From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
Xuan Wu, Jia-Rui Li, Yu Fu, Dan-Yang Chen, Hao Nie,*, Zhou-Ping Tang,*
Abstract Ischemic stroke is one of the most common causes of mortality and disability worldwide.However,treatment efficacy and the progress of research remain unsatisfactory.As the critical support system and essential components in neurovascular units, glial cells and blood vessels (including the bloodbrain barrier) together maintain an optimal microenvironment for neuronal function.They provide nutrients, regulate neuronal excitability, and prevent harmful substances from entering brain tissue.The highly dynamic networks of this support system play an essential role in ischemic stroke through processes including brain homeostasis, supporting neuronal function, and reacting to injuries.However, most studies have focused on postmortem animals, which inevitably lack critical information about the dynamic changes that occur after ischemic stroke.Therefore, a high-precision technique for research in living animals is urgently needed.Two-photon fluorescence laser-scanning microscopy is a powerful imaging technique that can facilitate live imaging at high spatiotemporal resolutions.Twophoton fluorescence laser-scanning microscopy can provide images of the whole-cortex vascular 3D structure, information on multicellular component interactions, and provide images of structure and function in the cranial window.This technique shifts the existing research paradigm from static to dynamic, from flat to stereoscopic, and from single-cell function to multicellular intercommunication,thus providing direct and reliable evidence to identify the pathophysiological mechanisms following ischemic stroke in an intact brain.In this review, we discuss exciting findings from research on the support system after ischemic stroke using two-photon fluorescence laser-scanning microscopy,highlighting the importance of dynamic observations of cellular behavior and interactions in the networks of the brain’s support systems.We show the excellent application prospects and advantages of two-photon fluorescence laser-scanning microscopy and predict future research developments and directions in the study of ischemic stroke.
Key Words: astrocytes; blood-brain barrier; calcium signaling; glymphatic system; ischemic stroke;microglia; network; remodel; two-photon fluorescence laser-scanning microscopy; vessels
Introduction
Ischemic stroke is a major cause of mortality and morbidity worldwide (GBD 2019 Stroke Collaborators, 2021).Currently, reperfusion therapy performed within the appropriate time window for rescuing neurons in the ischemic penumbra is recommended as the first-line treatment (Powers et al.,2019; Turc et al., 2019; Berge et al., 2021).However, many patients cannot undergo reperfusion therapy due to contraindications.Even with emergency reperfusion treatments, there is still a considerable portion of patients with poor prognoses (Bhaskar et al., 2018; Sun et al., 2018; Rabinstein et al., 2019; Wollenweber et al., 2019; Valkonen et al., 2022).Removing the initiating factor of ischemia alone without addressing the subsequent cascade responses is not enough to achieve ideal outcomes.
Due to a lack of understanding of the communication among neuronal networks, glial cells, and vessels, the efficiency of alternative proneurogenesis or neuroprotective therapy remains uncertain (Li et al., 2018;Shi et al., 2018; Bang and Kim, 2019; Hatakeyama et al., 2020; Xu et al.,2020; Paul and Candelario-Jalil, 2021).As the critical support system for the brain and essential components of the neurovascular unit, glial cells and vessels (including the blood-brain barrier [BBB]) maintain an optimal microenvironment for neuronal function (Ransohoff, 2016b).They provide nutrients, regulate neuronal excitability, and prevent harmful substances from entering brain tissue (Allen and Lyons, 2018; Verkhratsky and Nedergaard,2018; Caporarello et al., 2019; Garcia-Caceres et al., 2019; Liu et al., 2019;Sweeney et al., 2019; Schaeffer and Iadecola, 2021).The synchrony and coordination of neurons, glia, and blood vessels allow the brain to function well.Based on the idea that“structure determines function,”the functional network concept was first proposed for connective neurons.However, both neurons and the support system form functional networks, and the support system is involved in the regulation of neuronal networks (Kabba et al.,2018; Santello et al., 2019; Kirst et al., 2020; Schaeffer and Iadecola, 2021;Sun et al., 2021).These complex, highly heterogeneous, multicomponent,dynamic, and topologically heterogeneous networks of the support system in the brain ensure neuronal function and are involved in the development of neuropsychiatric disorders (Freeman, 2010; Chai et al., 2017; Iadecola,2017; Greenhalgh et al., 2020; Kirst et al., 2020; Schaeffer and Iadecola, 2021;Endo et al., 2022; Silvin et al., 2022).Direct and reliable information about the dynamic and subtle changes in the networks of the support system for the brain can only be acquired from living tissue (Schaeffer and Iadecola,2021).However, commonly used paradigms in ischemic stroke research do not provide high-level spatiotemporal observations in living systems (Table 1).It is difficult to reconstruct the dynamic changes in the networks of the support system from postmortem brain sections.Emerging fluorescent microoptical sectioning tomography techniques can be used to rebuild whole brain networks but fail to reflect dynamic network functions, such as blood flow direction and velocity.Thus, techniques that can be used to understand the dynamic changes in the support system during ischemic stroke are essential to explain the pathophysiological processes and identify potential therapeutic targets.In 1990, Denk et al.first presented two-photon fluorescence laserscanning microscopy (2PLSM; Figure 1), which has been widely used in live imaging.In this method, two long-wavelength photons are used to simultaneously excite one fluorescent probe from the ground state to an excited electronic state.Compared to conventional laser scanning confocal microscopy, 2PLSM uses infrared light (IR) for excitation, which greatly reduces absorption and scattering in biological tissues, allowing it to illuminate deepertissues.This imaging protocol restricts fluorescence and photobleaching to the focal plane, greatly reducing damage to specimens.In addition, a widefield photon detector without the use of pinholes recovers the scattered emission photons and further increases imaging depth.Thus, 2PLSM can be used to observe pathophysiological processes down to 600–800 μm in livingtissue, depending on the degree of absorption and scattering and the powerof the femtosecond laser (Helmchen and Denk, 2005; Diaspro et al., 2006).Nowadays, modern 2PLSM systems have now achieved imaging depths of up to 1000 μm throughout almost the entire gray matter of the mouse neocortex(Theer et al., 2003).

Table 1 |Commonly used ischemic stroke study paradigms
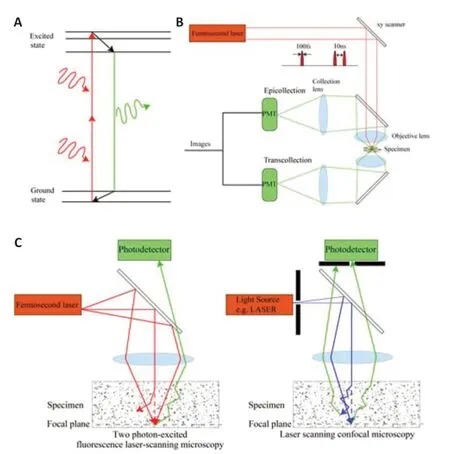
Figure 1|Simplified imaging strategy of 2PLSM and comparison of 2PLSM with laser scanning confocal microscopy.
By introducing different kinds of fluorescence probes, 2PLSM can be used to observe dynamic changes in morphologies and functions in cells in living brains before and after ischemic stroke (Ricard et al., 2018).By combining 2PLSM with other techniques, researchers can obtain multimodal,in situ,time-resolved, and spatial-sequential brain imaging data and information in living animals.2PLSM imaging turns static research into dynamic research,revealing structural and functional changes and reconstructing the functional networks in ischemic stroke, which can help identify new therapeutic targets and refine the time window for ischemic stroke.In this review, we first show the simple steps required to perform 2PLSM imaging.We mainly discuss the major findings from 2PLSM-based research on the support system during ischemic stroke, focusing on interactions between glial cells, vessels, the BBB, and neuronal networks.We hope this review will help researchers gain a better understanding of the pathophysiological processes of the support system after ischemic stroke.
Search Strategy and Selection Criteria
Studies cited in this review were obtained from searching the PubMed database (https://pubmed.ncbi.nlm.nih.gov) using the following keywords:stroke, ischemia, ischemic, astrocyte, microglia, vessel, blood-brain barrier,two-photon microscopy, multiphoton microscopy, reprogram, and glymphatic system.Studies cited in this review were published between 1989 and 2022.The literature search was completed by the author YF on July 25, 2022.
