The role of fibronectin in multiple sclerosis and the effect of drug delivery across the blood-brain barrier
Shuang-Shuang Wei, Le Chen, Feng-Yuan Yang, Si-Qi Wang, Peng Wang
AbstractRemyelination failure is one of the main characteristics of multiple sclerosis and is potentially correlated with disease progression.Previous research has shown that the extracellular matrix is associated with remyelination failure because remodeling of the matrix often fails in both chronic and progressive multiple sclerosis.Fibronectin aggregates are assembled and persistently exist in chronic multiple sclerosis, thus inhibiting remyelination.Although many advances have been made in the mechanisms and treatment of multiple sclerosis, it remains very difficult for drugs to reach pathological brain tissues; this is due to the complexity of brain structure and function, especially the existence of the blood-brain barrier.Therefore, herein, we review the effects of fibronectin aggregates on multiple sclerosis and the efficacy of different forms of drug delivery across the blood-brain barrier in the treatment of this disease.
Key Words:blood-brain barrier; brain delivery; exosomes; extracellular matrix; fibronectin aggregates;fibronectin; intestinal flora; multiple sclerosis; remyelination failure; remyelination
Introduction
Multiple sclerosis (MS) is associated with a complex array of symptoms,including visual impairment, limb weakness, paresthesia, and ataxia.This disease often occurs in young and middle-aged individuals and is characterized by repeated attacks (Feinstein, 2007).The most harmful effects of MS are disability and deformity; these effects can affect a patient’s quality of life, thus resulting in the loss of labor, along with remarkable psychological and emotional pain.The predominant pathological feature of MS is demyelination and remyelination failure has been shown to lead to the progression of MS (Franklin, 2002; Bhargava, 2021; den Dunnen et al., 2021).Pharmaceutical treatments for MS have yielded positive results; several of the currently available drugs work by suppressing the immune system and reducing inflammation.
As a primary source of new remyelinating cells, oligodendrocyte precursor cells (OPCs) are widely and abundantly distributed in the adult central nervous system (CNS).Given their ability to self-renew and generate certain neurons, OPCs can reasonably be deemed to represent a type of adult neural stem cell (Nunes et al., 2003).It is possible that remyelination failure arises due to an insufficient supply of OPCs or the lack of OPCs differentiating into remyelinating oligodendrocytes (Smith et al., 1979; Cherchi et al.,2021).In toxin-induced demyelinating lesions, an intracavitary injection of fibronectin (Fn) aggregates (aFn) was shown to hinder OPC differentiation and remyelination, thus indicating that aFn probably leads to remyelination failure in MS patients (Stoffels et al., 2013).As Fn mRNA is rarely detected in chronic MS, aFn is synthesized extracellularly, thus indicating that aFn is the result of an Fn clearance disorder and not the consequence of an upregulation in Fn expression.Consequently, degrading aFn effectively might be an alternative option with which to cure MS.Protein histochemistry analysis previously revealed 18 predicted secreted proteins in aFn derived from rat astrocytes,including Fn, thrombospondin-1 (TSP1), tenascin-C (Tn-C), cysteine-rich61(CYR61) and heat shock proteins HSP70, HSP47, and HSP90 (Sikkema et al.,2018).
Many factors can preserve remyelination and are expected to become potential therapeutic drugs.Exogenous ganglioside-1a is known to inhibit aFn on (re)myelination through a protein kinase A-dependent signaling pathway (Qin et al., 2017).Furthermore, platelet-derived growth factor(PDGF) and fibroblast growth factor-2 are predicted to generate remyelinated oligodendrocytes (Murtie et al., 2005).In another study, the clinical symptoms and histopathological manifestations of a mouse model of experimental autoimmune encephalomyelitis (EAE) treated with a mixture of lactobacillus probiotics were alleviated to varying degrees (Lavasani et al., 2010).Clinical intervention with intestinal probiotics might provide a new concept for the treatment of MS.Indeed, the incubation of MS microflora with human peripheral blood mononuclear cells can induce pro-inflammatory reactionsin vitro, thus indicating that MS microflora lack beneficial microorganisms for regulating self-immunity and excessive pro-inflammatory bacteria (Cox et al.,2021).Considering the role of intestinal disorders in promoting susceptibility to MS, an attractive treatment might be to restore the balance of microflora,or to use the communication between the immune system and intestinal microbes to suppress the autoimmune reaction.The blood-brain barrier (BBB) prevents medications to treat brain diseases from entering the brain; in fact, even the very smallest molecules cannot penetrate the BBB (Nair et al., 2018).Nano-drug delivery systems have become a promising method given their abilities to protect drugs from degradation, to transport drugs to action targets, to prolong the blood circulation time, and reduce toxicity to the human body.Of these systems,exosomes play an important role in the exchange of information between cells and are widely distributed in the human body.Exosomes can cross the cell membrane, do not readily cause an immune response, and have unique advantages as drug carriers (Zhang and Yu, 2019).At present, there is marked interest in exosomes in the medical field and we are anticipating the future use of exosomes as new delivery carriers and treatment methods; this may provide hope for patients with MS.Hence, in this review, we intend to discuss the effects of aFn components on myelin regeneration and drug delivery systems that can cross the blood-brain barrier to treat MS.
Search Strategy
We performed electronic searches using PubMed and Google Scholar databases.The publication time and article type were unlimited and reviews published within the past 5 years up to March 15, 2022 were preferred.The key words were“multiple sclerosis + extracellular matrix”,“fibronectin +multiple sclerosis”,“multiple sclerosis + remyelination failure”,“remyelination failure + fibronectin aggregates”,“multiple sclerosis + intestinal flora”,“brain delivery + blood-brain barrier”,“blood-brain barrier + exosomes”and“brain delivery + exosomes”.Selected literature were further screened by reading the titles and abstracts.
Multiple Sclerosis and Remyelination Failure
MS
MS is a chronic autoimmune disease of the CNS.The pathogenesis of MS remains unknown although it is evident that autoimmune responses, viral infections, environmental factors, and genetic susceptibility contribute to its occurrence.Moreover, vitamin D deficiency, lack of sunlight, and smoking may induce this disease (Compston and Coles, 2008).Given the diverse array of affected areas, the clinical manifestations of MS are complex and varied.Optic neuritis, myelitis, and brainstem or cerebellar disorders may result in the most common initial clinical features, such as visual disturbance, limb powerlessness, activity obstacle, and ataxia (Goldenberg, 2012).Based on the progression of MS, patients can be divided into four major categories:relapsing-remitting MS, secondary progressive MS, primary progressive MS,and progressive-relapsing MS.In general, remission always alternates with relapse; this is often followed by progressive neurological deterioration over time (Goldenberg, 2012).In the advanced stages of MS, disability and deformity can cause patients to leave the labor force.
