A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain
Angelina S.Bortoletto, Ronald J.Parchem
AbstractType 2 diabetes mellitus patients have a markedly higher risk of developing dementia.While multiple factors contribute to this predisposition, one of these involves the increased secretion of amylin, or islet amyloid polypeptide, that accompanies the pathophysiology of type 2 diabetes mellitus.Islet amyloid polypeptide accumulation has undoubtedly been implicated in various forms of dementia,including Alzheimer’s disease and vascular dementia, but the exact mechanisms underlying islet amyloid polypeptide’s causative role in dementia are unclear.In this review, we have summarized the literature supporting the various mechanisms by which islet amyloid polypeptide accumulation may cause neuronal damage, ultimately leading to the clinical symptoms of dementia.We discuss the evidence for islet amyloid polypeptide deposition in the brain, islet amyloid polypeptide interaction with other amyloids implicated in neurodegeneration, neuroinflammation caused by islet amyloid polypeptide deposition, vascular damage induced by islet amyloid polypeptide accumulation, and islet amyloid polypeptide-induced cytotoxicity.There are very few therapies approved for the treatment of dementia, and of these, clinical responses have been controversial at best.Therefore, investigating new, targetable pathways is vital for identifying novel therapeutic strategies for treating dementia.As such, we conclude this review by discussing islet amyloid polypeptide accumulation as a potential therapeutic target not only in treating type 2 diabetes mellitus but as a future target in treating or even preventing dementia associated with type 2 diabetes mellitus.
Key Words:Alzheimer’s disease; amylin; amyloid; dementia; diabetes; human islet amyloid polypeptide; islet amyloid polypeptide; protofibrils; type 2 diabetes mellitus; vascular dementia
Introduction
Dementia refers to neurological symptoms that include impaired memory,learning, language, problem-solving, and emotional regulation (Alzheimer’s Association, 2021).Although associated with advanced age, dementia is pathologic and is not a normal part of aging.The most common cause of dementia is Alzheimer’s disease (AD), which is estimated to account for up to 80% of dementia cases (Alzheimer’s Association, 2021).Other causes of dementia include cerebrovascular disease, Lewy body disease, frontotemporal lobar degeneration, Parkinson’s disease (PD), and hippocampal sclerosis(Brenowitz et al., 2017; Kapasi et al., 2017).Despite recent controversy, the underlying pathology in many of these diseases is believed to be driven by the accumulation of misfolded proteins (Table 1), including amyloid-beta (Aβ),Tau, alpha-synuclein (ɑ-Syn), and TAR DNA binding protein-43.Over 50 million people worldwide have dementia, but this number is estimated to triple by 2050 (Alzheimer’s Association, 2021; World Health Organization, 2022).Although dementia is considered a disease of the elderly, equally concerning is the incidence of dementia in younger adults, which increased by 200% from 2013 to 2017 (BCBSA, 2020).Thus, dementia represents a significant financial and social burden worldwide.Similar trends of increasing disease burden have been reported in type 2 diabetes mellitus (T2DM).T2DM is a metabolic disease of misregulated glucose homeostasis that currently affects over 400 million people worldwide and is expected to increase to over 700 million people by the year 2045.Similar to early-onset dementia, the recent increase in prediabetes and T2DM in adolescents and young adults is especially concerning.Prediabetes, also known as impaired glucose tolerance, occurs when blood glucose levels are above normal yet not at the level of T2DM.However, 70% of prediabetic individuals progress to over T2DM within their lifetime (International Diabetes Federation, 2021) In 2019, 20% of adolescents and 25% of young adults were considered prediabetic (Viner et al., 2017; Magliano et al., 2020) Recently,studies have determined that dementia is linked with T2DM.It is estimated that nearly 40% of AD patients have diabetes and upwards of 80% of AD patients have impaired glucose tolerance (Janson et al., 2004; Alzheimer’s Association, 2021).Studies have also described the increased incidence of dementia in T2DM patients, and T2DM is now considered a predictive factor in the development of AD and other neurodegenerative diseases (Ott et al.,1999; Haan et al., 2003; Gudala et al., 2013; Ninomiya, 2014; Biessels and Despa, 2018; Raimundo et al., 2020).
