Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
Aziz Unnisa, Nigel H.Greig, Mohammad Amjad Kamal
AbstractToxic aggregated amyloid-β accumulation is a key pathogenic event in Alzheimer’s disease.Treatment approaches have focused on the suppression, deferral, or dispersion of amyloid-β fibers and plaques.Gene therapy has evolved as a potential therapeutic option for treating Alzheimer’s disease, owing to its rapid advancement over the recent decade.Small interfering ribonucleic acid has recently garnered considerable attention in gene therapy owing to its ability to down-regulate genes with high sequence specificity and an almost limitless number of therapeutic targets, including those that were once considered undruggable.However, lackluster cellular uptake and the destabilization of small interfering ribonucleic acid in its biological environment restrict its therapeutic application,necessitating the development of a vector that can safeguard the genetic material from early destruction within the bloodstream while effectively delivering therapeutic genes across the bloodbrain barrier.Nanotechnology has emerged as a possible solution, and several delivery systems utilizing nanoparticles have been shown to bypass key challenges regarding small interfering ribonucleic acid delivery.By reducing the enzymatic breakdown of genetic components, nanomaterials as gene carriers have considerably enhanced the efficiency of gene therapy.Liposomes, polymeric nanoparticles, magnetic nanoparticles, dendrimers, and micelles are examples of nanocarriers that have been designed, and each has its own set of features.Furthermore, recent advances in the specific delivery of neurotrophic compounds via gene therapy have provided promising results in relation to augmenting cognitive abilities.In this paper, we highlight the use of different nanocarriers in targeted gene delivery and small interfering ribonucleic acid-mediated gene silencing as a potential platform for treating Alzheimer’s disease.
Key Words:Alzheimer’s disease; amyloid-β; BACE1; gene silencing; gene therapy; nanoparticle;neurotrophins; small interfering ribonucleic acid
I ntro duction
Alzheimer’s disease (AD) is one of the most prevalent neurodegenerative conditions, is characterized by progressive cognitive decline and impaired memory, and accounts for some 60–80% of late-onset dementia cases worldwide.Hallmarks of AD and essential requirements for its diagnosis are the presence of amyloid-beta protein (Aβ) in the brain as extracellular amyloid plaques and hyperphosphorylated Tau as intraneuronal neurofibrillary tangles(Muralidar et al., 2020).A further omnipresent feature is neuroinflammation.In this regard, the blood-brain barrier (BBB) is largely fundamental in the development and persistence of chronic inflammation in AD (McLarnon et al., 2021).The effective brain removal of neurotoxic Aβ is hampered by the defensive role of the BBB (Li et al., 2021).The accumulation of Aβ in the brain combined with BBB malfunction creates a feedback loop that leads to amyloid oligomers and plaque development (Cai et al., 2018), the triggering of tau hyperphosphorylation and other aberrant cascades and, ultimately,cognitive decline and dementia (de Oliveira et al., 2021).Consequently, many Alzheimer’s treatment strategies have focused on the suppression, deferral,or dispersion of Aβ oligomers, fibrils, and plaques (Sanati et al., 2019).This article reviews nanotechnology-based gene therapy approaches for treating AD across potential targets.
Beta-site amyloid precursor protein cleaving enzyme-1 (BACE1) is critical in generating Aβ, whether in physiological or pathological amounts.BACE1 initiates Aβ monomer production by cleaving amyloid precursor protein (APP)at the β-secretase site.Further cleavage by the γ-secretase complex results in Aβ monomers that can potentially clump together to create toxic oligomers and fibrils, and, ultimately, amyloid plaques (Jiang et al., 2022).BACE1 levels have been reported to be elevated in the autopsy of AD brains, implying that heightened BACE1 increases Aβ formation and drives initial disease progression.The amyloid pathway is most likely responsible for the BACE1 increase, which may then lead to a positive-feedback cycle in AD (Zhao et al.,2007).Golgi-localized γ-ear containing ARF binding protein-3 deficiency is also a leading contender for the mechanism driving the BACE1 rise (Kim et al.,2018).