Remyelination failure is one of the main features of MS
The myelin sheath is a layer of membrane covering the axons which acts as an insulator, prevents the transmission of electrical impulses from one neuron axon to another, protects the axon, and induces axonal regeneration in cases of axon damage.Demyelination impairs Langfei’s knot, thus jeopardizing effective saltatory conduction.As a chronic inflammatory demyelinating disease, MS leads to focal lesions in the gray and white matter and diffuse neurodegeneration in the entire brain that is characterized by primary demyelination with variable degrees of axonal injury (Haider et al., 2016).In the initial stages of lesions, perivenous demyelination fuses into confluent plaques, which expand into the surrounding normal white matter.This gives rise to a typical sign called Dawson fingers.Although there are many possible interrelations between inflammation, demyelination, and axonal damage, the sequence of inflammation and demyelination remains controversial.However,it has been confirmed that these factors can reinforce each other (Stadelmann et al., 2011).In MS, demyelination is associated with the activation of astrocytes in active tissue injury and the formation of gliotic scars in inactive lesions.Owing to the recruitment and differentiation of oligodendrocyte progenitor cells, MS lesions can be remyelinated, at least to some extent.Furthermore, remyelination can repair structural recovery and aid in the recovery of axonal conduction properties.Interestingly, remyelination failure is a main characteristic of MS and may correlate with the relentless evolution of this disease (Franklin, 2002).It would be possible to postpone the progress of MS if measures can be implemented to intervene after determining the mechanism of remyelination and the causes of remyelination failure.
Immunopathology of MS
As the pathogenesis of MS has not been fully clarified, it is widely considered that the main mechanisms involved may involve predisposing factors that act on patients with genetic susceptibility in a specific environment, thus inducing abnormal autoimmune reactions and causing MS.A central aspect of MS is immunopathology, including adaptive and innate immunity (Lassmann et al.,2007).
For adaptive immunity, CD4+T cells, CD8+T cells, and regulatory T cells are all known to enhance remyelination, while Th17 lymphocytes exhibit an inhibitory effect on remyelination (Baxi et al., 2015; Dombrowski et al., 2017).The expansion of Th17 lymphocytes or a reduction in the number of CD4 T/CD8T/regulatory T cells may induce MS.Therefore, to a certain extent,MS is a type of T cell-mediated autoimmune disease.However, the role of humoral immunity in MS remains unclear.It has been speculated that B cells and plasma cells may be abnormally stimulated to produce antibodies.These antibodies might combine with antigens that induce MS, damage the myelin sheath, or be involved in regulating the course of MS (Comi et al.,2021).In an EAE model, B cells were shown to act as antigen-presenting cells and interacted with CD4+T cells to initiate an adaptive immune response,thus causing inflammation of the myelin sheath antigen (Parker Harp et al.,2018).In addition, the inflammatory and clinical manifestations of EAE in rats were improved by inhibiting B cell immunity (Myhr et al., 2019).It has been suggested that B cell immunity plays an essential role in regulating the inflammatory response of the CNS and may be involved in the pathogenesis of MS.
In the innate immune system, mast cells release molecules to recruit and activate T cells or macrophages; these can present myelin antigens to T cells and destroy the BBB to allow activated T cells to infiltrate the brain(Xu and Chen, 2015).Activated natural killer cells can regulate immune and inflammatory responses by secreting immune regulatory factors and killing target cells in MS.Evidence indicates obvious cell infiltration, CNS inflammation, and demyelination in animals with depletion of natural killer cells, thus indicating that natural killer cells might perform protectively in the progression of MS (Kaur et al., 2013).Given their ability to clear myelin debris,macrophages and microglia contribute to myelin wrapping (Kotter et al., 2001;Lloyd and Miron, 2019).Demyelination and neurodegeneration are associated with macrophage accumulation and microglial activation in injured tissues(Voet et al., 2019).Infiltrating macrophages produce numerous inflammatory mediators that are crucial for autoimmune diseases (Di Benedetto et al.,2019).These not only promote active invasion and tissue destruction, but also support the regeneration of lesions and remyelination.These different functions of macrophages suggest that they exist as proinflammatory (M1)or anti-inflammatory (M2) phenotypes.Under normal circumstances, nonactivated microglia help to maintain the stability of the nervous system (Shields et al., 2020).Once activated, microglia support inflammatory responses such as phagocytosis and the clearance of debris, the production of growth factors,and the formation of neuronal circuits (Loane and Byrnes, 2010).In addition to supporting inflammation and impairing nerves, activated microglia can protect nerves and release cytokines, chemotactic factors, nitric oxide (NO),and reactive oxygen species (Minagar et al., 2002).Cytokines, interleukin (IL)-1β, tumor necrosis factor α, and reactive oxygen species, can induce mitogenactivated protein kinases, thus leading to the transcriptional enhancement of matrix metalloproteinases (MMPs) and the reduction of tissue inhibitor of metalloproteinases, inducing extracellular matrix (ECM) remodeling (van Horssen et al., 2007).
Remodeling of the Extracellular Matrix
The ECM is located between neurons and gliacytes and accounts for 10-20% of the cumulative volume of the brain.As a highly organized network composed of macromolecular proteins, the ECM plays a significant role in regulating cell growth, polarity, shape, migration, and metabolic activities,thus forming a complex and dynamic microenvironment (de Jong et al.,2020).In addition to providing physical support for neurons and glial cells,the ECM also acts as a reservoir for signaling molecules, such as growth factors, indirectly influencing cell behavior.Different organs or tissues have different ECM components, and almost all cell types can produce and secrete ECM molecules (Barthes et al., 2014).The ECM is composed of several macromolecules that can be approximately divided into five categories:collagen, non-collagen protein, elastin, proteoglycan, and glycosaminoglycan.The holistic biological characteristics of the ECM depend on the various compositions and structures of the ECM components; these characteristics can influence signal transmission and cellular responses.
For instance, chondroitin sulfate proteoglycans, the main proteoglycans in the CNS, generally plays an inhibitory role by binding to cell surface receptors or interacting with growth factors and cytokines to regulate cellular physiology.Laminin is a large extracellular heterotrimeric glycoprotein that exhibits a range of significant effects in the CNS by interacting with integrin and nonintegrin receptors.In addition to diffusing from the ECM, ECM molecules can also condense into extracellular compartments, including peri-neuronal nets,peri-synaptic nets, and the basement membrane related to the BBB.
The dynamic and sophisticated ECM manipulates development, functional regionalization, and fight-or-flight responses in the CNS via the interplay among ECM molecules, various membrane receptor molecules, and soluble signals, as well as ECM remodeling.The defective assembly, reduced production, and excessive accumulation of ECM proteins are associated with the pathology of MS; these processes lead to detrimental changes in the composition of the ECM (Satoh et al., 2009).Remodeling of the ECM involves transient changes in the ECM proteins that are regulated by matrixdegrading enzymes; these alter behavior to promote recovery in damaged lesions, although this process occurs in both healthy and pathological cases and is a finite process in the CNS of healthy adults (Barthes et al., 2014).ECM remodeling often fails in chronic and progressive MS lesions; this is known as remyelination failure (Lindner et al., 2015).
Fibronectin Aggregates Lead to Remyelination Failure
Fn and aFn
Fn is an ECM component that is instantaneously produced as a dimer after demyelination; Fn is only present in blood vessels and not in the interstitial ECM in the CNS of healthy adults.However, the expression of Fn increases in the white matter of the CNS and blood vessels in lysophospholipid-induced models of demyelination; furthermore, the levels of Fn decrease with the progression of regeneration (Stoffels et al., 2013).The upregulated expression of Fn in MS lesions results from the leakage of plasma across the BBB and the synthesis of astrocytes (Zhao et al., 2009; Hibbits et al., 2012).In vitroexperiments showed that Fn promoted the migration and proliferation of OPCs, whereas Fn coatings disturbed the growth of OPCs and prevented the formation of myelin membranes (Stoffels et al., 2015).Fn is specifically located in demyelinating areas and is removed during remyelination (Stoffels et al., 2013).