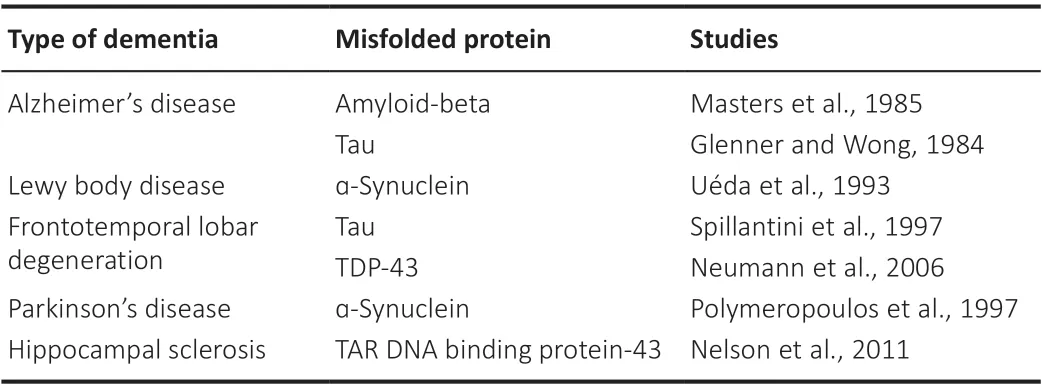
Table 1 |Misfolded proteins implicated in neurodegenerative diseases
Multiple factors likely contribute to the increased risk of dementia in T2DM patients.One component involves the toxic accumulation of amylin, or islet amyloid polypeptide (IAPP).Biologically active monomeric IAPP is cosecreted with insulin from pancreatic β cells and functions as a hormone that aids in metabolism regulation and satiety signaling (Hay et al., 2015; Zhang et al., 2016).As insulin secretion increases with hyperglycemia in diabetic and prediabetic patients, a simultaneous increase in IAPP secretion also occurs.IAPP is prone to aggregation, and minimal increases in IAPP levels can lead to the formation of toxic oligomers in the pancreas, similar to other amyloidogenic peptides, such as Aβ.Although amyloid fibrils and plaques can lead to cell death, recent data suggests that the most cytotoxic form of amyloids is the pre-fibrillar or protofibril form.As monomeric IAPP is soluble in serum and IAPP protofibrils can form in the serum and cross the bloodbrain barrier, it is believed that IAPP aggregation in the brain contributes to neurodegeneration (Banks et al., 1995; Rodriguez Camargo et al., 2018).Adult pancreatic β cells and neurons both have minimal regenerative capacity.Thus, identifying new therapeutic approaches to prevent cell death is of great interest in T2DM and dementia.
In this review, we consider the evidence supporting the role of IAPP aggregation in dementia as well as future perspectives on IAPP as a therapeutic target.We discuss the physiological role of IAPP and highlight the current evidence demonstrating IAPP deposition in the brain, the interaction of IAPP with other amyloidogenic peptides, IAPP-induced inflammation, IAPPinduced vascular damage, and IAPP-induced cytotoxicity.Finally, we introduce recent studies suggesting that targeting IAPP may be a novel therapeutic approach for treating or preventing dementia in the future.
Search Strategy and Selection Criteria
The selection criteria for this review included a literature search of the PubMed database of works published between 2000 and 2022.Most of the studies included in this review (> 70%) were published between 2010 and 2022, and any studies published before 2000 are included for historical reference.Search criteria for this review included the following terms in the abstract or the title: IAPP and Alzheimer’s disease, amylin and Alzheimer’s disease, type 2 diabetes mellitus and Alzheimer’s disease, IAPP and dementia,amylin and dementia, type 2 diabetes mellitus and dementia.Any additional articles were included from suggestions from citation tracking.