General Procedures for In VivoTwo-Photon Fluorescence Laser-Scanning Microscopy Imaging of Experimental Ischemic Stroke
The first procedure forin vivo2PLSM imaging of experimental ischemic stroke is the preparation of the cranial window for imaging in living animals.The thick and inhomogeneous structures of intact skulls cause severe optical aberrations and scattering noise, which greatly reduces image quality(Helmchen and Denk, 2005; Yoon et al., 2020).Thus, craniotomy or thinnedskull cranial window surgery using dental drills is needed (Xu et al., 2007;Marker et al., 2010; Yang et al., 2010).Different types of cranial windows are shown in Figure 2A–C.In general, craniotomy enables researchers to perform operations on the brain parenchyma, such as direct dye coverage and stereotactic injection of viruses or substances.Acute cranial windows can be combined with electrophysiology to measure neuronal electrical signals with electrodes simultaneously with 2PLSM imaging.Compared with acute or chronic craniotomy, the thinned-skull cranial window minimizes damage to brain tissue at the expense of imaging quality.Thinned-skull cranial window surgery is widely used, especially for imaging sensitive cells, such as microglia, or for structures that do not require high precision, such as imaging amyloid plaques (Xu et al., 2007; Yang et al., 2010).The second procedure for imaging of experimental ischemic stroke is visualization the components of interest in the brain, which is the selection of the fluorescent labeling strategies (Trachtenberg et al., 2002).A schematic of 2PLSMin vivoimaging of neurons and support systems is shown in Figure 2D.The fluorescent labeling strategies can be roughly divided into cell morphology and cell function labeling, namely, labeling the soma and processes to show cell morphology or labeling important intracellular functional ions, such as intracellular calcium,to show cell function.The commonly used fluorescent labeling methods are shown in Table 2 (Hartmann et al., 2015a; Hierro-Bujalance et al., 2018;Tong et al., 2021).The third procedure is the selection of specific imaging strategies using a 2PLSM system, including different excitation wavelengths different fluorescent labels as well as frame acquisition rates and timescales for different components.This aspect requires extensive experience and practical tests.The relevant parameters used are summarized in other articles(Benninger and Piston, 2013; Fumagalli et al., 2014; Ricard et al., 2018;Adhikari et al., 2021; Leben et al., 2022).The fourth procedure is inducing the experimental ischemic stroke.There are two commonly used experimental ischemic stroke models forin vivo2PLSM imaging, middle cerebral artery occlusion (MCAO) and photothrombotic stroke (PT).A schematic diagram and images ofin vivo2PLSM imaging for MCAO and PT are shown in Figure 2E–H.MCAO is a widely used model of experimental ischemic stroke.To simulate stroke infarction and reperfusion, a single filament is introducedinto the internal carotid artery from the external carotid artery and advanced to block the origin of the middle cerebral artery, blocking blood flow, and left in place for a period of time before being removed (Longa et al., 1989;Chiang et al., 2011).MCAO can form the ischemic penumbra, but the infarct area is large and the corresponding damage to the animals is also large(Sigler and Murphy, 2010; Krafft et al., 2012).In PT, the infarct is induced by the systematic application of a photosensitive dye (usually rose bengal)and illumination with light of a specific wavelength to block focal cortical blood flow (Kim et al., 2000; Labat-gest and Tomasi, 2013).This approach results in a rapid, stable, sharp-edged infarct suitable for studies of cortical plasticity; however, the infarct is permanent and lacks the ischemic penumbra(Nishimura et al., 2006; Zhang and Murphy, 2007; Krafft et al., 2012; Labatgest and Tomasi, 2013; Li and Zhang, 2021).

Table 2 |The commonly respective used fluorescent labeling methods for vessels, astrocytes and microglial cells
Table 2 |Continued
Vessel Dysfunction and Recovery after Ischemic Stroke
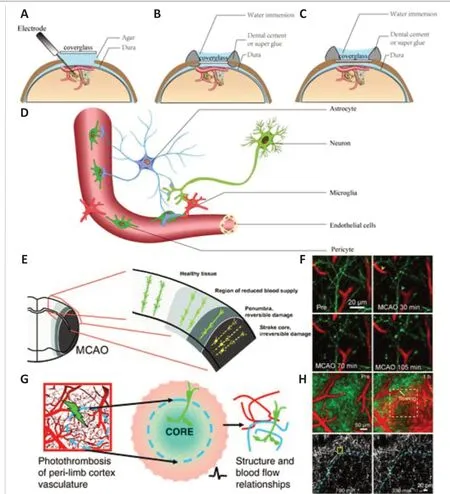
Figure 2|Schematic ofin vivo2PLSM imaging for experimental ischemic stroke.
Blood vessels, as pipelines for blood circulation, supply neurons and glial cells with essential nutrients, carry away metabolic waste, and form the barrier between the blood and cerebral parenchyma.In intact brains, blood vessels constitute a unique hierarchical 3D network (Kirst et al., 2020).Dysfunction and recovery in hemorheology, hemodynamics, and architecture of the vessel network significantly affect the pathological process of ischemic stroke and are associated with prognosis.In the acute phase of ischemic stroke, reperfusion therapy saves neurons in the ischemic penumbra and reduce mortality; this is regarded as the first-line therapy in ischemic stroke (Powers et al., 2019; Turc et al., 2019; Berge et al., 2021).However, timely and effective recanalization is not always accompanied by downstream tissue reperfusion, which has been described as the“no-reflow phenomenon”(Kloner, 2011; Bai and Lyden,2015).Good collateral circulation is an independent predictor of improved outcomes and is a potential therapeutic target (Vernieri et al., 2001; Miteff et al., 2009; Shuaib et al., 2011; Vagal et al., 2018; Guglielmi et al., 2019; Broocks et al., 2020).In the recovery phase of ischemic stroke, angiogenesis in the peri-infarct region has been correlated with longer survival times in patients and coupled with neuronal remodeling (Kanazawa et al., 2019; Hatakeyama et al., 2020).Therefore, detailed intravital information on post-stroke hemodynamics, hemorheology, and vascular network architecture can help to gain a comprehensive understanding of the pathophysiological mechanisms of ischemic stroke and to develop corresponding therapies.By applying intravenous vascular tracers to label blood plasma, researchers can efficiently study the hemorheology, hemodynamics, and structure of hierarchical vessels(Figure 3A–C).Using transgenic fluorescent markers in animals, researchers can further image different cellular components of microvessel networks(Table 2).In this section, the network of vessels mainly refers to the cerebral microvessel system.
Hemodynamics and hemorheology in the acute phase of ischemic stroke
The strong and rapid compensatory capacity and plasticity of the vascular network against local ischemia are important for the survival of neurons in the infarct core in the acute phase of ischemic stroke.Schaffer et al.(2006)found that, after photothrombotic occlusion to individual pial arterioles,the blood flow was rapidly re-established at the first branch downstream without neuronal apoptosis or peri-photothrombosis, revealing a robust redistribution and compensatory ability of blood flow after surface arteriole occlusion.Shih et al.(2009) found that the MCAO model caused red blood cell flow reversal in surface arterioles in the penumbra to ensure blood flow in penetrating arterioles, which compensated for the incomplete recovery of blood flow during reperfusion.However, this compensatory capacity is related to the structural level of the occluded vessel, and not all levels can be compensated by endogenous mechanisms of blood flow after vascular occlusion.Using 2PLSM, researchers can obtain cortical vascular network structures, classify vascular hierarchy, and target vessels for embolization (the 3D network structure of cortical vasculature is shown in Figure 3A).They can identify the surface communication network, the subsurface microcirculation network, and penetrating arterioles that connect the two.After occlusion of penetrating arterioles, nearby penetrating and surface arterioles did not dilate, and there was no restoration of blood flow in occluded penetrating arterioles, although the capillaries directly downstream from the occluded arteriole dilated (Nishimura et al., 2007; Nishimura et al., 2010).To further assess the response of different levels of vessels and collateral blood flow to focal cortical ischemia, Luo et al.(2017) further usedin vivo2PLSM to observe interactions between the surface and subsurface microcirculatory networks after ischemia.They performed five different combinations of occlusion of the target arterioles, their collateral surface vessels, and penetrating arterioles(Luo et al., 2017).Afterin vivostaining of dead cells, behavioral tests, and histological analysis, they observed protective roles of collateral flow within the subsurface microcirculation network in neural circuits in the ischemic penumbra (Luo et al., 2017).They first demonstrated that collateral blood vessels at different levels of the endogenous cerebrovascular hierarchy compensated for decreased blood flow during ischemia by penetrating arterioles (Luo et al., 2017).These findings demonstrated the important compensatory function of collateral blood flow during the acute phase of ischemic stroke and highlighted the different responses and different roles of vessels at different levels of the vascular network during acute ischemic conditions.However, the specific compensatory mechanisms of the cerebral arteriole system in response to ischemic stroke is not clear and may be related to the response of precapillary sphincters, smooth muscle cells, pericytes, and even astrocytes to hypoxia and diffuse depolarization as shown in previous studies (Chuquet et al., 2007; Fernández-Klett et al., 2010; Mishra et al., 2016;Alarcon-Martinez et al., 2020; Hartmann et al., 2021; Meza-Resillas et al.,2021; Zambach et al., 2021).