Fn has two forms: plasma fibronectin (pFn) and cellular fibronectin (cFn).pFn leaks through the damaged BBB while cFn is mainly synthesized by astrocytes but is also secreted by microglia, macrophages, and endothelial cells (Hibbits et al., 2012).cFn may contain alternately spliced domains.For example,rodents possess extra type III repeat A (EIIIA), extra type III repeat B, and V-regions while humans possess extra domain A of Fn, extra domain B of Fn,and a type III connecting segment (Schwarzbauer et al., 1987).Following the conditional knockout of cFn, the number of OPCs recruited for demyelinating lesions decreased significantly; in contrast, the number of OPCs remained unchanged after pFn conditional knockout.However, after the conditional knockout of cFn and pFn, remyelination was normal.The EIIIA and extra type III repeat B domains of cFn are expressed after demyelination.In vitroexperiments showed that the EIIIA domain of cFn mediates the proliferation of OPC, but not migration.Although the EIIIA domain of cFn mediates OPC proliferation, it is not necessary for successful remyelination (Stoffels et al.,2015).
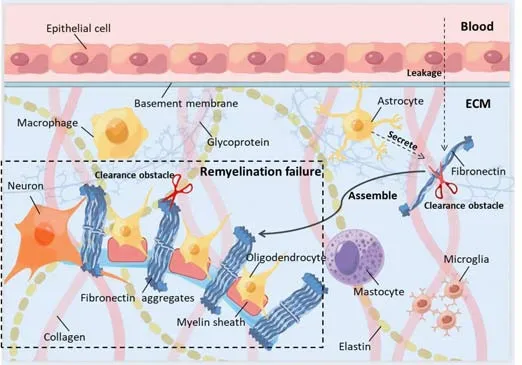
Figure 1|The formation and dysfunction of fibronectin aggregates in MS.
Given the persistent presence of Fn in chronic MS lesions, pFn and cFn assemble into stable aFn, which acts in a similar manner to dimer Fn, thus inhibiting the formation of myelin membranes and myelination in individual and combined culture systems (Sikkema et al., 2018).Moreover, the intraluminal injection of aFn hinders OPC differentiation and remyelination in toxin-induced demyelinating lesions, thus implying that aFn might lead to remyelination failure in patients with MS (Stoffels et al., 2013).Fn mRNA is rarely detected in chronic MS lesions and aFn is synthesized extracellularly,thus indicating that aFn is the outcome of Fn clearance obstruction and not the result of an upregulation in the expression of Fn (Mao and Schwarzbauer,2005; Figure 1).Fn and aFn similarly induce several pro-inflammatory traits that are relevant to microglia and bone marrow-derived macrophages (BMDMs); these traits are primarily associated with the amoeboid morphology of microglia and BMDMs, enhanced microglial proliferation, and improved phagocytosis of BMDMs (Sikkema et al., 2018).Consequently, aFn might represent the appearance of a persistent mode of pFn signaling, although it still exhibits other functions, such as stimulating the microglia and BMDMs to release NO and increasing the expression and activity of arginase-1 in macrophages.This might be due to the accumulation of aFn-based proteins, such as HSP70 and TSP1, which induce NO release rather than associating with integrin.Once microglia and BMDMs are activated by aFn, their general influence remains uncertain (Sikkema et al., 2018).BMDMs phagocytize myelin debris and help remyelination but can also aggravate injury of the myelin sheath and axon 21(Yang et al., 2011).NO enhances immune activity, eventually leading to myelin damage.
aFn impedes microglia or macrophages to express normal activation phenotypes
The activated phenotype of microglia and macrophages determines their precise functional roles.The classically activated phenotype is induced by interferon-γ or lipopolysaccharide, which plays a pro-inflammatory role, whereas the alternate-activated phenotype is stimulated by IL-4 or IL-13, which has an anti-inflammatory effect (Liu et al., 2014).The classically activated microglia and macrophage phenotypes are required for remyelination in the initial stage, although these require transformation into an alternatively activated phenotype to facilitate remyelination in the later period (Miron et al., 2013).After demyelination, Fn is expressed instantaneously, thus inducing the classically activated microglia and macrophage phenotype, which promotes remyelination (Sikkema et al., 2018).However, evidence indicates that persistent aFn stimulates a phenotype for macrophages, and perhaps also includes microglia, thus simultaneously representing both the classically and alternatively activated phenotype; this may hinder remyelination (Sikkema et al., 2018).In general,in addition to impeding the differentiation of OPCs, the inability of microglia or macrophages to express normal activation phenotypes may represents a potential factor responsible for aFn-induced remyelination failure.Therefore,the mechanisms underlying aFn degradation can be considered as effective targets with which to rescue remyelination.
aFn components
In toxin-induced lesions,in situhybridization colocalization studies have shown that astrocytes, microglia, macrophages, and endothelial cells might produce fibronectin (Stoffels et al., 2013).However, it is unclear whether these cells also secrete and deposit fibronectinin vivo.Furthermore,in vitroexperiments have shown that fibronectin is primarily produced by astrocytes.Previous research showed that only astrocytes could actively synthesize and deposit fibronectin and that fibronectin in the plasma does not aggregate without astrocytes.Proteomic analysis revealed that 18 predicted secreted proteins were present in rat astrocyte-derived aFn, including Fn, TSP1, Tn-C,CYR61, and HSP70, HSP47, and HSP90 (Table 1; Stebbins et al., 1997; Shimo et al., 1999; Yoshida et al., 2013; Randell and Daneshtalab, 2017; Sikkema et al., 2018; Tabibian et al., 2019; Xia et al., 2019; Wang and Liu, 2021; Li et al.,2022; Ruzha et al., 2022).
HtrA serine peptidase (HTRA), also known as DegP, is a membrane protein with heat shock properties that acts as a molecular chaperone at low temperatures and as sericin at high temperatures; this enzyme can degrade intracellular misfolded proteins.HTRA1 is a non-glycosylated serine protease that can degrade a variety of substrates, such as the ECM.Furthermore,HTRA1 can interact with a variety of transforming growth factor-β (TGF-β)family proteins to prevent receptor activation and inhibit the conduction of signaling molecules such as bone morphogenetic protein 4, bone morphogenetic protein 2, and TGF-β1 (Oka et al., 2004).HTRA1 can regulate cell differentiation through the TGF-β1/small mothers against decapentaplegic(SMAD) signaling pathway (Li et al., 2018).
As an important member of the cellular communication network family,cysteine-rich protein 61 is an ECM molecule with a wide range of biological characteristics.This protein can regulate cell proliferation, adhesion,migration, and apoptosis, and promote angiogenesis and formation of the ECM.By activating the transcription of cysteine-rich protein 61, injury lesions can induce the growth, adhesion, and proliferation of fibrocytes and vascular endothelial cells, and enhance the expression of MMPs (Lau, 2011).Cysteinerich protein 61 initiates the body injury response, activates p53, induces Rasrelated C3 botulinum toxin substrate 1-nicotinamide adenine dinucleotide phosphate oxidase 1, and stimulates the p16/retinoblastoma protein signaling pathway (Calcinotto et al., 2019).