Physiological Function of Islet Amyloid Polypeptide
Monomeric IAPP is a hormone that modulates satiety signaling in the brain(Lutz et al., 1994, 1998; Chapman et al., 2007).The receptors for IAPP are heterodimeric complexes between the calcitonin receptor, a G proteincoupled receptor, and one of three receptor activity-modifying proteins(Christopoulos et al., 1999; Coester et al., 2020).The binding of IAPP to the IAPP receptor leads to an increase in cyclic adenosine monophosphate concentrations and activation of various downstream signaling pathways(Figure 1A; Boccia et al., 2020).IAPP receptors have been identified in multiple areas of the brain, including the medial preoptic area, the arcuate nucleus, the ventromedial hypothalamus, the dorsomedial hypothalamic nucleus, the nucleus accumbens, the area postrema (AP), the caudal nucleus of the solitary tract, the organum vasculosum of the lamina terminalis, the choroid plexus, the median eminence, and the dorsal raphe (Paxinos et al.,2004; Boccia et al., 2020).More recently, however, whole-brain imaging showed that IAPP uptake primarily occurs in the AP, the organum vasculosum of the lamina terminalis, the arcuate nucleus, the choroid plexus, and the median eminence (Skovbjerg et al., 2021).
The binding of IAPP to its receptor in the AP has been extensively studied.IAPP receptor binding on noradrenergic neurons in the AP leads to the activation of nuclei located in the lateral parabrachial nucleus, which then increases satiety signaling (Figure 1B and C; Lutz et al., 1998, 2001; Potes et al., 2010).AP neurons can also signal to the ventral tegmental area, which modulates dopaminergic reward signaling.AP neurons activated by IAPP also stimulate the vagus nerve, which innervates the stomach to delay gastric emptying.Delayed gastric emptying is another mechanism of appetite suppression and satiety signaling (Wickbom et al., 2008; Zakariassen et al.,2020).In addition to signaling in the AP, IAPP modulates satiety signaling by interacting with leptin to increase leptin signaling in the hypothalamus, thus decreasing food intake and hunger (Roth et al., 2008; Seth et al., 2012; Li et al., 2015).Finally, IAPP can bind receptors in the arcuate nucleus, reducing food intake and increasing energy expenditure (Li et al., 2015).
Aside from modulating energy homeostasis, studies have established a role for IAPP in nociception, as long-term IAPP administration in rats leads to an increased response to neuropathic pain (Figure 1F; Almeida et al., 2019).IAPP-calcitonin signaling in the medial preoptic area of female mice has also been shown to promote mother-pup bonding in maternal adaptation (Dobolyi,2009; Szabó et al., 2012) and to promote affiliative social behaviors between adult female mice (Figure 1D and E; Fukumitsu et al., 2022).Although most studies of IAPP signaling have been centered on the brain, researchers have also shown that IAPP can affect blood pressure.IAPP can bind receptors in the kidney, and a small human study found that systemic infusion of IAPP led to increased plasma renin levels, suggesting that IAPP may induce hypertension(Cooper et al., 1995).However, other studies have shown that IAPP infusions lead to a relaxation of vascular tone, implying a vasodilatory effect of IAPP(Westfall and Curfman-Falvey, 1995; Edvinsson et al., 2001).
Pathophysiology of Increased Islet Amyloid Polypeptide Secretion
One hallmark feature of T2DM is insulin resistance.During homeostasis,pancreatic β cells sense increased blood glucose levels via GLUT2-mediated glucose uptake.In response to intracellular glucose, β cells secrete insulin and IAPP through the constitutive secretory pathway (Germanos et al., 2021).Circulating insulin binds insulin receptors in various tissues, including skeletal muscle, the liver, the kidneys, the gastrointestinal tract, adipose tissue,and the brain.Insulin receptor binding then triggers multiple mechanisms designed to normalize blood glucose levels.In tissues such as muscle, liver,and adipose tissue, insulin receptor binding triggers the cellular uptake of glucose via GLUT4 glucose transporters (Galicia-Garcia et al., 2020).Additionally, insulin receptor binding activates signaling cascades that inhibit gluconeogenesis, promote glycogen storage in the liver, and inhibit lipolysis in adipose tissue.These cellular events all contribute to maintaining glucose homeostasis.
