Eph receptor A4 regulates motor neuron ferroptosis in spinal cord ischemia/reperfusion injury in rats
Yan Dong, Chunyu Ai, Ying Chen, Zaili Zhang, Dong Zhang, Sidan Liu, Xiangyi Tong, Hong Ma
AbstractPrevious studies have shown that the receptor tyrosine kinase Eph receptor A4 (EphA4) is abundantly expressed in the nervous system.The EphA4 signaling pathway plays an important role in regulating motor neuron ferroptosis in motor neuron disease.To investigate whether EphA4 signaling is involved in ferroptosis in spinal cord ischemia/reperfusion injury, in this study we established a rat model of spinal cord ischemia/reperfusion injury by clamping the left carotid artery and the left subclavian artery.We found that spinal cord ischemia/reperfusion injury increased EphA4 expression in the neurons of anterior horn,markedly worsened ferroptosis-related indicators, substantially increased the number of mitochondria exhibiting features consistent with ferroptosis, promoted deterioration of motor nerve function, increased the permeability of the blood-spinal cord barrier, and increased the rate of motor neuron death.Inhibition of EphA4 largely rescued these effects.However, intrathecal administration of the ferroptosis inducer Erastin counteracted the beneficial effects conferred by treatment with the EphA4 inhibitor.Mass spectrometry and a PubMed search were performed to identify proteins that interact with EphA4, with the most notable being Beclin1 and Erk1/2.Our results showed that inhibition of EphA4 expression reduced binding to Beclin1, markedly reduced p-Beclin1, and reduced Beclin1-XCT complex formation.Inhibition of EphA4 also reduced binding to p-Erk1/2 and markedly decreased the expression of c-Myc, transferrin receptor 1, and p-Erk1/2.Additionally, we observed co-localization of EphA4 and p-Beclin1 and of EphA4 and p-ERK1/2 in neurons in the anterior horn.In conclusion,EphA4 participates in regulating ferroptosis of spinal motor neurons in the anterior horn in spinal cord ischemia/reperfusion injury by promoting formation of the Beclin1-XCT complex and activating the Erk1/2/c-Myc/transferrin receptor 1 axis.
Key Words:Beclin1; c-Myc; EphA4; Erk1/2; ferroptosis; motor neuron; p-Erk1/2; rat; spinal cord ischemia/reperfusion injury; transferrin receptor 1
Introduction
Spinal cord ischemia/reperfusion injury (SCIRI) occurs when, after a period of ischemia in the spinal cord, blood circulation is restored.When the spinal cord is reperfused, the neurological function is not improved but rather further aggravated, and a series of complications occur, including obvious neurological dysfunction, paraplegia, and even death.Clinically, SCIRI generally occurs after some spine surgeries and cardiovascular operations that require temporary occlusion of the lumbar artery or thoracic and abdominal aorta.The mechanism of SCIRI is extremely complex (Coselli et al., 2000; Chen et al.,2022a).A combination of mechanisms eventually leads to spinal cord neuron death and neurological dysfunction.Blocking a signaling pathway that leads to cell death did not completely inhibit neuronal death (Fricker et al., 2018).Therefore, it is important to investigate the mechanisms of neuronal death that occur during SCIRI to prevent cell death and improve neurological function.
Neurons undergo several forms of cell death, such as necrosis (Xu et al.,2016), apoptosis (Zhang et al., 2021), autophagic cell death (Wang et al.,2020b), and pyroptosis (Li et al., 2019a), in SCIRI.Ferroptosis is a recently discovered type of programmed cellular death, originally reported by Dixon et al.(2012), that involves the accumulation of lethal reactive oxygen species(ROS) induced by an increased intracellular iron ions and oxidative damage caused by lipid peroxidation.Previous studies have shown that ferroptosis occurs in multiple organs suffering from ischemia/reperfusion injury (IRI) such as the intestine, liver, kidney, and lung (Li et al., 2019b; Yamada et al., 2020;
Dong et al., 2021; Wang et al., 2021).Moreover, the characteristic changes associated with ferroptosis have also been observed in nerve tissue and cerebral tissue subjected to IRI (Tuo et al., 2021; Suo et al., 2022).Inhibition of ferroptosis can effectively improve neurological function and increase nerve cell survival (Guan et al., 2019).Therefore, in this study, we asked whether SCIRI can lead to ferroptosis of spinal motor neurons.