Thrombospondins are a glycoprotein family that inhibits angiogenesis; of these proteins, TSP1 is secreted by astrocytes and known to support the growth of fresh synapses and protect neurons.TSP1 interacts with CD36 to inhibit the proliferation and migration of endothelial cells (Li et al., 2002).In addition to cell surface receptors, TSP can interact with soluble growth factors.Growth factors moderate TSP synthesis; for example, TSP1 is regulated by PDGF (Majack et al., 1987).Moreover, the expression of TSP is regulated by growth factors; the environment of growth factors, in turn, can also regulate the functionality of these factors.Thus, TSP plays a role in ECM remodeling.Astrocytes secrete TSP1, a protein that is regulated by various growth factors (Scott-Drew and ffrench-Constant, 1997).TSP1 is also known to promote the adhesion and migration of central glia 4 OPCs, which may influence remyelination (Li et al., 2002).
As a chaperone, HSP70 protects neurons from protein aggregation, toxicity,apoptosis, and inflammation, plays an auxiliary role in antigen presentation,and participates in immune response in autoimmune diseases.This may be due to its chaperone activity, which inhibits various latently toxic elements.However, in some cases of HSP70 overexpression or brain dysfunction,the beneficial effects of HSP70 are uncertain.In MS, HSP70 may exert a positive influence due to the downregulated immune response while immunoreactivity is enhanced due to the development and recruitment of extra intra-lesional antigenic targets (Turturici et al., 2011).Without combination with integrin, aFn induces the release of NO from bone marrowderived macrophages and microglia; this might be due to the accumulation of other proteins, such as HSP70, in aFn as a scaffold.The classical and alternative activation phenotypes of macrophages are known to increase when macrophages are co-incubated with aFn (Oka et al., 2004).Similar to aFn, extracellular HSP70 can significantly increase the expression of iNOS in macrophages.HSP70 increases the expression of collagen and Fn via TGF-β1 signal transduction.Extracellular HSP90β directly binds to Fn, which promotes the formation of aFn, which is insoluble in deoxycholate (Cid et al., 2004).HSP70 and HSP90β are both associated with the pathology of MS.HSP47 acts as a receptor for collagen fibrillogenesis in MS, although the specific role of these HSPs in aFn has yet to be determined.
The tenascin family consists of five known members, Tn-C, Tn-R, Tn-X, Tn-Y,and Tn-W.Elevated Tn expression is likely to represent a protective response to inhibit inflammation.Astrogliosis is a feature of inactive MS lesions and central chronic active lesions which exhibit a small number of leukocytes,wide immunoreactivity and express an abundance of ECM molecules, such as Tn-C and Tn-R.Astrogliosis has been shownin vitroto curb the migration of oligodendrocyte type-2 astrocyte (O-2A) progenitor cells (Groves et al., 1993).In the healthy CNS, Tn-C is mainly produced by astrocytes and restricted to the white matter, whereas in acute and sub-acute lesions, the expression of Tn-C is downregulated, even beyond the edges of plaques, which has the densest population of macrophages, extending into the apparently healthy alba (Gutowski et al., 1999).Tn-C is abundantly expressed in the developing brain and disappears when the body matures; this protein also exhibits a negative effect on the expression of myelin basic protein (MBP) and myelin sheath formation (Czopka et al., 2010).However, the effect of Tn-C on neurocyte development is lineage-dependent.For instance, Tn-C benefits thegrowth of axons from hippocampal neurons during embryonic development but does not have any effect on retinal neuron axons (Rigato et al., 2002).Tn-C expression is downregulated at the edge of acute MS lesions but is upregulated in subacute lesions (Udalova et al., 2011).The expression of Tn-C was also found to be increased in the serum of patients with MS.The expression levels of Tn-C in chronic MS plaques were shown to be similar to that in the adjacent white matter.The downregulation of Tn-C in acute lesions appears to be due to the enzyme-mediated destruction of ECM; an increase in Tn-C may produce a glial scar, thus hindering remyelination.
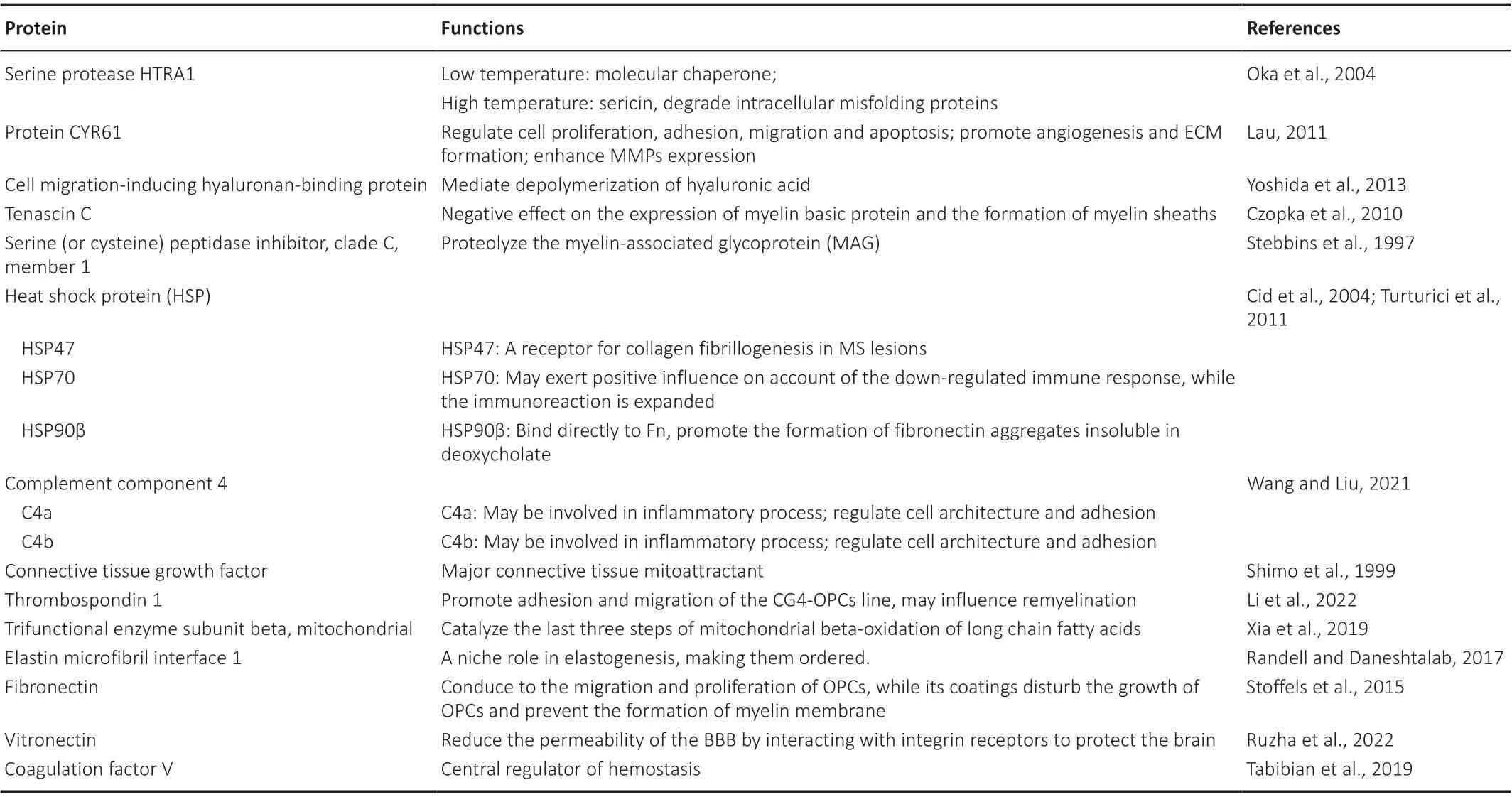
Table 1 |Function of estimated proteins in astrocyte-derived fibronectin aggregates
Elements That Affect Therapeutic Strategy
Discoveries that differ between in vitroandin vivo studies
In vitrotests refer to the use of microorganisms, cells, or biological molecules outside of their normal biological context.In vivotests are performed at the level of whole, living organisms, usually animals, but including humans.When performingin vivoexperiments, feedback is regulated by the wholebody system whereasin vitroexperiments are only regulated by the selected system of interest.In vitroresearch previously revealed an increase in fibronectin mRNA and a slight increase in protein expression in astrocytes treated with lipopolysaccharide, an inflammatory mediator (Stoffels et al., 2013).The aggregation of fibronectin also increased significantly after lipopolysaccharide treatment in rats (Stoffels et al., 2013).However,in vivoandin vitroexperiments have not always been consistent.In vitro,OPCs were placed in the astrocyte matrix of rat astrocytes treated with lipopolysaccharide.When OPCs were cultured on an astrocyte-derived matrix for 7 days, a small increase in the number of MBP-positive cells was observed,thus indicating successful differentiation.The formation of the myelin sheath membrane is defined by MBP-positive membrane cells distributed between cell processes.However,in vivoexperiments have shown that this process may prevent the formation of myelin membrane (Stoffels et al., 2013).These results indicate that aFn arising from astrocytes mainly interferes with the differentiation and reconstruction of OPCsin vivo, and the that the results arising fromin vitroandin vivoexperiments might be different.Thus, the results ofin vitroexperiments cannot always be reproducedin vivo.