Numerous declines in the levels of key proteins have been reported in the AD brain, such as endogenous neurotrophic factors whose concentrations are essential to maintaining the health and survival of neurons.For example,the first growth factor identified, nerve growth factor (NGF), acts on basal forebrain cholinergic nerve cells, supporting their physiological function in providing the bulk of acetylcholine release across the hippocampal and cortical regions.In AD, these nerve cells dramatically deteriorate, resulting in cognitive decline (Nyakas et al., 2011).NGF deficiency promotes cholinergic abnormalities (Do Carmo et al., 2021).The findings of phase I clinical studies employing theex vivogene therapy technique in 8 individuals with moderate AD (Stage V/VI) were published in 2005 (Tuszynski et al., 2005).Cognitive abilities, brain metabolic activity, and the morphological status of cholinergic neurons were reported to improve in this set of people (Stepanichev et al.,2020), highlighting the promise of gene therapy if used adroitly.Following up on this example, within this review article, we evaluate gene therapy strategies to mitigate AD, the targets chosen, and the technologies applied to optimize the treatment strategy.
Search Strategy
We searched PubMed, Google Scholar, Chinese National Knowledge Infrastructure (CNKI), and Excerpta Medica (EMBASE) databases for articles related to nanotechnology-based gene therapy for treating AD from inception to February 2022.The following search terms were used: (gold nanoparticles or treatment or diagnosis) AND (Alzheimer’s disease) in English.
Inclusion criteria
The inclusion criteria are as follows: (1) The article is an experimental study of AD and nanotechnology-based gene therapy; (2) The article also includes AD and nanotechnology-based gene therapy clinical studies, pharmacy studies,and pharmacokinetic studies.
Exclusion criteria
The following are the exclusion criteria: (1) Failure to report the use of nanotechnology-based gene therapy in AD; (2) failure to report the studies related to pharmaceuticals, clinical trials, toxicity, and pharmacokinetics; (3)Studies reported without a control group; (4) letters, case reports, editorials,clinical guidelines, and comments; (5) repeated publications.
Quality assessment
The quality standards are primarily based on a seven-item modified scale developed collaboratively from the collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies: (1) Peer-reviewed publications; (2) blind evaluation of behavioral results; (3) adequate sample size; (4) compliance with animal welfare laws and regulations; (5) declaration of potential conflicts of interest; (6) temperature control; and (7) random assignment to groups.
Search results
The article processing is shown in the flow diagram provided inFigure 1: We screened 190 articles, from which the following were excluded.
After excluding the articles irrelevant to our study title, we included 101 articles for the review.

Figure 1|Flow diagram elucidating the methodology of the scientific literature search.
Gene Therapy for Alzheimer’s Disease
Gene therapy can increase enzyme activity while balancing the low levels of bioactive substances.It has been increasingly explored in numerous animal AD models with encouraging outcomes.The first clinical trials employingex vivogene therapy (relating to NGF) have been completed, with promising reported improvements in AD pathology (Tuszynski et al., 2005).Gene therapy has potential benefits, such as enhanced safety and gene expression selectivity.Furthermore, in specific cases, it may be more effective than smallmolecule treatments for the“up-regulation”of an enzyme (Alves et al., 2016).The various etiologies of AD provide a multitude of potential gene therapy targets (Figure 2), including Aβ-generating proteins (BACE1 and APP),neurotrophins such as brain-derived neurotrophic factor (BDNF) and NGF that support and maintain neuronal health/growth, apolipoprotein E (APOE)and Aβ degradation associated enzymes (endothelin-converting enzyme,cathepsin B and neprilysin) that are targets for small interfering ribonucleic acid (siRNA) mediated gene silencing (Pfundstein et al., 2022).Research has targeted the genes mentioned above and obtained the outcomes detailed in Table 1.