To enhance the compensatory capacity of the collateral circulation and neural remodeling, remote ischemic conditioning is considered an effective treatment (Liao et al., 2019; McDonald et al., 2021).Remote ischemic preconditioning is a kind of remote ischemic conditioning which involves inducing acute transient ischemia in the distant limb after the onset of ischemic stroke but before reperfusion treatment (Ma et al., 2020a, b).Ma et al.(2020a, b) used aged rats to mimic human ischemic stroke and found that using remote ischemic preconditioning before experimental ischemic stroke could enhance collateral flow by preventing the stroke-induced narrowing of pial arterioles, which reduced the infarct area.However, the mechanisms of enhanced collateral blood flow induced by remote ischemic preconditioning was not further discussed in this study.The role of humoral factors needs to be further studied.
Recovery of hemodynamics, hemorheology, and vascular network architecture after ischemic stroke
Dynamic changes seen with embolismsin vivoare important because thromboses are the main initiator of ischemic stroke and are thought to be closely related to revascularization and prognosis (Powers et al., 2019; Turc et al., 2019; Berge et al., 2021).In 2010, usingin vivo2PLSM, Lam et al.(2010)first observed dynamic changes in microemboli in cerebral microvessels in living mouse brains.They used fluorescently labeled microemboli and found that embolus extravasation is an alternative recanalization mechanism when hemodynamic forces and the fibrinolytic system fail to clear the thrombus.The process of embolus extravasation occurs 2–7 days after thrombosis and is mediated by a novel mechanism of microvascular plasticity (Figure 3G; Lam et al., 2010).The rate of embolus extravasation is significantly reduced in aged mice or after inhibiting matrix metalloproteinase 2/9 activity (Lam et al., 2010).El Amki et al.(2020) induced a fibrin rich clot thrombin in the middle cerebral artery (MCA) followed by intravenous t-PA thrombolysis to mimic stroke and intravenous thrombolytic therapy, which differed from the classical MCAO model using filament to induce ischemia.They fluorescently labeled neutrophils, usedin vivo2PLSM to image the distal capillary flow after recanalization in mice and demonstrated that the no-reflow phenomenon after reperfusion therapy might be due to cortical microvascular occlusion caused by neutrophils (El Amki et al., 2020).Thesein vivo2PLSM studies, using fibrin thrombi or cholesterol emboli, closely simulated the pathophysiological process of human ischemic stroke, which enabled researchers to dynamically observe changes in embolismin vivowith high spatiotemporal resolution.By monitoring blood flowin vivoafter PT,Schrandt et al.(2015) tracked long-term vascular changes and found that a flow deficit remained even 35 days after occlusion, suggesting that more time was necessary for full perfusion recovery.
Endothelial cells are indispensable components of the vasculature, and labeling endothelial cells rather than plasma can enable imaging of the whole vascular system without missing any capillaries (Williamson et al., 2020).Williamson et al.(2020) used transgenic mice expressing fluorescent proteins in endothelial cells to observe changes in the microvessel system after ischemic stroke (Figure 3D).Usingin vivo2PLSM and multi-exposure speckle imaging, they visualized the processes of structural vascular plasticity and the reconstruction of peri-infarct blood flow.They associated the rebuilding of blood vessels with neuronal network function recovery based on 2PLSM imaging of microvessels, multi-exposure speckle imaging of blood flow, and behavioral testing.They demonstrated that the extent of vascular structural plasticity predicted local blood flow reconstruction, which predicted the recovery of neuronal function.
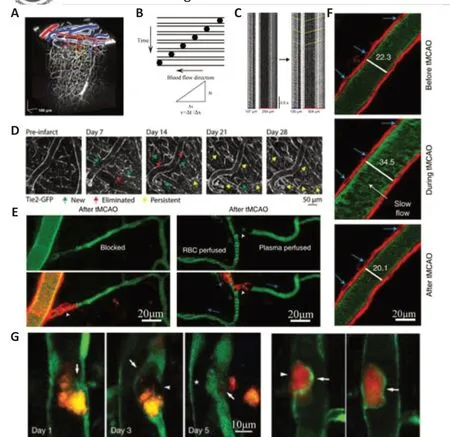
Figure 3|Vessels observed by 2PLSM.
Breakdown of the BBB and the roles of peripherally derived cells
The BBB is a critical diffusion barrier between the brain parenchyma and cerebral capillaries that can prevent the influx of most toxic substances and provide essential nutrients (e.g., oxygen and glucose) to maintain brain homeostasis (Liebner et al., 2018; Vanlandewijck et al., 2018; Sweeney et al., 2019; Cheng et al., 2022).The BBB mainly comprises endothelial cells and tight junctions, astrocyte endfeet, and pericytes (Liebner et al., 2018;Sweeney et al., 2019; Profaci et al., 2020; Zou et al., 2021).To date, all of these components are considered to be indispensable to maintain the integrity and function of the BBB (Liebner et al., 2018; Caporarello et al.,2019; Heithoff et al., 2021).BBB dysfunction is commonly involved in the pathological processes that occur during the acute phase of ischemic stroke and is associated with ischemic stroke outcomes (Lasek-Bal et al., 2019; Li et al., 2019b; Bernardo-Castro et al., 2020).The integrity of the BBB is essential for maintaining homeostasis and supporting the function of neuronal and glial networks, which can be easily represented by permeability to fluorescent dyes duringin vivo2PLSM (see detailed information of calculation methods in Figure 4).In this section, we focus on the roles of endothelial cells and pericytes in the breakdown of the BBB and the roles of peripherally derived immune cells in ischemic stroke, while the role of astrocytes in maintaining the BBB is detailed in the section“Roles of communication between astrocytes and multiple cell types in the acute phase of ischemic stroke”.
Researchers usingin vivo2PLSM found that the PT model was more inclined to produce early BBB injury than MCAO, revealing that the two commonly used models of experimental ischemic stroke may differ in their mechanisms(Frederix et al., 2007; Bragin et al., 2016).This makes photochemical embolization a useful tool to study the pathological mechanisms of BBB breakdown.However, whether the pathophysiology of BBB damage caused by thromboembolism and that caused by direct photodamage are different remains to be studied (Frederix et al., 2007; Kleinschnitz et al., 2008; Labatgest and Tomasi, 2013; Bragin et al., 2016; Cotrina et al., 2017).
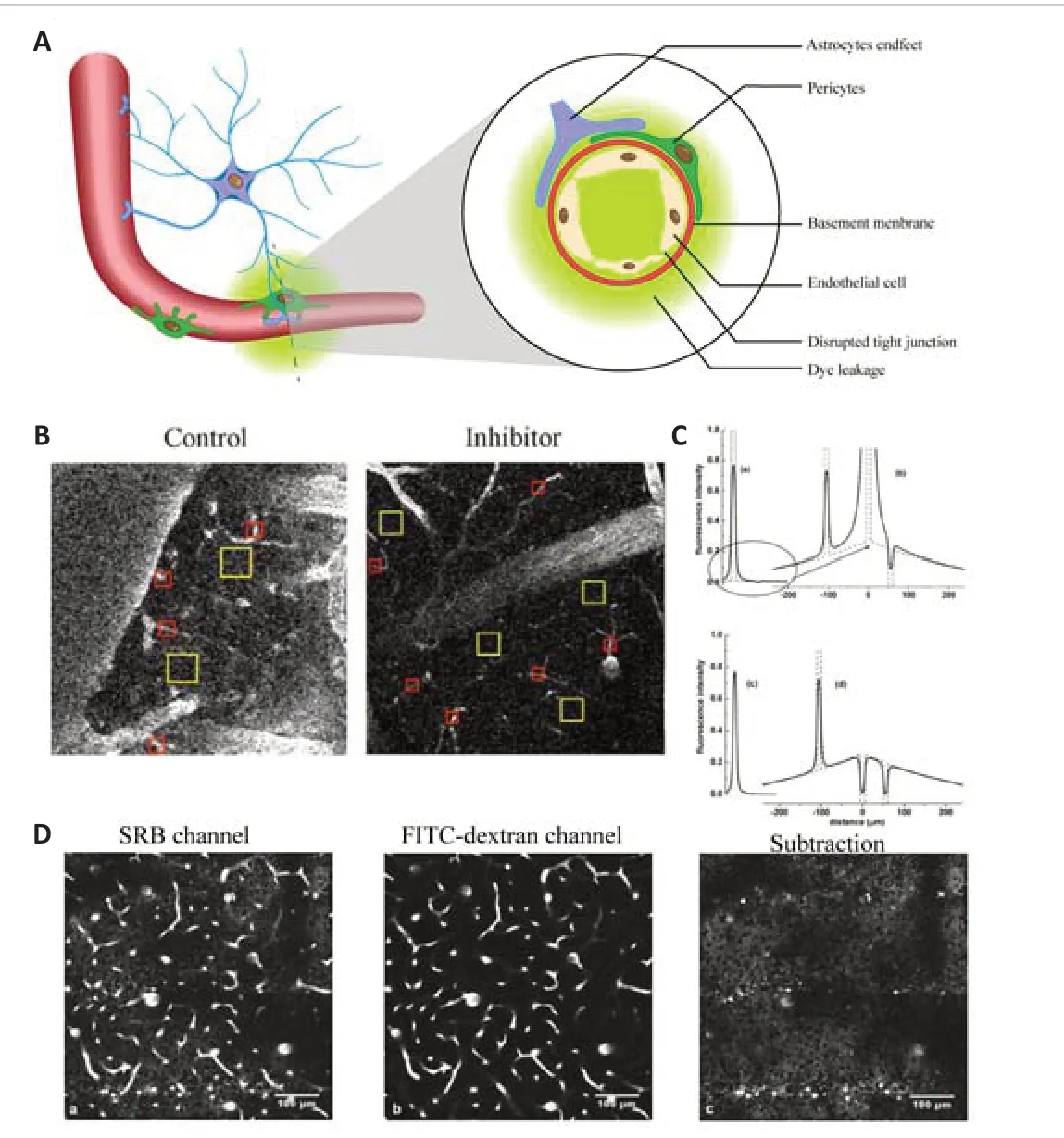
Figure 4|Calculation of BBB permeability.