Differences between mouse models and human patients
The entire structure of the immune system of mice is very similar to that of humans, although mice have a significant amount of bronchial-related lymphoid tissue; such tissue is essentially absent in healthy humans (Haley,2003).MS is a multifactorial disease; autoimmunity has been shown to promote the progression of MS.EAE is a widely used model that simulates demyelination of the central and peripheral nerves in MS.A previous study showed that interferon-γ exerts a protective effect in EAE because it neutralizes antibodies that may aggravate the course of disease by blocking the induction or activation of inhibitory activity (Lublin et al.,1993).Unexpectedly, when clinical trials were conducted, the results were frustrating: interferon-γ aggravated the disease rather than acted as a rescue factor.Studies in mice have shown that blocking very late antigen-4 (α4β1 integrin)-vascular cell adhesion molecule 1 interaction may contribute to MS;this molecule has been successfully tested in humans (Yednock et al., 1992;Miller et al., 2003).These studies warn us that we can never be too careful when generalizing the experimental results from mouse models to humans for clinical trials but also show that mouse models can successfully predict the effects of some therapies for human diseases.
Corresponding treatments for different types of MS
MS treatment is generally divided into acute and remission stages.In the acute stage, regardless of its classification, the consensus mainly recommends that the first-line treatment is high-dose glucocorticoids, which are clinically popular (Neuroimmunity Branch of Chinese Society of Immunology and Neuroimmunology Group Chinese Society of Neurology, 2018).Diseasemodifying therapies are recommended treatments for the remission stage and are currently attracting much attention.During the therapy of MS, special attention should be paid to the corresponding treatment for different types of MS.Moreover, MS has a continuous disease spectrum which must be viewed from a dynamic perspective.Different types of MS have different pathological manifestations, and according to the degree of MS activity, these types can be divided into active and inactive plaques.In the early stages of MS, such as clinically isolated syndrome and relapsing-remitting MS, plaques are active, with many infiltrating lymphocytes accompanied by activated microglia, macrophages, and astrocytes.However, primary progressive MS and secondary progressive MS are mainly characterized by inactivity; plaque boundaries are clear, with few cells, and axonal density is reduced (Qiu and She, 2021).This suggests that different types of MS may have generate different research results; whether the outcomes of one type are applicable to another type requires further elucidation.
Factors That Rescue Remyelination
Gangliosides are glycosphingolipids localized at the cell surface and play a role in cell growth, apoptosis, and differentiation.Exogenous ganglioside-1a(GD1A) reduces the aFn-mediated inhibition of remyelination via a protein kinase A-dependent signaling pathway (Qin et al., 2017).Ganglioside-1a may represent a potential factor for stimulating remyelination in MS.PDGF and fibroblast growth factor-2 have been predicted to function individually or cooperatively to generate remyelinating oligodendrocytes (Murtie et al.,2005).
Peripherally derived fibroblast growth factor-21 has been shown to promote remyelination in the CNS.In the toxin-induced demyelination model (Kuroda et al., 2017), factors derived from the peripheral tissue were shown to leak into the CNS and promote remyelination through interactions with β-klotho,an essential co-receptor of fibroblast growth factor-21.
TGF-β, a superfamily of creatine kinase proteins, plays important roles in neurogenesis, synapse formation, and development.TGF-β1-3 is known to be related to inflammation.TGF-β receptors I and II are expressed in neurons,astrocytes, OLs, and microglia.During the process of inflammation, TGF-β1 promotes OL differentiationin vitroby regulating the effect of PDGF (a determinant factor) to promote the differentiation of OPCs (Diniz et al., 2019).
Neuregulin-1 is an important factor for remyelination; a previous study showed that the persistent intra-spinal delivery of recombinant human neuregulin-1b1 near demyelinating lesions via microcarriers led to the enhanced growth of new oligodendrocytes and improved endogenous remyelination by both oligodendrocytes and Schwann cells in a state of demyelination (Kataria et al., 2018).Moreover, neuregulin-1 also increased the thickness of myelin in newly regenerated axons.