Neurotrophin gene therapy
Recent advances in gene therapy-assisted delivery of neurotrophins have yielded surprising results, including trophic responses of cholinergic neurons and increased cognitive abilities (Nasrolahi et al., 2022).NGF is part of the neurotrophin family, including BDNF, neurotrophin 3 (NT-3), NT-4/5, and NT-6 (Lübke et al., 2021).NGF, albeit the first neurotrophin discovered, has potential actions beyond its neurotrophic effects, and recent research found that depletion of NGF in PC12 (pheochromocytoma) cells triggers the process of Aβ biogenesis; thereby associating NGF with Aβ (Latina et al., 2018).It has been suggested that NGF gene therapy may alleviate AD’s clinical and behavioral manifestations (Peng et al., 2022).Some research has reported an association between the Val66Met mutation in BDNF with Alzheimer’s pathogenicity, while other research did not confirm this (Huang et al., 2007;Gao et al., 2022).Without changing the Aβ burden, BDNF gene delivery has been reported to exert a neuroprotective impact on cells of the entorhinal cortex, which is a key afflicted area in AD (Mueller-Steiner et al., 2006).
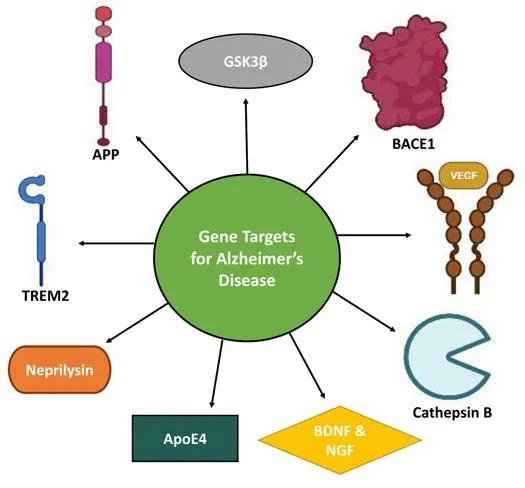
Figure 2|Targets for gene therapy in Alzheimer’s disease.
Nanotechnology-Based Gene Therapy
Due to recent nano-carrier-based gene therapy developments, scientific organizations have been provided with the technology and impetus to evaluate numerous potential treatment procedures (Wadetwar et al., 2021).In this regard, by reducing the enzymatic breakdown of genetic components,nanomaterials as gene carriers have considerably enhanced the efficiency of gene therapy (Yu et al., 2021).Organically modified silica nanoparticles, the most intriguing of all, were the first to be investigated forin vivogene delivery to the central nervous system by Bharali et al.(2005).
SiRNA-mediated gene therapy
Genes involved in disease pathology, individual genes, and viral pathogens are promising candidates for RNA interference (RNAi).RNAi can also find new essential genes in pathophysiological processes (Aigner et al., 2019).RNAi modulates gene expression by regulating protein production using a posttranscriptional gene silencing mechanism (Mody et al., 2021).This includes lengthy portions of double-strand RNA (dsRNA) that split into the siRNA fraction and activate RNAi.SiRNA is the most suited agent for the short-term silencing of genes that encode proteins (Mahfuz et al., 2022) and has garnered considerable attention in gene therapy in recent years owing to its ability to down-regulate genes with high sequence specificity and a limitless number of therapeutic targets, including those that are thought to be undruggable(Gupta et al., 2019).However, incorrect delivery carrier choice can diminish the gene-silencing activity of siRNA, change pharmacokinetics, and potentially increase off-target effects (Sioud et al., 2020).It was once assumed that siRNA was biochemically stable and did not necessitate delivery carriers forin vivosiRNA delivery (Artiga et al., 2019).However, several previous studies have revealed the need for the functionalization of siRNA to improve its stability and the use of a unique carrier system that can primarily enhance effective delivery to target tissues (de Fougerolles et al., 2007; Kim et al., 2007; Kuhn et al., 2007; Bumcrot et al., 2016).Nanotechnology has emerged as a possible option, and several delivery systems utilizing nanoparticles have been shown to bypass key delivery challenges regarding siRNA delivery, including the BBB(Zheng et al., 2018; Han et al., 2021).The mechanism of siRNA-nanoparticlemediated gene silencing is shown inFigure 3.Table 2shows the various nano complexes used in siRNA-mediated gene therapy in AD.