Endothelial cells and tight junctions play an essential role in maintaining the integrity of the BBB.Knowland et al.(2014) used Tg eGFP-Claudin5 transgenic mice to label tight junctions between endothelial cells (Knowland et al., 2014).They found that the recruitment of transcellular and paracellular pathways of endothelial cells, rather than tight junction breakdown, mediated earlystage BBB dysfunction in the transient MCAO experimental model (Knowland et al., 2014).This discovery revealed a new mechanism of BBB damage after ischemic stroke.In addition, Ernesto Caballero-Garrido et al.(2015) found that injection of an inhibitor of miR-155, a potential regulator of endothelial morphogenesis, could reduce BBB permeability and increase red blood cell flow in a distal MCAO model.
Regarding pericytes, the soma of pericytes is immobile, while the processes exhibit dynamic extension and retraction.After selective ablation of individual pericytes, neighboring pericytes extend their processes to contact uncovered regions of the endothelium (Berthiaume et al., 2018b).Pericyte-labeled transgenic mice were imaged usingin vivo2PLSM to examine the relationship between pericytes and the integrity of cortical capillaries after PT (Underly et al., 2017; Berthiaume et al., 2018a, b; Underly and Shih, 2020).This showed that, during the acute phase of PT, matrix-metalloproteinase-9 synthesis in pericytes mediated tight junction injury, as reflected by greater leakage of fluorescent dye in regions where pericyte somata adjoined the endothelium(Underly et al., 2017; Berthiaume et al., 2018a, b; Underly and Shih, 2020).Further study of the related molecular mechanisms showed that mice with pericyte-specific deletion of SENP1, a protein with reverse SUMOylation function, promoted the formation of cerebral microvascular thrombosis faster than that observed in wild-type mice and exacerbated poststroke neuronal damage after PT (Sun et al., 2020).
Peripherally derived cells that infiltrate into the brain parenchyma are also largely involved in the post-stroke pathophysiology (Jayaraj et al., 2019;Yang et al., 2019; Cai et al., 2022; Wicks et al., 2022).Combining intravital 2PLSM with flow cytometry and quantitative real-time PCR, Neumann et al.(2018) described the dynamics of tdTomato-labeled neutrophils and GFPlabeled microglia after PT in bone marrow chimeric mice.A cross of CX3CR1-GFP and LysM-eGFP mice was used to simultaneously image neutrophils and microglia.They found that neutrophils rapidly entered the brain parenchyma from the activated site of endothelial cells in ischemic stroke and mediated the microglial immune response (Neumann et al., 2018).Systemic blockade of VLA-4 could effectively reduce the entry of neutrophils and reduce postischemic injury (Neumann et al., 2018).This study confirmed neutrophil infiltration into the brain parenchyma and long-term contact with microglia(Neumann et al., 2018).However, the lethal irradiation used may destroy the BBB and be a limited reflection of the real pathophysiological processes of ischemic stroke (Li et al., 2004).
For imaging specific immune cells (T cells and B cells) after stroke, the observation time must be relatively prolonged.Using sagittal brain slices from adoptive lymphocyte transfer mice and hCD2-GFP transgenic mice after ischemic stroke, Ortolano et al.(2010) performed in situ real-time T-cell imaging in ischemic stroke.In 2011, Fumagalli et al.further used hCD2-GFP transgenic mice to exhibit GFP-labeled T cells combined within vivomultiphoton microscopy to visualize, define, and quantitatively analyze the movement and behavior of T cells.Li et al.(2017) used intravital 2PLSM imaging and showed that C–C chemokine receptor type 5 was critical for regulatory T cells to dock in the injured vessel wall and interact with blood-derived neutrophils/macrophages after MCAO.In vivoandin vitroexperiments showed that C–C chemokine receptor type 5 was important for regulatory T cells to reduce BBB damage after ischemic stroke.Ortega et al.(2020) used whole-brain volumetric serial two-photon tomography and a custom-developed image analysis pipeline to visualize and quantify B cell diapedesis throughout the brain in B cell adoptive transfer mice after temporary MCAO.
Dual Roles of Astrocytes in Ischemic Stroke
Astrocytes, highly heterogeneous glial cells in the brain, tile the entire CNS,form interconnected astrocytic networks, and interact with neurons and blood vessels (Nedergaard et al., 2003; Volterra and Meldolesi, 2005; Perea et al., 2009; Santello et al., 2019).Astrocytes assist in maintaining brain tissue homeostasis and supporting neurons (Liddelow and Barres, 2017; Verkhratsky and Nedergaard, 2018; Santello et al., 2019).Single-cell sequencing and transcriptome sequencing have shown that astrocytes transform into protective reactive astrocytes after ischemic stroke and are characterized by upregulated neurotrophic factors and extracellular matrix-associated protein expression (Zamanian et al., 2012; Liddelow et al., 2017).However,the exact impact of reactive astrocytes and the astrocytic scar remains controversial (Diniz et al., 2019; Yang et al., 2020; Zhou et al., 2020).On the one hand, reactive astrocytes regulate the extracellular matrix and form the astrocytic scar after ischemic stroke, which can isolate and limit the harmful environment of the infarction core.On the other hand, astrocytic scars may impair axonal regeneration and neuronal plasticity (Pekny et al., 2014;Sun et al., 2019; Shen et al., 2021).Using 2PLSM, researchers can observe the longitudinal morphological and functional changes in astrocytes; the communication among astrocytes, neurons, and vessels; and the influence of astrocytes on neuronal survival and blood vessel rebuilding after ischemic stroke.Thus, we can learn more about the dual roles of astrocytes in ischemic stroke and their effects on outcomes.
Roles of astrocytes in the acute phase of ischemic strokeChanges in astrocyte behaviors
2PLSM has been used to investigate the dynamic morphological and positional changes in astrocytes after ischemic stroke.Using anin vivocardiac arrest model andex vivooxygen/glucose deprivation (OGD) model in living cortical slices, Risher et al.(2009, 2012) found that swollen astrocytes are the primary sources of cytotoxic cerebral edema after ischemic stroke(Risher et al., 2009, 2012).They found that the spreading depolarization (SD)mediated the volume increase in astrocytes, and astrocyte volume changes could be reversed, possibly due to the distribution of aquaporin 4 (AQP4)protein on astrocytes (Risher et al., 2012).However, Rakers et al.(2017)used AQP4-knockout transgenic mice and found that AQP4 did not affect SDrelated edema of astrocytesin vivo.These findings illustrated a contribution of the astrocyte volume increase in post-stroke cell-derived edema, but the mechanisms of this volume increase were likely unrelated to AQP4.Although this water channel protein is specifically expressed in astrocytes, it is mainly densely expressed on the endfeet and is more likely to be associated with the maintenance of the BBB and has a limited relationship with astrocyte volume regulation (Manley et al., 2004; Friedman et al., 2009; Akdemir et al., 2014;Yao et al., 2015; Heithoff et al., 2021).