These processes can become dysregulated through genetic and environmental causes, leading to insulin resistance, prediabetes, and,eventually, T2DM (Galicia-Garcia et al., 2020).However, insulin resistance ultimately drives the increases in IAPP secretion.Insulin resistance can occur via multiple mechanisms, including decreased tissue responses to insulin and hormone secretion from excess adipose tissue that can interfere with insulin signaling (Galicia-Garcia et al., 2020).It is important to mention that insulin resistance can also occur in the brain and has been shown to contribute to neurodegeneration seen in AD (Wei et al., 2021).As a result of insulin resistance in these various tissues, the negative feedback system that normally tamps down insulin secretion as blood glucose levels fall is not activated, and thus insulin secretion remains high in response to continually high blood sugar levels.Another consequence of insulin resistance is β cell hypertrophy, which may also occur due to insufficient glucose control (Sasaki et al., 2021).Both β cell hypertrophy and increased insulin secretion by existing β cells contribute to abnormally high levels of IAPP, which ultimately results in toxic aggregation.
Although insulin and IAPP are primarily secreted through the canonical secretory pathway, recent studies have shown that intracellular IAPP and IAPP aggregates can be trafficked out of the cell through a type of extracellular vesicle called exosomes (Ribeiro et al., 2017; Burillo et al., 2021).While this mechanism is somewhat protective for β cells,in vitrostudies have shown that these IAPP-containing exosomes can induce apoptosis when added to hippocampal neuron cultures (Burillo et al., 2021).Intracellular Aβ and ɑ-Syn accumulation has been shown to induce exosome formation and the spread of Aβ and ɑ-Syn deposition within the brain (Song et al., 2020).Because of this, IAPP accumulation in the brain may also induce exosome formation and the spreading of IAPP deposition.However, despite the ability of exosomes to cross the blood-brain barrier and exacerbate neurodegeneration in the brain,further studies will be needed to determine the contribution of exosomalversusnon-exosomal IAPP to the pathology of dementia (Banks et al., 2020).
Islet Amyloid Polypeptide Accumulation in the Brain
Because monomers and small oligomers of IAPP can cross the blood-brain barrier (Banks et al., 1995), it is possible for IAPP oligomers to accumulate within the brain, as it does within the islets of the pancreas.IAPP deposition has been shown to occur in the brains of both T2DM and AD patients (Jackson et al., 2013; Fawver et al., 2014).Various studies have suggested that this accumulation is due to the deposition of circulating IAPP and not brainderived IAPP, as there is a minimal expression of IAPP mRNA in brain tissue(Banks et al., 1995; Banks and Kastin, 1998).Human IAPP (hIAPP) transgenic rodent models used for studying IAPP deposition in T2DM pathology have also been used to study IAPP deposition in the brain.For example, the human IAPP transgenic (HIP) rat model, which expresses human IAPP under the control of the rat insulin II promoter (Butler et al., 2004), was used to identify IAPP deposits in the brain.Compared to age-matched controls, HIP rats also exhibited significant decreases in coordination, learning, and memory(Srodulski et al., 2014).Another study using a similar transgenic mouse model identified hIAPP accumulation in the hippocampus of transgenic mice and that the level of IAPP depended on time and the presence of a high-fat diet.In addition, the increase in hippocampal IAPP also correlated with increased Aβ deposition and neuron toxicity, suggesting a potential interaction between IAPP and Aβ (Xi et al., 2019).Temporal lobe sections of AD patients with known mutations in presenilin 1 and amyloid precursor protein also showed the presence of IAPP within Aβ plaques (Ly et al., 2021).
Islet Amyloid Polypeptide and Amyloid-Beta
Because IAPP has been shown to co-localize with Aβ, various groups have also studied the idea that IAPP can pathologically interact with Aβ and other amyloidogenic peptides implicated in multiple forms of dementia.Interestingly, IAPP and Aβ share about 25% sequence homology and 50%sequence similarity, further supporting the possibility that they interact(O’Nuallain et al., 2004).Indeed, two regions (aa 11–21 and aa 23–37) of the pathological forms of Aβ, Aβ40, and Aβ42, exhibit a high binding affinity for IAPP.In contrast, two analogous areas on IAPP (aa 8–20 and aa 21–37)exhibit a corresponding affinity for Aβ40and Aβ42(Andreetto et al., 2010).In support of this hypothesis, multiple studies have shown that IAPP can act as seeds in the formation of Aβ aggregates bothin vitro(O’Nuallain et al.,2004; Ono et al., 2014; Ge et al., 2018) andin vivo(Oskarsson et al., 2015;Moreno-Gonzalez et al., 2017).One reason IAPP deposits may act as seeds for Aβ aggregation is that IAPP oligomerizes more quickly than Aβ.Similar to most amyloids, IAPP oligomerization involves multiple steps.Linear IAPP initially forms small multimers in an ɑ-helix structure before forming a β-fold structure.Subsequently, it forms a polymer and a fiber structure before finally forming nondegradable plaques (Figure 2A).However, compared to Aβ, the formation of IAPP aggregates occur much more quickly, with a far shorter lag phase (Figure 2B; Bharadwaj et al., 2020; Al Adem et al., 2022).The quicker formation of IAPP oligomers may provide points of seeding for Aβ oligomerization.