Nanoparticle-based gene therapy
Liposomes
The adsorption affinity of nanoliposomes coated with an anti-Aβ monoclonal antibody was studied by Canovi et al.(2011).The ability of Aβ-monoclonal antibody -coated liposomes to attach to Aβ plaques in AD brain autopsy samples provided evidence for using such liposomes as potential disease diagnostics and therapeutics.To prevent the cytotoxicity of cationic nanoparticles, anionic siRNA-nanocomplexes were created using polyethylene glycol (PEG)-liposomes (anionic) and targeting peptides (cationic), and,through the use of these nano complexes, BACE1 suppression was accomplished (Tagalakis et al., 2014).For plasmid encoding apolipoprotein E2 delivery, Dos Santos Rodrigues et al.(2019) employed transferrin-penetratin entrapped liposomes and found a remarkable elevation in apolipoprotein E2 levels in mice.

Figure 3|Mechanism of gene silencing by small interfering ribonucleic acid (siRNA).
Polymeric nanoparticles
A glycosylated polymeric siRNA nanocomplex (Gal-NP@siRNA) was created and evaluated in a transgenic AD model that selectively suppressed BACE1 and decreased Aβ levels.This mitigated damage to myelin, aided clearance of by-products (owing to its biocompatibility) and helped restore cognitive performance in the mice (Kim et al., 2007).A polymer-based gene carrier having the glycemic phenylalanine-leucine-glycine sequence that is considered sensitive to cleavage by cathepsin B, a lysosomal cysteine protease with a role in intracellular proteolysis, was used to improve plasmid DNA (pDNA) delivery intracellularly (Lee et al., 2017).Cathepsin B levels are considered elevated in AD and neurodegenerative conditions such as following traumatic brain injury,with the protein leaking from the lysosome to the cytosol compartment where, in addition to pathological roles on Aβ generation/clearance, it could more efficiently release pDNA from an appropriately designed nanocarrierbased gene delivery system.
Glycemic monitoring was used as an external stimulus to establish a method for the brain delivery of systemic antisense oligonucleotides by traversing the BBB.Antisense oligonucleotides can be encapsulated in glucose-installed polymeric nanoparticles that may be coupled to glucose transporter-1 (Min et al., 2020).These antisense oligonucleotides target messenger RNA (mRNA)for APP or its processing enzymes to reduce toxic Aβ levels (Grabowska-Pyrzewicz et al., 2021).Malhotra et al.(2013) developed nanoparticles for siRNA delivery using a new cell-targeting peptide called transactivator of transcription, PEG, and chitosan polymer.The Chitosan-PEG-transactivator of transcription complex was produced and utilized to create nanoparticles with a diameter of approximately 5 nm coupled with siRNA for delivery to nerve cells to suppress the ataxin-1 gene, which is responsible for BACE1 expression and is involved in Aβ pathology (Suh et al., 2019).In another investigation,a poly-siRNA/thiolated glycol chitosan nanoparticle was utilized to silence vascular endothelial growth factor, which is found to be elevated in AD brain and has been associated with loss of pericytes, elevated BBB permeability,more severe AD and particularly Tau pathology, and greater clinical signs of AD (Lee et al., 2012).Various polymer-siRNA nanoconjugates used in gene therapy are schematically depicted inFigure 4.
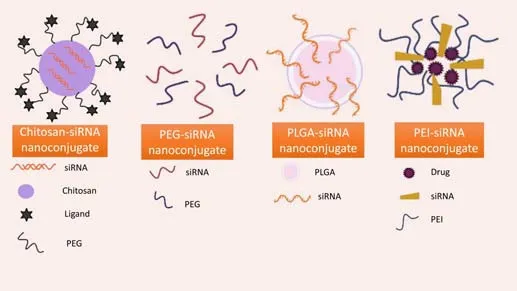
Figure 4|Types of polymer-siRNA nanoconjugates.