Roles of communication between astrocytes and multiple cell types in the acute phase of ischemic stroke
Traditionally, astrocytes are considered to be electrically silent cells.However,recent studies have found that the critical role of multiple ion channels in modulating cell membrane potential in astrocytes (McNeill et al., 2021;Armbruster et al., 2022).Using calcium imaging techniques, researchers have found that astrocytes frequently bidirectionally communicate with surrounding glia, neurons, and microvessels, and this communication process can be reflected by changes in intercellular signaling in astrocytes (Perea et al.,2009).Astrocytes integrate and respond to signals from neurons and vessels.2PLSM is well suited for tracing dynamic intercellular signaling changes in astrocytes through calcium imaging techniques in live tissue.Interestingly, the[Ca2+]itransients in the processes of astrocytes differ from those in the soma in terms of spatiotemporal characteristics (Bazargani and Attwell, 2016).[Ca2+]itransients in astrocytic processes originate from two pathways: extracellular calcium influx through ion channels (i.e., transient receptor potential channels) and release from intracellular stores.[Ca2+]itransients in the soma,on the other hand, depend largely on release from intracellular stores, which is mainly mediated by IP3R2 signaling (Bazargani and Attwell, 2016; Bindocci et al., 2017).The [Ca2+]itransients in astrocytes may contribute to the Ca2+-dependent release of glial glutamate after ischemic stroke, which mediates excitotoxicity in neuronal networks (Takano et al., 2009; Siracusa et al., 2019;Kirdajova et al., 2020).
Bidirectional communication between astrocytes and neurons received attention as early as the 1990s, and the term“tripartite synapse”was proposed to describe this bidirectional communication (Cornell-Bell et al.,1990; Charles et al., 1991).Changes in communication between astrocytes and neurons are worthy of exploration.
Ding et al.(2009) usedin vivo2PLSM imaging and found that, during the acute phase of PT, [Ca2+]itransients increased synchronously in astrocytes and propagated as waves in the astrocytic network.Using BAPTA to selectively inhibit [Ca2+]itransients in astrocytes could significantly reduce infarct volume.Additional studies have shown that IP3R2 receptor knockout mice and TRPV4 knockout mice, which exhibit reduced internal calcium release and external calcium inflow in astrocytes, respectively, also exhibited reduced glia-dependent glutamate release and performed better after experimental ischemic stroke (Ding et al., 2009; Dong et al., 2013; Rakers and Petzold,2017; Rakers et al., 2017).Moreover, in aged mice, spontaneous Ca2+activity in astrocytes and the infarct area were higher than those in adult mice after ischemic stroke, while spontaneous Ca2+activity in neurons was unchanged (Figure 5A) (Fordsmann et al., 2019; Murmu et al., 2019).These studies showed that, after experimental ischemic stroke, [Ca2+]iincreased in astrocytes, which may aggravate neuronal excitotoxicity through gliadependent glutamate release (Ding et al., 2009; Dong et al., 2013; Rakers and Petzold, 2017; Rakers et al., 2017; Fordsmann et al., 2019; Murmu et al.,2019).Calcium waves in astrocytes and peri-infarct depolarizations are shown in Figure 5B.Shinotsuka et al.(2014) used acute cortical slices under OGD and showed differing results regarding the calcium wave in astrocytes.They found that the astrocytic gap junction network acted as a buffer for intercellular calcium fluctuations in neurons during the acute phase of ischemia.After blocking gap junctions between astrocytes in mouse cortical slices under OGD, the SD occurred earlier (Shinotsuka et al., 2014), showing a protective effect of astrocyte networks on neurons.However, inex vivostudies, the severity of ischemia and hypoxia in brain tissue may differ from that inin vivostudies, affecting the roles of astrocytes after ischemia.
After quantifying the contacts between astrocytes and vesselsin vivousing 2PLSM, almost all cortical astrocytes were in direct contact with blood vessels, which provided the structural basis for synapse-astrocyte-vessel communication (Hӧsli et al., 2022).Astrocytes integrate signals from synapses and vessels and are involved in the functioning of the neurovascular unit,which has been widely recognized in healthy live brains (Zonta et al., 2003;Perea et al., 2009; Bazargani and Attwell, 2016).2PLSM can simultaneously observe the [Ca2+]ifluctuations in astrocytes and vessels diameters and selectively release Ca2+from astrocytes using photolysis of the Ca2+cage DMNP-EDTA.Studies in normal live brain slices showed that elevated [Ca2+]iin astrocytes could regulate the diameter of arterioles adjacent to the endfeet to modulate blood flow for adaptation to neuronal activity, mainly through the phospholipase A2-arachidonic acid pathway, which produces metabolites prostaglandin and epoxyeicosatrienoic acid for vasodilation and 20-hydroxyeicosatetraenoic acid for vasoconstriction (Zonta et al., 2003;Mulligan and MacVicar, 2004; Metea and Newman, 2006; Gordon et al.,2008; Rosenegger et al., 2015).However, otherin vivophotolysis experiments using caged Ca2+in astrocytic endfeet adjacent to cortical penetrating arterioles showed that the increase of [Ca2+]iin astrocyte endfeet was related to vasodilation via release of prostaglandin messengers to ensure basal blood flow in the brain (Takano et al., 2006).Whether the elevated [Ca2+]iin astrocytes causes vasodilation or constriction may be related to the preexisting tone of the vessel and the astrocyte microenvironment (Gordon et al.,2008; Attwell et al., 2010; Rosenegger et al., 2015).Thus, the specific role of astrocytes in the regulation of the neurovascular unit during the acute phase of ischemic stroke needs to be better determinedin vivorather than ex vivo;intravital experiments are thought to more precisely show communication between astrocytes, neurons, and mural cells (Grubb et al., 2021).Chuquet et al.(2007) imaged the neurovascular unitin vivoduring KCl and cardiac arrestinduced SD and found that the hyperacute vasoconstriction of arterioles that occurred after SD was associated with fast [Ca2+]iwaves in astrocytes,mediated by a phospholipase A2 derivative.This vasoconstriction of arterioles mediated the hyperemic phase and might have contributed to endogenous compensation after ischemic stroke (Chuquet et al., 2007).However, this study partially ignored the contribution of mural cells after ischemic stroke,which construct the walls of blood vessels and are thought to be the crucial components for regulating vasomotor tone (Grubb et al., 2021).Therefore,labeling mural cells and calcium signaling in mural cells as well as clarifying the sequential order of the communication between astrocytes and mural cells and responses to ischemia are essential.Moreover, it is necessary to differentiate the vascular hierarchy, as the structures in the arterioles and capillaries are different (Grubb et al., 2020).Mishra et al.(2016), usingin vivo2PLSM imaging, showed that the role of astrocytes in the regulation of hemodynamics was related to the vascular level; astrocytes seemed to regulate capillaries but not arterioles.They applied calcium coupling agents and several receptor inhibitors and simultaneous imaging of astrocyte calcium signals and vessel diameters both in acute brain slices andin vivo(Mishra et al., 2016).The increased [Ca2+]iin astrocytes mediated capillary dilation through ATP-gated channels P2X purinoceptor 1, but the increased [Ca2+]iin astrocytes did not mediate arteriole dilation (Mishra et al., 2016).However,there is a lack of studies related to astrocyte-vessel communication and the roles of astrocytes in regulating hemodynamics in experimental ischemic stroke.
Moreover, astrocytes are the essential component of the BBB (Heithoff et al., 2021).However, Tóth et al.(2019) found no changes in astrocyte-vessel connections and no plasma extravasation over two hours of time-lapse 2PLSM imaging in PT and bilateral carotid ligation.Only photochemical embolization,a direct vascular injury, resulted in fluid extravasation (Tóth et al., 2019).This surprising result suggested that morphological changes in astrocytes in response to injury may be much slower than previously thought.However,several previous studies have shown that AQP4, a protein densely expressed on astrocytic endfeet, was associated with stroke-related vascular disruption(Manley et al., 2004; Friedman et al., 2009; Akdemir et al., 2014; Yao et al.,2015).Researchers found that AQP4–/–transgenic mice exhibited lower brain parenchymal water content and better neurological function, and animals with a normal AQP4 phenotype showed reduced AQP4 expression after ischemic stroke (Manley et al., 2004; Friedman et al., 2009; Akdemir et al.,2014; Yao et al., 2015).