MMPs are a large family that are named after their demand for metal ions such as Ca2+and Zn2+as cofactors.This family consists of 26 members, 24 of which exist in mammals and are divided into six subgroups.It is generally acknowledged that MMPs can degrade all components of the ECM.MMPs are known to degrade Fn and contribute to the development of the nervous system (Wells et al., 2015).Moreover, aFn is a product of the Fn clearance barrier; the benefits of aFn degradation were discussed earlier in this review.From this perspective, MMPs might also be capable of achieving therapeutic effects.MMP3 can promote oligodendrocyte differentiation and myelination by regulating various signaling pathways or degrading components that inhibit oligodendrocyte differentiation and maturation, such as chondroitin sulfate proteoglycans, Fn, and myelin debris (Muir et al., 2002).MMP3 is an effective activator of other MMPs such as MMP7 and MMP9.MMP7 was previously confirmed to directly degrade aFn, forming a fragment containing 13kDa EIIIA (16 kDa, extra domain A of Fn) (Wang et al., 2018).These fragments from degrade aFn inhibited the differentiation of OPC to a greater extent,compared with unbroken aFn (Wang et al., 2018).However, these authors also pointed out that this represents the first step in the degradation of aFn,given that other proteasesin vivomay help to degrade aFn further.MMP7 might represent a potential drug for inflammatory demyelinating diseases.Moreover, IL-4-activated microglia and macrophages can secrete significant levels of proMMP7; aFn has been shown to hinder the transformation of the activated phenotype of macrophages and microglia, which supports OPC differentiation, while IL-4 can promote this transformation.Thus, IL-4 may be used to overcome aFn-mediated remyelination failure.
Research has also shown that the intestinal flora can promote the development of the BBB and regulate the inflammatory and immune responses of the CNS (Table 2; Zhou et al., 2020).Several recent studies have shown that MS is closely related to the intestinal flora (Adamczyk-Sowa et al., 2017; Bao et al., 2018).Studying the role of an imbalance in the intestinal flora in the pathogenesis of MS represents a window for understanding the connection between intestinal microecology and central autoimmune diseases; research in this area is expected to identify a new treatment direction for MS.In a previous study, Lavasani et al.(2010) found that the clinical symptoms and histopathological changes of a mouse model of EAE were relieved to varying degrees following treatment with a mixture of lactobacillus probiotics; this interventional experiment, involving intestinal probiotics, provided a new concept for the treatment of MS.
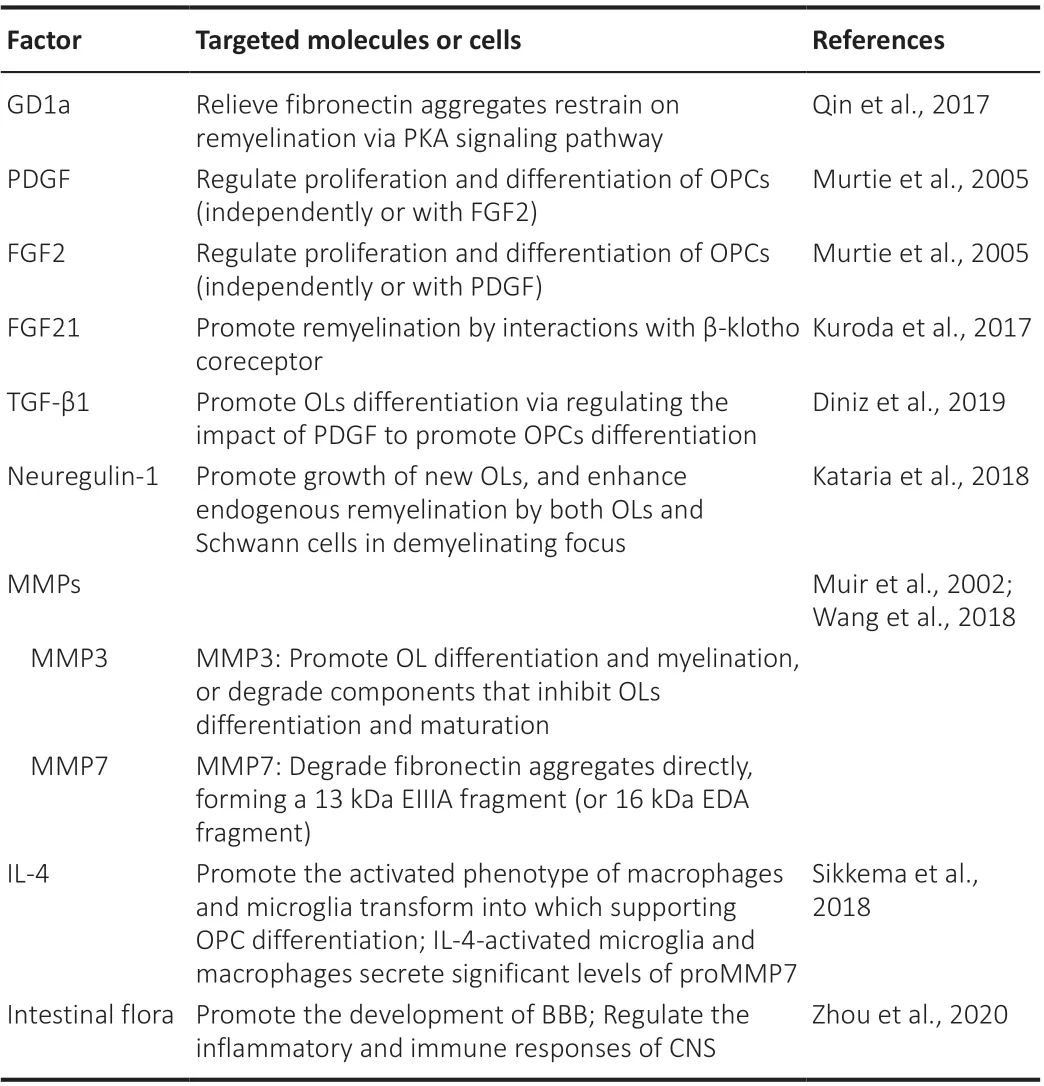
Table 2 |Targeted molecules or cells of factors to rescue remyelination
Approaches to Deliver Drugs through the Blood-Brain Barrier
Despite several advances in the mechanisms and treatment of MS, the clinical intervention of brain diseases is proceeding slowly owing to the complexity of the structure and function of the brain.In particular, the BBB makes it more difficult for drugs to reach pathological tissues in the brain and exert their effects.The BBB is a barrier system that exists between the blood circulation and brain tissue.This is a tight structure composed of brain capillary endothelial cells, pericytes, the terminal feet of astrocytes, and the vascular basement membrane.The BBB maintains a relatively stable internal environment for the brain tissue and ensures normal physiological functions in the CNS and the transmission of nutrients into the brain.However, the BBB also hinders the delivery of diagnostic and therapeutic medicines into the brain; approximately 98% of small-molecule drugs, and almost all macromolecular drugs, cannot be delivered into the brain (Nair et al., 2018).Many researchers have recognized the hindrance created by the BBB with regards to drug delivery; thus, many researchers have attempted to develop new technologies to allow the penetration of the BBB and formulate new drug delivery strategies (Nair et al., 2018).There are three commonly used approaches to administer molecules through the BBB, including systemic absorption, nasal, and intracerebroventricular methods (Figure 2 and Table 3; Wan et al., 2020).However, in general, systemic absorption via the BBB is easier than the other methods.Nano drug-loading systems have the advantage of protecting drugs from degradation, transporting them to the action target, prolonging the blood circulation time, and reducing toxicity to the body; thus, these systems have become promising new methods for drug delivery (Figure 3).An acceptable method should at least convey sufficient active ingredients to the desired location; furthermore, the selected drug must be released at a steady rate.