Dendrimers
Zhang et al.(2017, 2019) coated dendrimer nanoparticles with NL4 peptide and apolipoprotein A–I.They then employed these dendrimer nanoparticles in a unique dual-targeting drug delivery system to selectively transport siRNA into neuronal tissue to suppress BACE1 and, thereby, reduce Aβ generation.A multifunctional nanocarrier was created in a separate study using PEGylated dendrigraft poly-L-lysines to deliver desired genes and peptides to the brain via systemic injection.The delivery of non-coding ribonucleic acid (RNA)plasmids was used to down-regulate the main enzymes involved in Aβ production (Ouyang et al., 2022).
Lipid nanoparticles
CD45 (cluster of differentiation 45) expression has been reported to be elevated in microglial cells in AD patients (Singh et al., 2021).In line with this finding, Basha et al.(2011) utilized lipid nanoparticles for CD45-directed siRNA delivery to immune cells.A further study employed chitosan-coated and uncoated solid lipid nanoparticles for nose-to-brain siRNA delivery by exploiting the olfactory and trigeminal nerve pathways.It was discovered,using a Caco-2 epithelial cell model, that siRNA released from chitosancoated solid lipid nanoparticles penetrated the monolayer more effectively to support the targeting of BACE1 (Rassu et al., 2017).
Magnetic nanoparticles
A straightforward strategy for knocking down the expression of several genes, including triggering receptors expressed on myeloid cells 2 (TREM2; a gene involved in regulating microglial inflammatory responses and revealed by genome-wide association studies to have variants associated with AD pathology and impairment), was demonstrated by utilizing siRNA linked with magnetic nanoparticles (Carrillo-Jimenez et al., 2018).In another study,PEGylated magnetic nanoparticles were employed to deliver siRNA to nerve cells via endocytosis to suppress the BACE1 gene (Lopez-Barbosa et al., 2020)to lower Aβ generation.
Micelles
Shyam et al.(2015) used a micellar nanoparticle technique based on a copolymer comprising polyethyleneimine (PEI) and PEG to target BACE1 and APP via siRNA delivery.This study demonstrated that the design of siRNAconjugated nanoparticles is a crucial predictor of their distribution and genesilencing efficacy in the central nervous system (Shyam et al., 2015).By reducing neurodegeneration and improving Aβ clearance, intranasal delivery of neprilysin-coated extracellular vesicles mitigated behavioral impairments in a rat AD model (Izadpanah et al., 2020).Delivering neprilysin mRNA packaged in polyplex nanomicelles has also been reported to improve Aβ clearance(Meng et al., 2021).
Silicon nanoparticles
To create effective scaffolds for the transport of the siRNA strand, mesoporous silica nanoparticles were prepared and treated with PEI and aluminum (Badihi et al., 2021).Studies found that NGF-loaded porous silicon nanostructures elicited typical trophic responses (Mitra et al., 2021) and had a significant cytoprotective impact against damage caused by Aβ (Zilony-Hanin et al., 2019;Xu et al., 2021).
Miscellaneous nano complexes
Wang et al.synthesized siRNA nanocarriers that were modified with cingulin and ten-eleven translocation methylcytosine dioxygenase 1 peptides (CT/siRNA).The CT/siRNA nanocomplexes proved to be efficient in selectively targeting and decreasing the expression of BACE1 (Rassu et al., 2017).Nogo receptor-siRNA (NgR-siRNA) and BDNF were combined to create a new nanoparticle.Cell culture and rodent experiments revealed that the described nanoparticles could effectively overcome select shortcomings of traditional AD treatment approaches, supporting the brain cholinergic system and augmenting cognitive function in APP/PS1 AD mice (Wang et al., 2020).For siRNA-mediated gene silencing of glycogen synthase kinase three betas,carboxylated graphene oxide nanosheets were prepared and complexed with PEG and PEI, which downregulated amyloid cascade genes (APP and BACE1)(Gupta et al., 2021).CRISPR-Cas9 nanocomplexes were employed in two mouse AD models to target BACE1 and decrease Aβ-associated abnormalities and cognitive impairments (Park et al., 2019; Baker C, 2022).To improve the BACE1 targeting efficiency of siRNA and reduce Aβ in neurons, fluorescent quantum dots (Li et al., 2012) and exosomes-endogenous nano-vesicles(Yang et al., 2022) were utilized.In another study, Lv et al.(2020) conjugated epigallocatechin-3-gallate and BACE1 antisense shRNA-encoded plasmid with a multifunctional nanocarrier for downregulation of BACE1.
