormal EEG recordings in mice treated with KA by i.c.v.injection (Zheng et al., 2016).Suppression of several microRNAs was also found to have a positive effect on seizure behavior and these included miR-451 (Weng et al., 2020), miR-10a-5p, -21a-5p, -27a-5p, -142a-5p, and -431-5p (Venø et al., 2020), miR-155 (Fu et al., 2019), and miR-134 (Jimenez-Mateos et al., 2012).In the rat studies (Table 4), overexpression of miR-139-5p decreased neuronal damage in drug-resistant rats (Wang et al., 2020).Inhibition of several microRNAs had a positive effect on seizure behavior and/or reduced the loss of neuronal cells and these included miR-129-2-3p (Wang et al., 2021), miR-27a-3p (Lu et al.,2019), miR-155 (Huang et al., 2018), miR-134 (Reschke et al., 2017), miR-181a(Ren et al., 2016), and miR-146a (He et al., 2016).From the above mentioned findings, there are a considerable number of microRNAs whose expression has been shown to be altered in the hippocampus of mouse and rat models of epilepsy.Included among these were miR-132-3p, -146a-5p, -10a-5p, -21a-3p, -27a-3p, -142a-5p, -212-3p,-431-5p, and -155 which were upregulated in both mouse and rat models(Venø et al., 2020).Of these, suppression of miR-10a-5p, -21a-3p, -27a-3p,-142a-5p, -431-5p, -155, and -146a by antagomirs delivered i.c.v.decreased seizures and for several of these microRNAs had a neuroprotective effect(He et al., 2016; Huang et al., 2018; Venø et al., 2020).A combination of antagomirs against two or three of these microRNAs would be likely to have a much greater effect than a single antagomir.In addition, miR-137 and miR-219 were downregulated in mouse models (Zheng et al., 2016; Wang et al., 2018) and miR-139-5p was downregulated in a rat model (Wang et al.,2020).Using agomirs delivered to the hippocampus, overexpression of miR-137 and miR-219 decreased seizures in mouse models (Zheng et al., 2016;Wang et al., 2018) while overexpression of miR-139-5p decreased neuronal damage in drug-resistant rats (Wang et al., 2020).Downregulation of miR-139-5p in brain tissues was shown to distinguish drug (phenobarbital)-resistant rats from drug-responsive rats (Wang et al., 2020).Several agomirs of these downregulated microRNAs given together are likely to have a more pronounced effect on seizure behaviors.
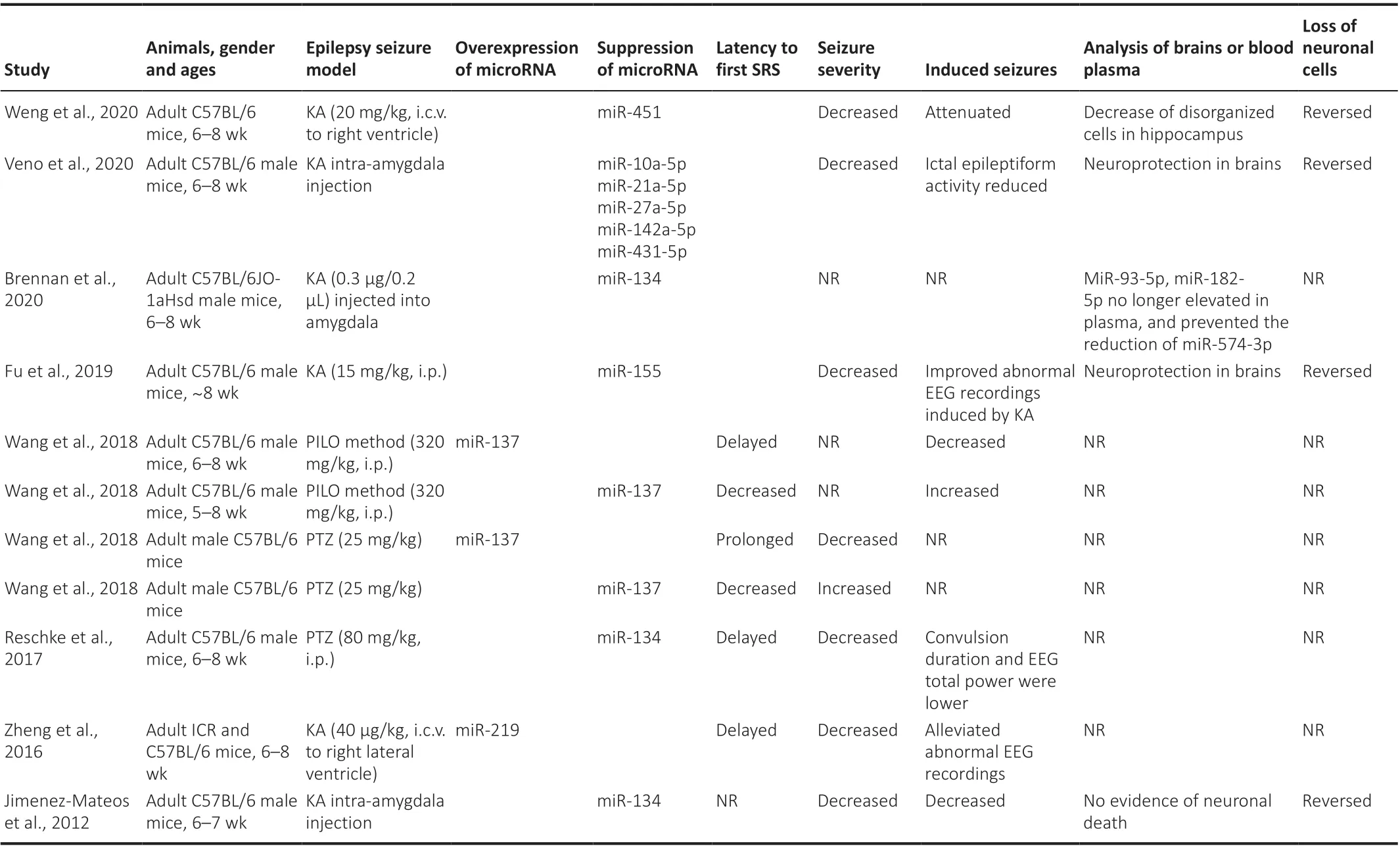
Table 3 |Changes in mouse models of epilepsy following overexpression or suppression of specific microRNAs