Intracerebroventricular drug delivery
Early invasive methods, such as craniotomy or intracerebral injections, are often used to deliver drugs to the brain.Invasive administration is based on neurosurgery, which can be used as an approach to treat local neuropathy.Using this method, therapeutic factors can be directly introduced into target foci by a catheter.Implantable infusion systems have been used in a single center to deliver valproic acid to cerebrospinal fluid for an extended period in five patients with drug-resistant focal epilepsy (Cook et al., 2020);all patients showed good tolerance to implantation and their quality of life improved markedly.This study showed that the chronic intraventricular administration of valproic acid is safe and effective for patients with medically refractory epilepsy.However, an inevitable disadvantage of this technique is that it can easily cause surgical trauma and cause diffuse rupture to the BBB;furthermore, it is necessary to maintain high intracranial pressure during this form of drug administration.
Nasal drug delivery
Intranasal delivery of therapeutic drugs to the brain could be used as a potential method to bypass the BBB.This form of delivery is associated with high bioavailability, is convenient to use, and can avoid stimulation of the gastrointestinal tract and the firstpass elimination of the liver.This form of delivery system has been widely investigated.For example, a previous study found that the intranasal administration of nerve growth factor can increase the survival rate of newborn neurons in adult rats after focal cerebral ischemia, promote the maturation of neurons in the striatum, and effectively improve neurological deficits in rats (Zhu et al., 2011).Another study described minimally invasive nasal depot technology, a new form of drug delivery that acts directly through the nasal CNS.This method overcomes the challenges of dose variability and efficiency that are associated with traditional local nasal strategies by directly delivering all therapeutic doses to the olfactory submucosal space.The efficacy of implanting a depot containing drugs was close to 40% for the intracerebroventricular route (Padmakumar et al., 2021).Nasal administration has great potential, although many challenges remain.First, the nasal mucosa epithelium is a natural barrier to nasal administration, and usually, only approximately 1% of a drug dose can reach the brain via this method (Illum, 2004).In addition, the expression of efflux transport proteins in the nasal mucosa can significantly limit brain uptake.Drug-metabolizing enzymes and tight junction proteins are also present in the nasal epithelium; these can further limit the efficiency of intranasal delivery to the CNS.Moreover, CNS diseases often require long-term drug administration; this may lead to nasal irritation.Another consideration is that some specific drugs may have potential adverse effects on the olfactory or trigeminal nerves.
Inorganic nanomaterials
As drug carriers, inorganic nanoparticles can be easily modified to enhance the targeting, persistence, and controllability of drugs, prolong the half-life of drugsin vivo, expand the distribution of drugs in lesions, and reduce adverse reactions.Inorganic nanomaterials can enhance permeability and increase retention time to achieve targeted drug administration cross the BBB (Estelrich et al., 2015).Currently, common materials include gold nanoparticles,magnetic iron oxide nanoparticles, silicon dioxide nanoparticles, and carbon nanotubes.Neurotrophic peptides have been immobilized on AuNPs through direct and lipid-mediated interactions; this practice led to the retainment of neurotrophic effects.The ability to deliver drugs through the BBB can be finely controlled by their size, surface charge, hydrophobicity, shape, coating,and chemistry (Di Pietro et al., 2017).In addition, highly biocompatiblecalcium-doped mesoporous silica nanoparticles have been prepared and functionalized with polysorbate-80 for use as a targeting ligand to deliver carbasalatin into the brain by crossing the BBB (Fateh Basharzad et al., 2022).

Table 3 |Approaches to deliver drugs through BBB
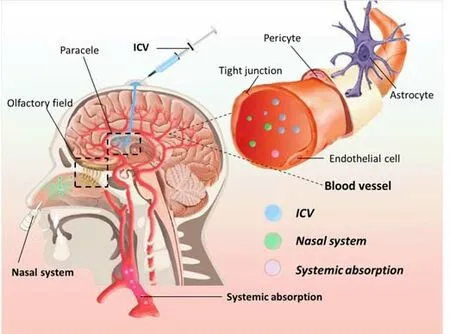
Figure 2|Three types of commonly used therapies.
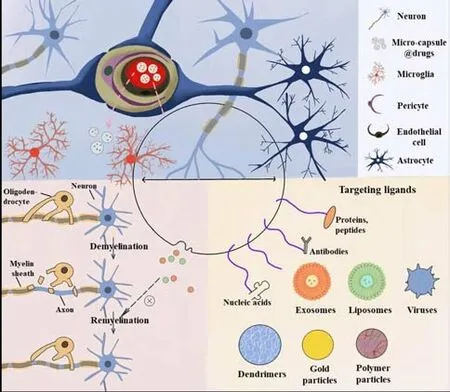
Figure 3|Non-invasive delivery of nano-carriers.
Natural polymer materials
Drug-loaded natural polymer materials can increase the action time of drugs, improve drug selectivity, reduce drug toxicity, and overcome the difficulties encountered in drug configuration.In a previous study, Yadav et al.(2017) engineered brain-targeted Tween-80 chitosan nanoparticles loaded with doxycycline hydrochloride; these nanoparticles exhibited marked antipsychotic activity and represented a new method for the treatment of psychosis that was delivered through the BBB.Song et al.(2016) constructed a bionic high-density lipoprotein nanocarrier using recombinant apolipoprotein E and synthetic lipids.Compared with control liposomes, these nanocarriers showed higher brain delivery efficiencyin vivo.
Synthetic nanomaterials
The variability of synthetic nanomaterials can endow drugs with many new characteristics that have attracted extensive levels of attention.Polylactic acid is a polymer obtained by polymerization with lactic acid as the main monomer and is a biodegradable and synthetic high-molecular-weight material with good biocompatibility, safety, and no toxicity; thus, this polymer might represent an alternative method for drug delivery.For example, Goyal et al.(2018) synthesized a polyethylene glycol-S-S-polycaprolactone nanodrug delivery system with pH and redox sensitivity.This system can be used for the treatment of neurodegenerative diseases and also the treatment of glioma and other CNS diseases.Polylactic-co-glycolic acid was prepared by the random polymerization of lactic acid and glycolic acid, which has similar characteristics to polylactic acid.Loperamide polylactic-co-glycolic acid nanoparticles were shown to pass effectively through the BBB and exert analgesic effects (Fornaguera et al., 2015).
Extracellular vesicles
Extracellular vesicles (EVs) are extracellular structures that are surrounded by a double layer of lipids and are secreted by almost all known cell types.EVs can be divided into two categories: ectosomes and exosomes.Ectosomes are vesicles shed from the plasma membrane surface through outward buds,including microbubbles, microparticles, and large vesicles with diameters ranging from 50 nm to 1 μm.Exosomes originate from endosomes with diameters ranging from 40 to 160 nm (Kalluri and LeBleu, 2020).Challenges remain in establishing purification and analytical procedures for exosome research that may lead to heterogeneous EVs populations, including exosomes.In this review, we mainly focus on the drug-carrying function of exosomes, although some of research findings may inevitably reflect the results of exosomes that are mixed with other types of EVs.Previous studies have shown that exosomes can carry proteins, microRNAs, mRNA, and DNA during cellular communication (Dragomir et al., 2018).For example, Alvarez-Erviti et al.(2011) used immature dendritic cells as maternal cells to load siRNAs, and first proposed and conceptually verified the feasibility of using exosomes as drug carriers.