The interaction between IAPP and Aβ has also been studied as a possible therapeutic modality in AD.IAPP analogs have been shown to prevent Aβ fibrillizationin vitroby acting as a“sponge”for excess Aβ.It is thought that instead of Aβ self-assembling, IAPP analogs act as a competitive inhibitor for Aβ self-binding to prevent Aβ aggregation (Yan et al., 2007).In vivostudies also showed that treating amyloid precursor protein/presenilin 1 transgenic AD mice with pramlintide, an IAPP drug analog, provided a neuroprotective effect on animals (Patrick et al., 2019).As IAPP analogs cannot oligomerize like native IAPP, IAPP-analog/Aβ oligomeric heterocomplexes do not form.Thus, this neuroprotective effect is likely due to monomeric IAPP interacting with monomeric Aβ, preventing Aβ oligomerization.
Islet Amyloid Polypeptide and Other Amyloid Peptides (Tau and α-Synuclein)
More recently, studies have been published that suggest IAPP can interact with Tau, another aggregation-prone peptide that has been causatively linked with AD and other types of dementia.Tau pathology is driven by the intracellular aggregation of hyperphosphorylated Tau filaments into Tau tangles.In vitrostudies have shown that even at low concentrations of IAPP and Tau, the formation of hetero-complexes still occurs (Arya et al., 2019).Further substantiating these data,in vivostudies utilizing brain tissue from AD patients showed co-localization of Tau with IAPP (Zhang et al., 2022).In addition, the presence of IAPP during Tau aggregation leads to an increase in the formation of highly toxic Tau oligomer species (Zhang et al., 2022).In the same study, IAPP-Tau heterocomplexes were found to be highly toxic to neurons, leading to neuroinflammation and clinical signs of neuronal dysfunction in mice.
Another protein implicated in Lewy body disease and PD is ɑ-Syn.Although no studies suggest that IAPP oligomers act as seeds for ɑ-Syn accumulation in the brain, recent studies have shown that ɑ-Syn and IAPP interact within pancreatic islets to promote IAPP oligomerization (Mucibabic et al., 2020;Martinez-Valbuena et al., 2021).Other studies have also demonstrated that circulating IAPP levels are increased in PD patients (Sánchez-Gómez et al., 2020).Together, these data suggest that the IAPP aggregation may also contribute to the neuropathology of Lewy body disease and PD.
Islet Amyloid Polypeptide and Neuroinflammation
Aside from the possible pathologic interaction between IAPP and other commonly misfolded proteins, IAPP oligomers may exert toxicity by inducing a neuroinflammatory response within the brain (Figure 3A).Neuroinflammation has been extensively studied in various forms of dementia, and most researchers agree that it contributes to neurodegeneration (Azevedo and Foguel, 2019).One study in the HIP rat model showed that IAPP deposits within the brain induced neuroinflammation and microglia clustering around IAPP deposits.Brain protein homogenates from HIP rat brains also exhibit increased levels of pro-inflammatory cytokines such as tumor necrosis factor-ɑ and interleukin (IL)-6 (Srodulski et al., 2014).IAPP aggregation has also been shown to cause inflammation and β cell death in pancreatic islets in T2DM by inducing IL-1β secretion by islet-resident macrophages.In β cells, IL-1β induces mitochondrial oxidative stress and results in cell death (Westwell-Roper et al., 2014).Microglia act as the tissue-resident macrophages of the brain, and activation of IAPP receptors on microglia by IAPP also induces the secretion of the pro-inflammatory cytokines tumor necrosis factor-ɑ and IL-1β (Fu et al., 2017), which may cause inflammationmediated neuron damage similar to β cell damage in the pancreas.