The glymphatic system
An exciting discovery from 2PLSM-based research in astrocytes is the glymphatic system.Iliff et al.(2012) first described the glymphatic system as“a sewage system in the brain”that promotes cerebrospinal fluid (CSF)flow through the brain parenchyma and removes interstitial solutes.They demonstrated that the glymphatic system consists of peri-arterial CSF inflow,CSF mixed with interstitial fluid, and a mixture of CSF and interstitial fluid flowing out through the peri-venous space and leaving the brain along the cranial and spinal nerves (Iliff et al., 2012).CSF mixing with interstitial fluid is mediated by the AQP4 protein densely expressed on astrocytic endfeet (Iliffet al., 2012).This sewage system in the brain has never been represented in postmortem animal tissue sections, which inevitably have damage to the CSF flow pathway due to fixation (Benias et al., 2018; Mestre et al.,2018).In contrast,in vivo2PLSM imaging provides high-resolution dynamic evidence of fluid flow.Iliff et al.(2012) usedin vivo2PLSM to record the movement of fluorescent tracers of different molecular weights injected into the ventricles.In Aqp4 gene knockout mice, the medium molecular-weight tracer could flow into the perivascular space while the movement from the perivascular space into the brain parenchyma was blocked (Iliff et al., 2012).The glymphatic system could perform clearance functions, including clearing soluble amyloid β, a key protein in Alzheimer’s disease (Iliff et al., 2012).Xie et al.(2013) further explored 2PLSM imaging of tetramethylammonium diffusion in awake, anesthetized, and sleeping mice and found that neither natural sleep nor anesthesia was associated with increased interstitial space,leading to a significant increase in convective exchange between CSF and interstitial fluid, suggesting that sleep drives metabolite clearance in the adult brain.As for studying ischemic stroke, they usedin vivo2PLSM and magnetic resonance imaging and demonstrated that CSF influx is an essential source of edema fluid after ischemic stroke (Mestre et al., 2020b).The influx of CSF with high sodium levels could further drive brain tissue swelling.Thus, they pioneered the term ionic edema to describe the intermediary brain edema phase between early cytotoxic and late vasogenic brain edema stages after ischemic stroke.They used genetically encoded calcium indicators to visualize the depolarization of neurons (Mestre et al., 2020b).They demonstrated that there was a temporal link between SD and CSF transport through the perivascular spaces to the brain parenchyma after ischemic stroke, indicating that SD likely mediated CSF transport to brain parenchyma through the glymphatic system (Mestre et al., 2020b).This emphasized the crucial role of the astrocyte network in post-stroke edema.Targeting AQP4 to treat brain edema after ischemic stroke may be a possible treatment option.
However, several studies have challenged the opinion that the glymphatic system mediates solute clearance from brain parenchyma through convective flow (Hladky and Barrand, 2014; Spector et al., 2015; Holter et al., 2017;Smith et al., 2017).Holter et al.(2017) used 3D electron microscopy to reconstruct interstitial solute transport in hippocampal tissue.Their results suggested that solutes were more likely to be transported through interstitial spaces by diffusion rather than by convective flow.Smith et al.(2017) used 2PLSM to photobleach dextran in the parenchymal extracellular space and to image the fluorescence recovery process.They found that fluorescent dextran transport in brain parenchyma depended on the size of the molecule, was mainly diffusive rather than convective transport, and was unaffected after cardiac arrest (Smith et al., 2017).Further imaging in AQP4-deficient animals revealed that AQP4 deletion did not impair the transport of fluorescent solutes from the subarachnoid space to the brain parenchyma (Smith et al.,2017).Mestre et al.(2020a) published a paper, summarizing and responding to the controversies around the glymphatic system.They argued that mathematical models ignored the irregularities of the perivascular space and that AQP4 knockout mice should show a high degree of age uniformity, as the activity of the glymphatic system declines rapidly with aging.However, the specific role and mechanisms of astrocytic AQP4 in mediating the influx of CSF into the brain parenchyma in ischemic stroke remains to be investigated(Spector et al., 2015; Mestre et al., 2020a).
Roles of astrocytes in recovery phase of ischemic stroke
The roles of astrocytes in the recovery phase of ischemic stroke are also complex.Based on postmortem techniques, Li et al.(2015) found that astrocyte IP3R2 knockout mice showed attenuated excessive astrogliosis and relieved brain injury, neuronal death, and behavioral deficits compared to controls 14 days after PT.This indicated that the calcium signal pathway in astrocytes may be harmful to neural recovery after ischemic stroke.However, other studies using dynamicin vivo2PLSM showed that astrocytes played an essential role in angiogenesis, vascular network reorganization,and neural recovery.Heras-Romero et al.(2022) administered extracellular vesicles released by primary cortical astrocytes in the ventricles of mice and continuously observed the dynamic recovery process after ischemic stroke for 21 days.They found that the extracellular vesicles could mediate recovery of structure and function in neurons.Williamson et al.(2021)traced the morphology of astrocytes and the relationship between astrocytes and angiogenesis after PT for 28 days.They demonstrated that reactive astrocytes colocalized with and contacted newly formed vessels (Figure 5C).Chemogenetic ablation of peri-infarct reactive astrocytes dramatically impaired vascular remodeling and impeded the recovery of neurological function.Gӧbel et al.(2020) explored the specific mechanisms of astrocytes in vessel remodeling.They observed a prominent mitochondria-enriched compartment in astrocytic endfeet and mediation of vascular remodeling after stab-wound injury.However, this finding requires further study in ischemic stroke models.
The contradictory results regarding astrocytes in the recovery of ischemic stroke may be related to the differentiation of diverse subpopulations of astrocytes after injury; different subpopulations of astrocytes may perform different functions.Gene knockouts in all astrocytes without differentiating between subpopulations may obscure experimental results.In 2013, Bardehle et al.used time-lapse 2PLSM to observe dynamic responses of astrocytes to acute traumatic brain injury in live mice for up to 28 days.They found significant heterogeneity in the responses of astrocytes to injury, with only a distinct subset located at juxtavascular sites proliferating, one subset retaining their initial morphology, and another directing their processes toward the lesion (Bardehle et al., 2013).However, there is a lack of research on the dynamics of astrocyte behaviors in ischemic stroke.Thus, subtle subpopulation studies of astrocytes may be more practical for the study of ischemic stroke.
The Response of Microglia in the Acute Phase of Ischemic Stroke
Microglia, the critical resident immune cells in the brain, are dedicated to neuronal network homeostasis and function (Pena-Ortega, 2017).Microglia transform into activated microglia after ischemic stroke, which is a process marked by changes from ramified to amoeboid proliferative and morphological features, and activated microglia exhibit enhanced phagocytosis and release cytokines and growth factors (Otxoa-de-Amezaga et al., 2019; Qin et al.,2019; Song et al., 2019; Ronaldson and Davis, 2020).Complex functions and multiple phenotypes of reactive microglia have been widely demonstrated using single-cell sequencing and transcriptomics (Ransohoff, 2016a; Li et al.,2019a; Qin et al., 2019; Sierra et al., 2019; Stratoulias et al., 2019; Jurga et al., 2020).However, the specific roles of microglia in ischemic stroke have not been well studied.Microglia are highly dynamic and frequently communicate with neurons and vessels, meaning that postmortem animal studies inevitably miss a great deal of data on dynamic changes, and neuroimaging has limited spatiotemporal resolution.Furthermore, it is inevitable that different microglial activation states will occur duringex vivopreparations due to their high sensitivity.Therefore, with advanced fluorescent labeling techniques(Table 2),in vivotime-lapse 2PLSM is an excellent tool for studying microglial networks, especially in the immediate response after ischemic stroke.
Changes in microglia behaviors after ischemic stroke
The transformation from ramified into amoeboid morphological features is an essential indicator of microglial activation (Kreutzberg, 1996; Zhang, 2019).Using 2PLSM, Nimmerjahn et al.(2005) and Davalos et al.(2005) found that,even in the resting state, microglial processes were remarkably motile, likely to monitor surroundings and interact with neighboring cells (Davalos et al.,2005; Nimmerjahn et al., 2005).After external stimulation such as laser ablation or the application of ATP, microglia rapidly converged to the lesion site, even without complete activation (Hanisch and Kettenmann, 2007).This finding demonstrated that microglia are highly dynamic and play an important role in brain microenvironment homeostasis in both healthy and diseased brain tissue.
Wake et al.(2009) found that, in the normal resting state, microglial processes change dynamically and contact neuronal synapses at a specific frequency and contact duration.After transient cerebral ischemia, the contact time between microglia and synapses was prolonged, corresponding to the disappearance of the presynaptic bouton (Wake et al., 2009).Li et al.(2013) used a parabiosis model of CX3CR1-GFPand wild-type mice to demonstrate that proliferating microglia in the brain parenchyma after ischemic stroke were derived from resident microglia rather than circulating monocytes or myeloid progenitor cells.Masuda et al.(2011) demonstrated that the activation of microglia in the ischemic penumbra was associated with a decrease in blood flow, and that this activation was energy-dependent and required residual blood flow.However, further studies on the pathophysiological impacts of activated microglia after ischemic stroke have been controversial.Severalin vivo2PLSM studies further showed that blocking important receptors expressed on microglia, including chemokine receptors, colony-stimulating factor receptors,and complement receptors (CX3CR1, C3aR, CSF1R, and CXCR3), could have neuroprotective effects (Fumagalli et al., 2013; Walter et al., 2015; Surugiu et al., 2019; Hou et al., 2020).Other studies have shownin vivothat selective elimination of microglia increased infarct size; exogenously applied BV2 microglial cell lines reduced neural damage in organotypic hippocampal slice cultures, indicating that microglia are protective (Neumann et al., 2006; Szalay et al., 2016; Zhang, 2019).
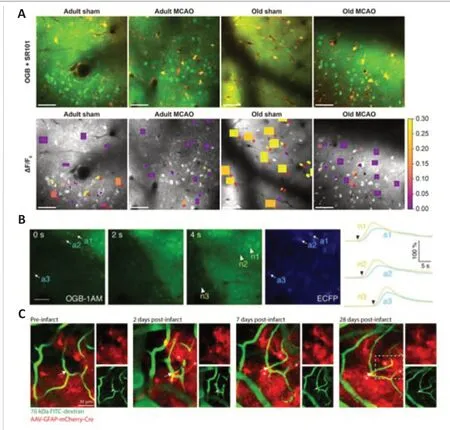
Figure 5| Images from 2PLSM observation of astrocytes.
In addition to the infarct core and ischemic penumbra, secondary neurodegeneration (SND) is also an important research focus.SND involves the progressive death of neurons associated with the site of infarction but not initially damaged during the ischemic stroke (Jayaraj et al., 2019; Stuckey et al., 2021).Researchers have used acute slices from the thalamus, a major site of SND, to observe morphological changes in microglia (Kluge et al.,2017, 2019).In these 2PLSM studies, they found that microglial specificity became nonresponsive in SND, as evidenced by weakened microglial process movements (Kluge et al., 2017, 2019).In contrast, phagocytosis and the levels of classic microglial molecular markers remained elevated.The appearance of nonresponsive microglia was strongly associated with neuronal damage.
Microglia communication with multiple cell types after ischemic stroke
As a kind of non-excitable cell, microglia highly depend on changes in intracellular [Ca2+]ito perform cellular functions (Heo et al., 2015).Usingin vivotwo-photon calcium imaging, Eichhoff et al.(2011) found that most (80%)microglia showed no spontaneous Ca2+transients at rest or under conditions of strong neuronal activity or intercellular astrocytic Ca2+waves.However,microglia reliably responded with large, generalized Ca2+transients to damage individual neurons (Eichhoff et al., 2011).This suggested that changes in microglial calcium signaling are primarily involved in pathological processes in response to neuronal injury, contrary to the results ofin vitroexperiments.Usingin vivo2PLSM, they found frequent Ca2+transients in microglia after triggering cortical SD, which was induced by applying exogenous KCl solution(Tvrdik et al., 2019; Kearns et al., 2020, 2022; Liu et al., 2021).This linked neuronal damage, neuronal electrical signals, and microglial functional.A calcium wave in cortical microglia during ischemic stroke is shown in Figure 6B.This study also provided a reliable paradigm for studying the relationship between microglia and neuronal networks after ischemic stroke.
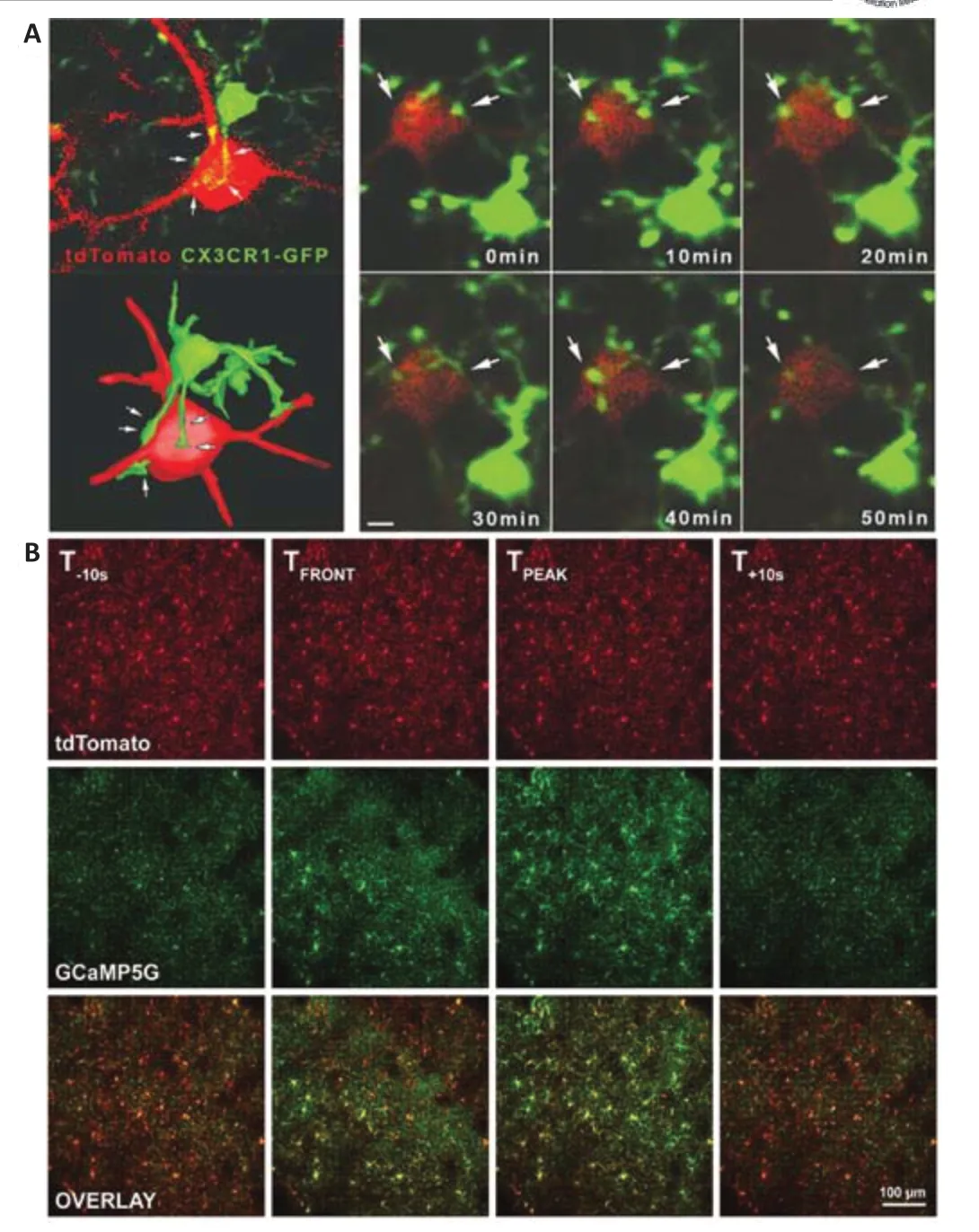
Figure 6| Images of in vivo 2PLSM of microglia.
Moreover, Cserép et al.(2020) used post hoc confocal laser scanning microscopy and electron microscopy to further illustrate the highly dynamic ultrastructure of microglia-neuron junctions in healthy brains, which provided valid structural evidence for the novel microglia-neuron junction concept(Figure 6A).They demonstrated that the microglia-neuron junctions consisted of closely apposed mitochondria, reticular membrane structures, intracellular tethers, and associated vesicle-like membrane structures within the neuronal cell body (Cserép et al., 2020).They used mitochondrial fluorescent labeling andin vivo2PLSM to demonstrate that the foundation of microglia-neuron junctions relied on mitochondria-dependent neuronal exocytosis release signals (Cserép et al., 2020).This signaling process was highly correlated with ATP and ADP, which are important ligands for the regulation of microglial processes via the microglial purinoceptor P2Y12 (Cserép et al., 2020).They proposed that healthy neurons may constitutively release ATP and other signaling molecules at the microglia-neuron junction and that P2Y12 receptors on microglia receive the relevant signals, thus allowing microglia to monitor neuronal status (Cserép et al., 2020).Blocking microglial P2Y12 receptors after MCAO led to a strong increase in neuronal calcium load and to a significantly larger lesion volume than those observed in control mice, which may indicate that microglia play a neuroprotective post-stroke role through microglia-neuron junctions (Cserép et al., 2020).