Numerous drug carriers have emerged over recent times.Liposome-based and polymer-based carriers are two types of drug carriers that are being investigated at present (Jakki et al., 2016; Male et al., 2016).However,these carriers are limited by their short circulation time in the blood, easy elimination by the human body, poor stability, and low targeting ability.Therefore, there is a clear need to develop a new carrier that can transport drugs effectively.Exosomes play an important role in the exchange of information between cells and are widely distributed throughout the human body.Exosomes can cross the cell membrane and are less likely to cause an immune response, thus making them unique drug carriers.Phase I clinical studies have been conducted on the application of exosomes for disease treatment; these studies have illustrated the safety of exosomes with regards to clinical application (Morse et al., 2005; Dai et al., 2008).
However, further research is required for the wider clinical application of exosomes.The main method used to purify exosomes is ultracentrifugation;this is less efficient, time-consuming, and relatively expensive.Furthermore,this method is not suitable for clinical application and requires further optimization.Although exosomes exhibit a certain targeting ability, this is weak, and ligands need to be modified to improve such targeting ability.There is also ample room for research to determine how to introduce modified exosomes to their targeting ligands.The targeting ligands are cell-penetrating peptides, trans-activated transcription proteins, and antimicrobial peptides,and have been widely used to transport therapeutic drugs to the brain via the BBB.In animal models, exogenous sialic acid has been shown to improve both learning and memory (Wang et al., 2007).A sialic acid-containing nanodrug carrier system uses sialic acid adhesion on endothelial cells to promote penetration of the BBB through receptor-mediated endocytosis.
Conclusions and Prospects
Myelin sheaths wrap around the surface of axons, protecting them and inducing remyelination after axonal injury.Demyelination impairs the nodes of Ranvier, thereby hindering effective saltatory conduction.MS results in focal lesions in the gray and white matter along with diffuse neurodegeneration throughout the brain that is characterized by primary demyelination with varying degrees of axonal injury.Remyelination failure is a predominant feature of MS and may be associated with disease progression.Owing to the persistent presence of Fn in chronic MS lesions, pFn and cFn can assemble into stable aFn that acts as an Fn dimer to inhibit myelin membrane formation and myelination in both single and combined culture systems.However, aFn also has other functions, such as stimulating the microglia and macrophages to release NO and increasing the expression and activity of arginase-1 in macrophages.
Proteomic analysis has revealed that 18 predicted secretory proteins are present in rat astrocyte-derived aFn (Sikkema et al., 2018).HSP70 can protect neurons from protein aggregation, toxicity, apoptosis, and inflammation,plays an auxiliary role in antigen presentation, and participates in the immune response in autoimmune diseases.Presumably, this is because the chaperone activity of HSP70 inhibits various potentially toxic elements.However, in some cases of HSP70 overexpression or brain dysfunction, the beneficial effects of HSP70 remain uncertain.In MS lesions, the overexpression of HSP70 may play an active role owing to downregulation of the immune response; however,due to the emergence and re-aggregation of additional antigen targets in the lesions, the immune response can expand.Tn-C is abundantly expressed in the developing brain and disappears when the body matures, thus resulting in negative effects on MBP expression and myelination.Further investigation of the aFn components is needed to further understand the inhibitory effects of aFn on remyelination, to degrade aFn, and delay the course of MS.
In addition, many factors are known to be be able to rescue remyelination;one particularly promising direction in this regard is the intestinal flora.Microbes affect CNS diseases by regulating the movement of immune cells from the intestinal tract to the brain to secrete neuroactive metabolites,change endocrine signaling pathways, and trigger afferent neurons (Fung et al., 2017).A disturbance in the intestinal flora is related to the occurrence and development of many neurodegenerative diseases (Bostanciklioğlu,2018; Gerhardt and Mohajeri, 2018).It has been suggested that autoimmune reactions can be suppressed by restoring balance in the microflora and utilizing communication pathways between the immune system and intestinal microbes.
Exosomes have attracted considerable attention as drug-loaded nano-systems to deliver drugs through the BBB.However, there are some limitations associated with exosome drug loading, including the low output of exosomes and poor efficiency of drug delivery; these factors represent serios limitations on the wider application of exosomes in clinical settings.Different cell culture media also affect the contents and types of exosomes.Furthermore, if a cell culture medium contains serum, then it is very likely that the medium will already contain exosomes; this complicates the analysis of harvested exosomes.Owing to the heterogeneity of exosomes, even exosomes secreted by the same cell may exhibit wide functional differences, this creating numerous issues for industrialization (Ferguson and Nguyen, 2016).The industrialization of exosome-loaded drugs is associated with many challenges,including scale, purity, cost, consistency, and standardization.For precise treatment, drug delivery carriers need to exhibit high targeting ability; it is imperative that we carry out more research if we are to modify exosomes in manner that will improve their targeting ability.
In view of the shortage of natural exosomes what we described above, an increasing number of studies have been conducted to develop artificial exosomes through nanotechnology, thus generating significant hope for the development of advanced drug delivery methods that combine the advantages of natural and synthetic nanocarriers (Li et al., 2021a, b; Staufer et al., 2021).In this review, we introduced the occurrence and development of MS from the perspective of remyelination failure, a condition that is caused by aFn.We propose that the degradation of aFn might represent a potential target for MS treatment.However, there is no effective method that can degrade aFn at present.The protein histochemistry analysis was performed to reveal that there were 18 predicted proteins in aFn derived from rat astrocytes; however,only several proteins were identified and the specific functions of the other proteins need to be further investigated.Moreover, there is a clear need for morein vitroanalysis of aFn derived from rat astrocytes; we also need to ascertain whether this can full represent aFn in MS patients.Various factors for rescuing remyelination are described herein; however, only their positive effects on remyelination and the mechanisms involved are briefly introduced.The specific targets and pathways of some factors are still unclear and need to be further investigated.In addition, as introduced earlier in this review,research results will inevitably be affected byin vivoandin vitrodesign and the use of mouse models and human patients; in addition, results will also be determined by the specific type/stage of MS.This review only introduces the experimental results of one or two cases of each each style of study and describes a potential trend; however, these studies may not fully represent results arising from patients in different stages of MS.This requires further study.In addition to invasive methods, such as intracerebroventricular and nasal drug delivery, nano-drug delivery systems represent a new method of non-invasive treatment; we introduce this new system herein.However, thus far, nanomaterial research remains in its infancy; the specific distribution of nanomaterials in the brain and their effects on metabolism and toxicity in the human body are not yet clear.Thus, nano-drug delivery systems may still be a long way from clinical application.To improve the targeting ability of nanodrug delivery systems, surface modification can be performed; we describe this option but not in significant detail.In the future, researchers should focus on the modification of nano-drug delivery systems so as to promote their vigorous development.
Acknowledgments:The authors specially thank Xue-Min Li (College of Water Conservancy and Agriculture Engineering, Tarim University, China) and Xiao-Xue Yan (Shandong Huayu University of Technology, China) who provide assistance in figure preparation.
Author contributions:SSW wrote the first draft.LC and FYY revised the manuscript.SQW polished the manuscript.PW provided ideas and guided manuscript modification.All authors approved the final version of this manuscript.
Conflicts of interest:The authors declare no conflict of interest.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Xiaoming Zhang, Brigham and Women’s Hospital, USA.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain