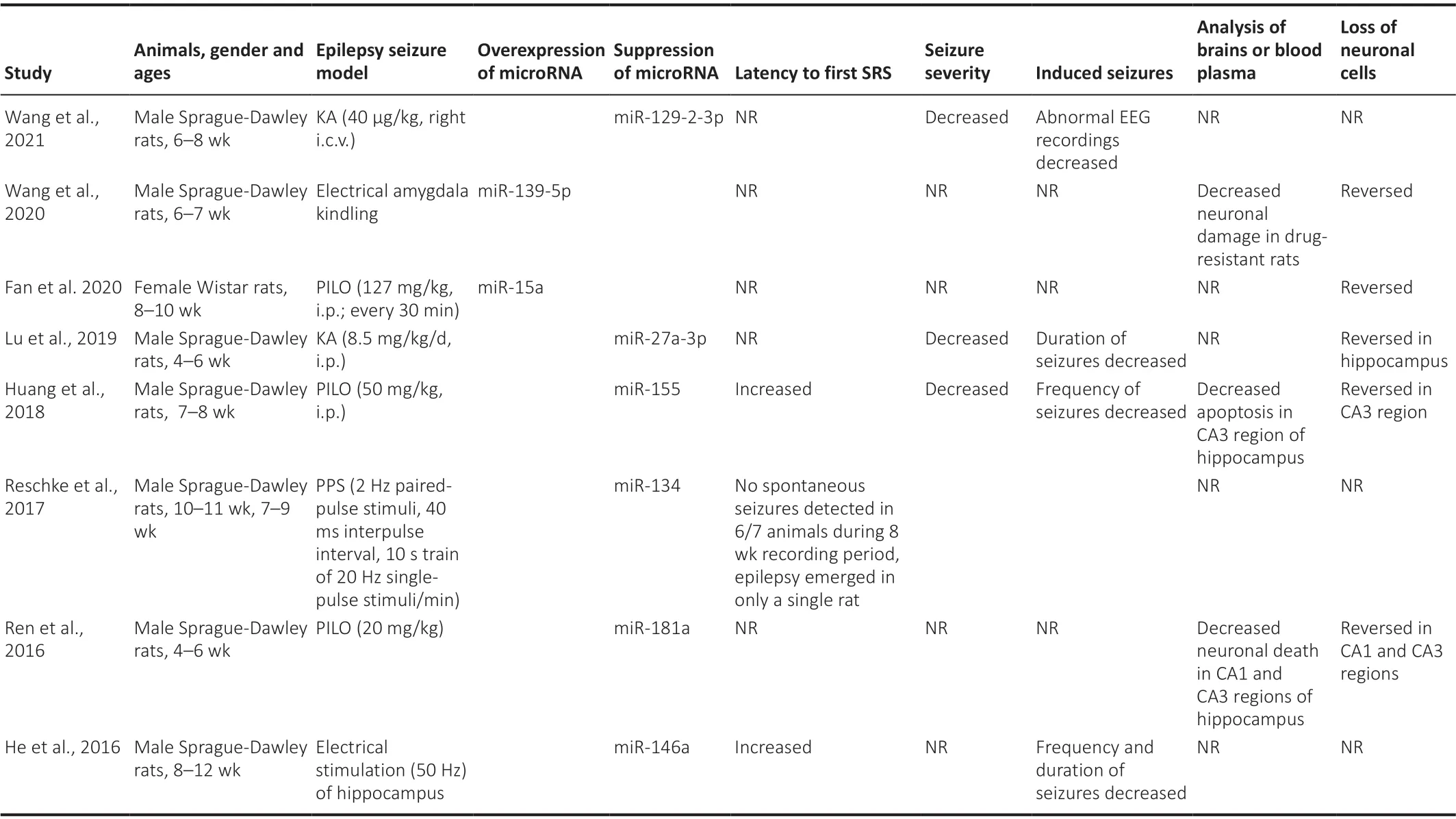
Table 4 |Changes in rat models of epilepsy following overexpression or suppression of specific microRNAs
Some of the studies have shown that inflammation is linked to epilepsy and modulation of microRNA expression levels can alter the levels of inflammatory cytokines and vice versa.For instance, the inflammatory factors IL-1β, IL-6, and TNF-α in the hippocampus tissues of the epileptic model group were significantly increased compared with the control group.MiR-27a-3p inhibitor significantly reduced the levels of IL-1β, IL-6, and TNF-α in hippocampaltissues of epileptic rats compared to the epileptic model group that received injection i.p.of KA only (Lu et al., 2019).Furthermore, when a miR-155 antagomir or antagomir control was injected i.c.v to epileptic rats 1 hour after SE, the hippocampal expression of TNF-α in the latent phase antagomir group was significantly downregulated compared with the latent phase antagomir control group or latent seizure group, which suggested that the miR-155 antagomir can inhibit TNF-α expression (Li et al., 2018b).In epileptic rats receiving i.c.v.injection of miR-155 antagomir or a scrambled nontargeting antagonist, TNF-α mRNA expression was decreased compared to the scrambled group (Huang et al., 2018).The expression levels of IL-1β, IL-6, and TNF-α were significantly decreased in epileptic rats that had received miR-15a mimic (Fan et al., 2020).To determine the ability of inflammation to regulate the expression of miR-146a in the hippocampus of epileptic rats, rats were injected with IL-1β in the chronic phase post-SE.MiR-146a expression was significantly upregulated compared with the control group (Li et al., 2018a).To confirm whether increased inflammation influences seizures in chronic epileptic rats, the rats were injected with IL-1β at week 7 (chronic phase) post-SE.IL-1β had a similar effect to miR-146a on seizures in the chronic epileptic rats.There was no obvious increase in convulsive motor seizures following the IL-1β injection, but an obvious increase in the abnormal waveforms detected by EEG monitoring (Li et al., 2018a).
With regard to possible targets and molecular mechanisms associated with dysregulated miRNAs in epilepsy models, proteomic data, RNA sequencing,and pathway analysis on predicted and validated targets of miR-10a-5p, -21a-3p, and -142a-5p has implicated derepressed TGF-β signaling as a shared seizure-modifying mechanism, with inhibition of the TGF-β signaling pathway occluding the antiseizure effects of antagomirs of these three miRNAs (Venø et al., 2020).Furthermore, upregulation of TNF-α mRNA in the CA3 region of the hippocampus was induced by SE in rats and Ant-155 decreased its level (Huang et al., 2018).TNF-α expression was downregulated in the latent phase 1 Ant-155 group of epileptic rats (Li et al., 2018b).Ant-155 caused an increase in rat Sestrin-3 expression, which is a gene known to counteract oxidative stress and may be one of the molecular links between brain damage and increased risk of seizures during damage by oxidative stress (Huang et al., 2018).In addition, miR-146a was shown to downregulate complement factor H expression in the hippocampus of epileptic rats (Li et al., 2018a).Suppression of complement factor H expression in rat hippocampus by stereotactic injection of LV-CFH-sh resulted in decreased latency of the first seizures and increased frequency and duration of seizures after SE (He et al.,2016).Interestingly, IL-1β expression was increased in epileptic rats (chronic phase) treated with miR-146a agomir, and could downregulate complement factor H expression (Li et al., 2018a).
In conclusion, a large number of microRNAs were found to be dysregulated in hippocampal tissues of mouse and rat models of epilepsy, and treatment with specific agomirs (mimics) or antagomirs (inhibitors) had the ability to decrease seizures.Where gender and age were reported, all the studies had used male mice and rats, mostly 6–8 weeks, apart from one that had used female rats,8–10 weeks (Fan et al., 2020).Further studies need to be performed using adult female and immature male and female animals (e.g., post-natal day 21).It would also be helpful to test the ability of specific agomirs and antagomirs to control seizure activity in a nonhuman primate model of epilepsy such as adult marmosets injected i.p.with PILO (Perez-Mendes et al., 2011) or cynomolgus monkeys given intrahippocampal injections of KA (Chen et al.,2013).
Author contributions:Both authors contributed equally and approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:No additional data are available.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain
- The role of fibronectin in multiple sclerosis and the effect of drug delivery across the blood-brain barrier