Thus, the nanoparticles mentioned above/nano complexes appear as promising gene delivery candidates in preclinical studies for consideration for translation to patients with AD.Whereas nanoparticle-based gene delivery strives to transfer the product with tolerability and selectivity to the target region, it should be noted that it becomes susceptible to degradation by intracellular enzymes after penetrating the cytoplasm.This can impact the final efficiency of the delivery system.The delivery of various neurotrophic factors using nanocarriers is tabulated inTable 3.
Challenges Regarding Nanotechnology-Based Gene Therapy
Even though nanotechnology-based gene therapy has the potential to become a breakthrough strategy to improve the treatment and management of ahost of debilitating diseases in the coming years, there remain key challenges associated with it - that require to be addressed.Nanotechnology has raised considerable concerns about nanoparticle-mediated cytotoxicity and undesirable effects from gene delivery.Regarding toxicity, various components of nano constructs may modify or disrupt the hemostatic mediator transportation within the central nervous system.Furthermore, internalized nanoparticles can potentially cause necrosis or apoptosis of neuronal cells via various mechanisms.Polymers appear to influence gene expression,which might cause significant issues with nucleic acid delivery to brain cells.The timing and duration between doses should be carefully considered in relation to minimizing nanoparticle-mediated undesirable effects.The design of gene therapy carriers for delivery across the brain is among the primary problems in optimizing therapeutic applications of gene therapy.To carry the gene to the designated targets, appropriate delivery techniques are required,along with the management of any off-target consequences.Because AD is a multifaceted disease, effectively treating it with a single gene transfer might not be possible as there appear to be multiple triggered cascades within the AD brain that lead to a host of neuronal impairments, pathology, and cognitive decline.

Table 1 |Potential genes targeted in AD-associated gene therapy
Consequently, it remains a significant challenge to determine how to assemble the many RNAi elements and transport them to a targeted site of interest.Which element(s) and targeted site(s) should best be selected, and at what stage during the disease process? As previously indicated, antibodies have also been frequently employed for targeting.However, a significant drawback of this particular strategy is the large size of antibody molecules,making it harder to attach them to nano vehicles.
Future Prospects
RNAi has evolved as a powerful research tool that has provided the AD field with a better understanding of AD pathophysiology and is now being evaluated as a practical strategy for therapeutic use.Consequent to its benefits over other existing therapies, RNAi technology is anticipated to help tackle unanswered concerns about AD throughin vitroandin vivotechniques.Optimizing this technology for clinical application remains a highly important focus of the required work in the immediate future.
Certainly, nanoparticles have considerable promise in bioengineering,especially for delivering bioactive drugs to patients with neurological illnesses.Nevertheless, it should be acknowledged that nanomaterials’formulation and development are not confined to a single system but embody a platformwherein the nanomaterials can be modified to potentially treat any disease by substituting the target-specific molecule on the nanoparticles’surface.This can be considered an advantage, as it offers considerable potential(i.e., numerous options) to optimize the therapy to overcome the specific challenges associated with ADversusa different neurological disorder or,indeed, a systemic disease.A possible disadvantage is that the technology platform offers many potential evaluation choices.So many that this may complicate the optimization process in relation to gaining both an enhanced understanding and in-depth knowledge of the methods employed to synthesize and utilize nanoparticles as gene carrier systems that are valuable and, necessarily, practical.Last but not least, future research should additionally focus on tolerability and undesired gene silencing difficulties caused by chemical modifications to the delivery vehicle.