Microglia also interact with blood vessels.Bisht et al.(2021) usedin vivo2PLSM to observe fluorescently labelled pericapillary cells in CX3CR1-GFPtransgenic mice.They confirmed that the pericapillary CX3CR1-GFPcells were capillary-associated microglia using molecular, morphological, and ultrastructural approaches.After elimination of perivascular microglia using CSF1R inhibitors, capillary diameters increased and cerebral blood flow measured by laser speckle imaging increased, while vascular responsiveness to CO2decreased.This demonstrated that, in the healthy brain, microglia communicate with blood vessels and play an important role in regulating vascular structure and function (Bisht et al., 2021).After ischemic stroke,changes in microglia-vessel communication also affects prognosis.Using microglia-labeled transgenic mice (CX3CR1-GFP), Jolivel et al.(2015) coimaged microglia and blood vessels.Soon after reperfusion in transient MCAO, the microglia in the penumbra were activated and began to expand towards adjacent blood vessels, phagocytize vascular endothelial cells, and mediate injury to the BBB.The role of interactions between different types of glia in ischemic stroke has also received a lot of attention (Garcia-Caceres et al., 2019; Liu et al., 2020;Matejuk and Ransohoff, 2020).However, regarding interactions between glial cells, current two-photon studies lack data on communication and cooperation between different types of glia and their specific roles in the network structure after ischemic stroke.
Limitations and Innovations
Although the high spatial and temporal resolution forin vivostudies using 2PLSM are remarkable for the longitudinal study of ischemic stroke, 2PLSM is far from perfect.With its development, many limitations have been already resolved.First, before imaging, skull window surgery is required.For an openskull glass window, damage to brain tissue and associated inflammatory reactions are inevitable (Xu et al., 2007; Marker et al., 2010; Yang et al.,2010).Researchers also need long training to complete the surgery.Thinned cranial windows limit damage to the brain, while performing relative longterm observation requires repeated thinning of the skull due to bone regrowth (Yang et al., 2010).Zhao et al.(2018) have developed a technique for safe clearing of a skull window, which resulted inin vivo2PLSM imaging of cortical structures at synaptic resolution without removing the skull or excessive thinning of the skull, minimizing damage to brain tissue.Second,the maximum imaging depth of 2PLSM is limited, making whole-brainin vivolive imaging not possible.Changes in neural circuits after stroke are important for neuronal plasticity and functional compensation (Fox, 2018; Herbet and Duffau, 2020).Stereotactic injection of tracer viruses offers a possible solution forin vivovisualization of neural circuits (Chatterjee et al., 2018; Zhang et al.,2018; Macknik et al., 2019; Poinsatte et al., 2019; De La Crompe et al., 2020;Tang et al., 2020).Third, the achievement of multicolor 2PLSM imaging is also a crucial problem in studies targeting intercellular communication.The emission spectra of different fluorescent probes may overlap, generating spectral crosstalk and making it hard to clearly distinguish the sources of emitted light, which limits imaging of neurovascular units.To achieve multicolor 2PLSM imaging, multiple pairs of lasers and detectors or filters and detectors are used to separate different fluorescence signals, but this increases the cost (Le Grand et al., 2008).Several post-processing techniques and the combination of 2PLSM with other optical imaging principles can be used to solve this problem (Ueki et al., 2020; Olson et al., 2022; Yan et al.,2022).Moreover, novel quantum dots have been used as fluorescent labels in 2PLSM, and their optical properties are suitable for multicolor imaging(Mashinchian et al., 2014; He et al., 2018; Qi et al., 2018; Wang et al., 2019;Qin et al., 2020).Fourth, for highly specific fluorescent labeling of certain components, such as microglia, the prior methodology for cell visualization uses fluorescent reporters, requiring cross-breeding of genetic lines of interest.This requires a fairly long breeding cycle.Moreover, commonly used 2PLSM devices need heavy shock-resistant benchwork that is too bulky to miniaturize.For ischemic stroke studies, the common imaging strategy is head fixation after anesthesia, and the results may not reflect the actual cellular function in the natural state (Groothuis et al., 2007; Cao et al., 2017;Guo et al., 2021; Bharioke et al., 2022).Therefore, it is important to have a two-photon imaging platform that can assess post-stroke pathophysiological processes in awake, behaving animals.Several studies have used 2PLSM to image awake animals (Stobart et al., 2018; Yang et al., 2018; Piatkevich et al., 2019; Chow et al., 2020).Zong et al.(2021) developed a miniature twophoton microscope called FHIRM-TPM 2.0, which can reflect the activity characteristics of neurons in mice in a natural state rather than with fixation or under anesthesia.Finally, imaging data obtained from 2PLSM are extensive,and image processing is highly complex.Researchers have introduced artificial intelligence to assist with data processing (Barbastathis et al., 2019; Borhani et al., 2019; Gur et al., 2020).However, the robustness and interpretability of results obtained using artificial intelligence need to be carefully evaluated.
The development of two-photon fluorescent probes is also continuing to progress.Novel, specific organelle or subcellular component-localizable,two-photon fluorescent probes are now under development, which would allow researchers to explore biological molecular-level changes in important subcellular structures, such as mitochondria, lysosomes, nuclei,the Golgi apparatus, and the endoplasmic reticulum (Collot et al., 2018;Dana et al., 2019; Ye et al., 2019; Zhao et al., 2020; Cheng et al., 2021).A novel multiplexed dynamic intravital multiphoton imaging method allowed researchers to distinguish seven fluorophore signals corresponding to various cellular and tissue compartments using four detector channels (Rakhymzhan et al., 2021).Label-free imaging is also a good alternative.Wu et al.(2020)were able to receive autofluorescence signals with short wavelength twophoton excitation.They developed a time-resolved two-photon excitation microscopy system using a homemade 520-nm femtosecond fiber laser as the excitation source, which could noninvasively achieve intravital high-resolution 3D imaging of a microvascular network.
Conclusions
After discussing and summarizing studies of ischemic stroke using 2PLSM over the past decades, we found that the pathophysiological processes after ischemic stroke cannot be fully explored by only observing changes in neurons after stroke without considering the neural support system represented mainly by blood vessels, astrocytes, and microglia.Moreover, when studying the roles of blood vessels and glia, it needs to be fully recognized that they constitute a large and sophisticated spatiotemporal network whose structural hierarchy, behavior, and function change dynamically with the environment and over time and have important implications for the fate of neurons after ischemic stroke.The same cellular component at different temporal and spatial nodes may have different functions.However, we can easily find that the specific roles of glia and blood vessels in ischemic stroke are still controversial.We believe that this phenomenon is partly due to different responses of different subgroups of glial cells and different hierarchies in vascular networks in different environments and that knockdown or overexpression certain genes, as well as imaging only a portion of cells in the neurovascular unit for a certain period of time, are likely to represent only a few aspects of cellular functions.This problem is partially ignored in a majority of studies but is gradually being recognized (Schaeffer and Iadecola,2021).
Despite the limitations of imaging depth, 2PLSM is invaluable for recording both structural and functional information of multiple cells and cell types simultaneously with subcellular resolution in live brains.Although 2PLSM provides solid and reliablein vivoevidence and reflects subtlein vivoprocesses that cannot be obtained from postmortem animals, results based on 2PLSM still vary between studies.The use ofin vivoanimal models or live brain slices, the age of the animal, the method of astrocyte labeling,and modeling methods can bias results away from the natural disease state.Moreover, the commonly usedin vivoexperimental ischemic stroke models(i.e., MCAO and PT) are far from reality, which is an obstacle in clinical translation (Sommer, 2017).Imaging evidence provided by 2PLSM is at the cellular or subcellular level.It is a challenge to connect the high-quality cellular-levelin vivoevidence provided by 2PLSM with the molecular-level evidence provided by biochemical techniques.Relying on specific ablation of cells or gene knockouts or upregulation may not perfectly merge the advantages of both.The emerging spatial transcriptomics technique gives a possibility to fuse the two types of technologies, but the tedious data analysis and interpretation processes are not easily applicable.
Overall, future research will shift from postmortem animal research to live animal research, from static research to dynamic research, from flat to threedimensional, and from single-cell function and structure to intercellular communication.2PLSM is a robust tool to translate study discovery from bench to bedside.
Acknowledgments:We are very grateful to Yunyun Han from Huazhong University of Science and Technology for the helpful comments on the manuscript, and we also thank her valuable suggestions on the content of the article.
Author contributions:Manuscript design and conception: XW and JRL;manuscript writing: XW; literature search: YF.Figure and table design and preparation: DYC.Language polishing, manuscript review and editing: HN and ZPT.All authors read and approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:No additional data are available.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain
- The role of fibronectin in multiple sclerosis and the effect of drug delivery across the blood-brain barrier