Islet Amyloid Polypeptide and Microvascular Injury
After AD, cerebrovascular or vascular dementia is the next most common cause of dementia.Vascular dementia refers to cognitive impairment driven by ischemic events within the brain (Iadecola, 2013) and individuals with T2DM are twice as likely to develop vascular dementia (Ahtiluoto et al., 2010).IAPP aggregation was recently shown to induce inflammation and cell death in pancreatic islet endothelial cells (Castillo et al., 2022), suggesting that IAPP aggregation may cause similar vascular dysfunction in the brain (Figure 3B).Indeed, studies of brain tissue from dementia patients with T2DM have shown IAPP deposition within the cerebral microvasculature (Ly et al., 2017;Schultz et al., 2017).In addition,in vitroexperiments have demonstrated that IAPP accumulation induces cell death in human cerebral vascular pericytes(Schultz et al., 2017).Microvascular damage can lead to white matter injury,which is corroborated by HIP rats showing increased incidents of intracerebral hemorrhage, microvascular injury, and perivascular recruitment of reactive astrocytes, culminating in diffuse white matter damage (Ly et al., 2017).
Mechanistic studies of how IAPP deposition induces microvascular damage in the brain have shown that IAPP deposits may induce damage through multiple processes.Intravascular IAPP deposits cause the activation of IL-1β-mediated inflammation and, ultimately, cell death (Verma et al., 2016).In addition,these IAPP deposits have also been shown to activate the hypoxia signaling through the activation of hypoxia-induced factors 1 & 2 (HIF-1 and HIF-2) in endothelial cells (Verma et al., 2020).Downstream effects of HIF-1 and HIF-2 activation include the dysregulation of nitric oxide signaling and subsequent increases in vascular smooth muscle tone, which may impair cerebral blood flow and lead to ischemia (Verma et al., 2020).Finally, IAPP aggregation within the circulatory system can promote microthrombi formation due to deposition on red blood cells (Verma et al., 2020).
Islet Amyloid Polypeptide and Neuron Cytotoxicity
Another mechanism by which IAPP oligomers may contribute to the underlying pathology seen in dementia is by directly inducing neuronal cell death.IAPP oligomers are known to be cytotoxic to various types of cells (Raleigh et al., 2017; Wu et al., 2017; Abedini et al., 2018).Pre-fibrillar IAPP oligomers, termed protofibrils, have been shown to interact with lipid bilayer membranes.This interaction can lead to membrane permeabilization,destabilization, and cell death (Figure 3C; Caillon et al., 2016; Saghir et al.,2021).IAPP has also been shown to cause mitochondrial dysfunction by disrupting oxidative phosphorylation and inducing the production of reactive oxygen species.Interestingly, this mechanism is similar to how Aβ causes mitochondrial dysfunction (Lim et al., 2010).
Indeed, multiple studies have shown that IAPP oligomers can induce cell death in various neuronal populations.The addition of human IAPP to primary cultured rat hippocampal and cortical neurons induces cytotoxicity after peptides begin to aggregate in solution, suggesting that IAPP oligomers and not monomers are cytotoxic to neurons.In addition, similar experiments using rat and mouse IAPP do not induce toxicity.These experiments further support the oligomeric state of IAPP as the toxic species, as neither mouse nor rat IAPP oligomerizes (Lim et al., 2008; Zhang et al., 2020).Zhang et al.(2020)also showed that adding human IAPP to cultured neurons induced neuronal cell death due to membrane permeabilization and a subsequent increase in reactive oxygen species.In vitrostudies using the PC-12 fetal neuron-like cell line also showed that both IAPP and IAPP-Aβ aggregates induced cell death(Al Adem et al., 2022).Further substantiating the neuronal toxicity of IAPP aggregates,in vitrostudies blocking IAPP aggregation have been shown to prevent neuronal cell death caused by oxidative stress and inflammation (Yu et al., 2015)
Islet Amyloid Polypeptide Aggregates as a Therapeutic Target
A plethora of recent data suggests a pathogenic role for IAPP aggregation in dementia.Thus, compounds that could prevent IAPP aggregation offer the potential for novel therapeutic strategies for the prevention of dementia.Inhibiting the aggregation of other misfolded proteins in dementia has been studied (Gupta et al., 2012; Bonito-Oliva et al., 2017, 2019), and the use of monoclonal antibodies preventing the deposition of misfolded proteins has shown some clinical promise (Vaikath et al., 2015; Arndt et al., 2018;Avgerinos et al., 2021).Recently, the inhibition of IAPP aggregation as a possible treatment for T2DM has gained attention.Immunization against IAPP and monoclonal antibodies targeting IAPP fibrils have shown a modest effect on clinical symptoms in mouse models of T2DM (Bram et al., 2017; Vogt et al., 2021).Our group recently published a study showing that treatment with monoclonal antibodies specific for IAPP protofibrils delayed and even prevented disease in a rapidly progressive murine model of T2DM (Bortoletto et al., 2022).Treatment with protofibril-specific antibodies directly inhibited apoptosis of pancreatic β cells and inhibited the increased islet macrophage infiltration in severely diabetic mice.Due to the evidence suggesting that IAPP oligomers induce neuron toxicity and neuroinflammation, targeting IAPP protofibrils may provide an exciting new opportunity for preventing or treating dementia in the future (Figure 3D).