Table 2 |Nanocomplexes used in siRNA-mediated gene therapy
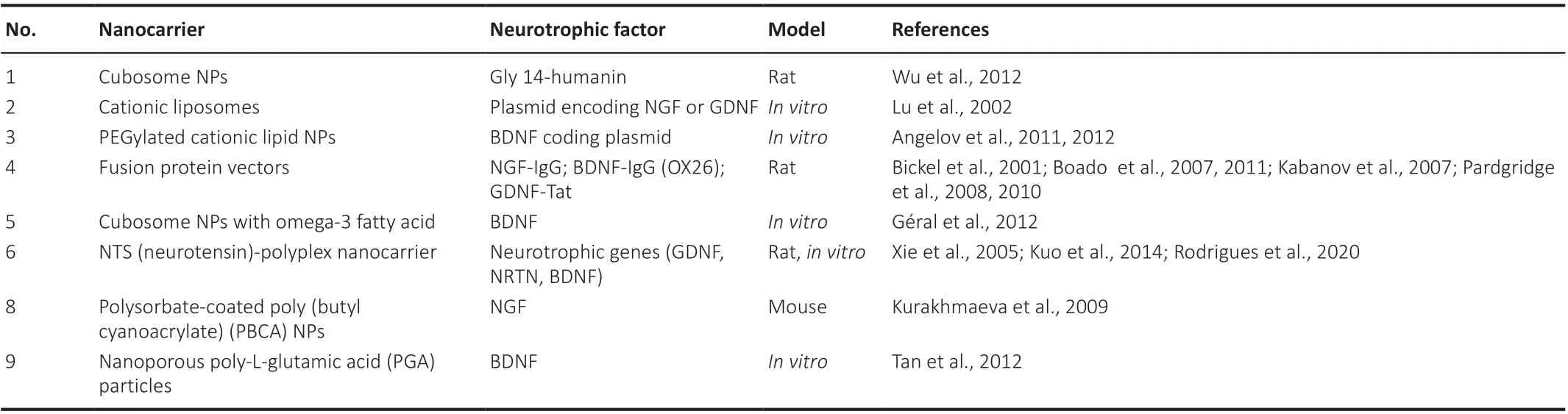
Table 3 | Therapeutic nanoparticle-based neurotrophin targeting for AD
Conclusion
Presently, no truly disease-modifying therapy for AD is available.There are numerous treatment approaches, and the amyloid cascade hypothesis, for good (Selkoe et al., 2021) or bad (Thambisetty et al., 2021), has emphasized amyloid-associated pathways and resulted in the invention of Aβ-generating enzyme (BACE1 and γ-secretase) inhibitors and Aβ clearance and antiaggregation monoclonal antibody therapeutics.Gene therapy can selectively access these same targets and bring about their inhibition or augmentation in a different but parallel manner, as well as potentially target other key proteins supported by other AD hypotheses, even those considered potentially undruggable.Thus, gene therapy is among the most innovative and promising new ways to treat AD.Combining gene therapy with nanotechnology has emerged as a valuable discipline for gene delivery across the BBB and other biological barriers.The development of multifunctional nanocomplexes for siRNA delivery has accelerated in recent years.These highly adaptable complexes provide various unique options as custom delivery carriers to improve the effectiveness and selectivity of gene therapy.This article reviewed many such proof-of-concept studies.However, there remain several issues in nanotechnology that need to be addressed before they can be practically utilized as gene delivery methods, such as low transfection efficacy and potential toxicity/tolerability issues.To address these constraints, it will be necessary to understand better diverse cell structure components, cell metabolism pathways, and microenvironments and support the creation of innovative nanostructures with specialized characteristics for neurological disorders.
Acknowledgments:All authors are grateful for the support from their associated scientific institutions.
Author contributions:Conception, literature search and evaluation, initial draft: AU; conception, literature evaluation, editing/revision: MAK; conception,editing/revision, literature evaluation: NHG.All authors approved the final version of the manuscript.
Conflicts of interest:There are no conflicts of interest.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Shaul Schreiber, Tel Aviv Sourasky Medical Center, Israel.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain
- The role of fibronectin in multiple sclerosis and the effect of drug delivery across the blood-brain barrier