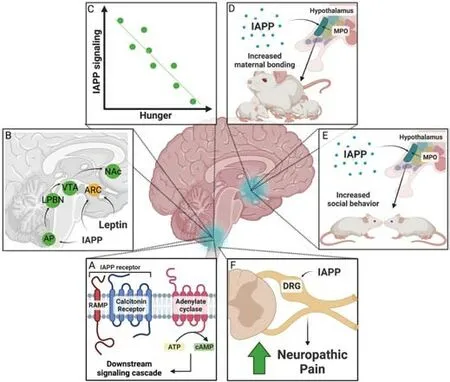
Figure 1|Overview of physiological IAPP signaling in the brain.
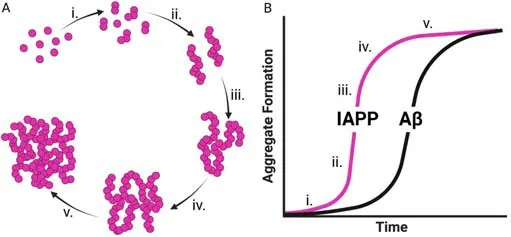
Figure 2|Kinetics of IAPP oligomerization.
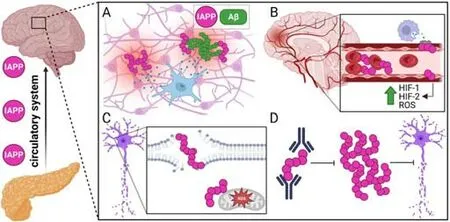
Figure 3|Overview of brain pathology induced by oligomeric IAPP and possible intervention.
Conclusions and Future Directions
In summary, current evidence suggests a strong connection exists between T2DM and dementia.Recent trends show that the size and proportion of older individuals in the population are expanding.This fact, combined with increases in early-onset dementia cases and adolescent and young adult T2DM, highlight an urgent need to identify new ways to modulate the increased risk of developing dementia in T2DM patients.Recent mechanistic studies have identified a role for the accumulation of the T2DM-associated peptide, IAPP, in driving brain pathology associated with dementia.Not only has IAPP been shown to interact with other misfolded proteins implicated in dementia, but IAPP oligomers have also been shown to induce neuroinflammation, microvascular damage, and neuron cytotoxicity.Similar to β cells in the pancreas, adult neurons retain a minimal capacity to regenerate.Therefore, there is a great interest in identifying new ways to preserve neuronal function and prevent neuron death in dementia.Combined with recent promising studies showing monoclonal antibodies targeting IAPP protofibrils as a novel therapeutic strategy in T2DM, these data suggest that IAPP aggregation may also be an appropriate target to prevent dementia.
Author contributions:ASB and RJP conceived and designed the review.ASB performed the literature review and drafted the manuscript.Both authors read and approved the final manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Carlos Guillén, Universidad Complutense de Madrid,Spain.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- The role of fibronectin in multiple sclerosis and the effect of drug delivery across the blood-brain barrier