Eph receptors comprise the largest known family of receptor tyrosine kinases and are extensively involved in development and pathophysiology (Kania and Klein, 2016).EphA4 is one of the most abundant Eph receptors in the nervous system.It is expressed at high levels in neurons of the brain and spinal cord(Carmona et al., 2009; Yang et al., 2014a; Chen et al., 2022b).Due to its ability to bind all type A ligands (ephrinAs) and most type B ligands (ephrinBs), it is speculated that EphA4 has a wider range of effects than other Eph receptors(Yang et al., 2018).EphA4 plays a crucial role in nervous system development(Helmbacher et al., 2000), neural progenitor cell proliferation (Chen et al.,2020), neuronal migration (Gatto et al., 2014), axon growth guidance (Gatto et al., 2014), synaptic plasticity (Filosa et al., 2009), and myelin formation(Harboe et al., 2018).It also plays an important role in conditions including Alzheimer’s disease (Fu et al., 2014) and subarachnoid hemorrhage (Fan et al., 2017).Furthermore, EphA4 is involved in the pathology of IRI (Li et al.,2012) and mediates motor neuron death in motor neuron disease (Zhao et al., 2021).In addition, the EphA4/ephrinA3 signaling pathway regulates glutamate excitotoxic neuronal death (Carmona et al., 2009).However,inhibition of EphA4 expression in the brain promotes glutamate uptake by astrocytes and improves neuronal survival (Yang et al., 2014a).Glutamate excitotoxic neuronal death is a key mechanism of nerve injury in nervous system IRI (O’Donnell et al., 2016).Recent research has shown that high concentrations of extracellular glutamate induce ferroptosis in neurons by inhibiting the function of system Xc–, and that this effect can be blocked by Fer-1 (ferroptosis inhibitor) in rat hippocampal slicesin vitro.The Xc–system is a cystine-glutamate exchange system consisting of Slc3a2 and Slc7a11(XCT) that facilitates the exchange of cystine and glutamate across the plasma membrane.Inhibition of the Xc–system function ultimately induces neuronal ferroptosis (Dixon et al., 2012).EphA4 signaling induces glutamate excitotoxic neuronal death and neuronal ferroptosis is an important mode of glutamateinduced excitatory neuron death.Therefore, the aims of this study were to determine whether EphA4 is involved in SCIRI regulation and whether this process is related to ferroptosis.
Methods
Animals
Eight-week-old healthy adult male Sprague-Dawley rats (specific-pathogenfree level, 230–270 g) were obtained from the Department of Animal Service of China Medical University (Shenyang, China; license No.SCXK (Liao) 2018-0004).Rats were reared in cages and allowedad libitumaccess to water and food for 1 week before the experiments were performed.The cages were maintained at 21–23°C and 50–60% relative humidity, with a 12/12-hour light/dark schedule.The study was approved by the Ethics Review Committee for Animal Experimentation of China Medical University (approval No.CMU2021336) on July 26, 2021.All experiments were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE)guidelines (Percie du Sert et al., 2020).
Experimental protocol
The experimental protocol is displayed in Figure 1.After neurological function assessment, the rats in all groups were sacrificed with 4% pentobarbital sodium (100 mg/kg) and perfused transcardially with normal saline.The L4–L6 spinal cord segments were harvested by means of retrograde tracing along the sciatic nerve (Wang et al., 2020a).Briefly, the experiment consisted of three parts.The aim of the first part was to determine the intervention time point (Figure 1A).The aim of the second part was to examine the changes in a series of indicators induced by intrathecal administration of AV-sh-EphA4 with or without Erastin (Figure 1B and C).The aim of the third part was to investigate the mechanism of EphA4’s effects on ferroptosis (Figure 1D–F).
SCIRI model
The rat SCIRI model was established as reported previously (Li et al., 2014).Concisely, the rats were anesthetized by intraperitoneal injection of 4%pentobarbital sodium (50 mg/kg, Beyotime Biotechnology, Shanghai, China).Thoracotomy was performed in the right decubitus position, and the aortic arch between the left carotid artery and the left subclavian artery was clamped to ensure effective ischemia for 14 minutes.Effective ischemia was assessed by the caudal arterial blood pressure dropping below 10 mmHg.The laser Doppler blood flow monitor showed that the caudal artery flow was decreased by 90% (Moor Instruments, Axminster, Devon, UK).After the blockade was lifted, the incision was sutured layer by layer, and each rat was reared individually in its own cage.In the sham group, thoracotomy was performed, and the aortic arch was exposed but not clamped.
Intrathecal administration
Intrathecal administration was performed as previously reported (Dong et al., 2011).A 25-μL microinjector (Gaoge, Shanghai, China) filled with 20 μL of various substances was inserted between the L5 and L6 vertebrae until the rat’s tail suddenly swung horizontally.Then, the substance was slowly injected.The substances included adenovirus (5 × 1010plaque-forming units/mL; IGE, Guangzhou, China), Erastin (10 pmol; MCE, Shanghai, China),U0126 (5 μg; MCE), and normal saline (20 μL).The injected adenoviruses included green fluorescent protein (GFP) adenovirus (NC-GFP), shRNA-EphA4 adenovirus (AV-sh-EphA4), and blank adenovirus (AV-sh-NC).The inserted AVsh-EphA4 sequence was as follows: 5′-CCG GGG AAG TAA GCA TTA TGG ATG ACT CGA GTC ATC CAT AAT GCT TAC TTC CTT TTT G-3′.Erastin is a ferroptosis inducer (Dixon et al., 2012) that was first identified by screening for synthetic compounds that are lethal to tumor cells (Dolma et al., 2003).U0126 is a specific inhibitor of mitogen-activated protein 1/2 that inhibits Erk1/2 phosphorylation (Favata et al., 1998).
Neurological assessment
The Tarlov score (Wrathall et al., 1985) and Basso-Beattie-Bresnahan (BBB)score (Basso et al., 1996) were used to assess hind limb motor function.The Tarlov score ranged from 0 to 4 (paraplegia to normal), while the BBB score ranged from 0 to 21 (paraplegia to normal).Three blinded observers assigned scores in accordance with the criteria.
Quantitative reverse transcription-polymerase chain reaction
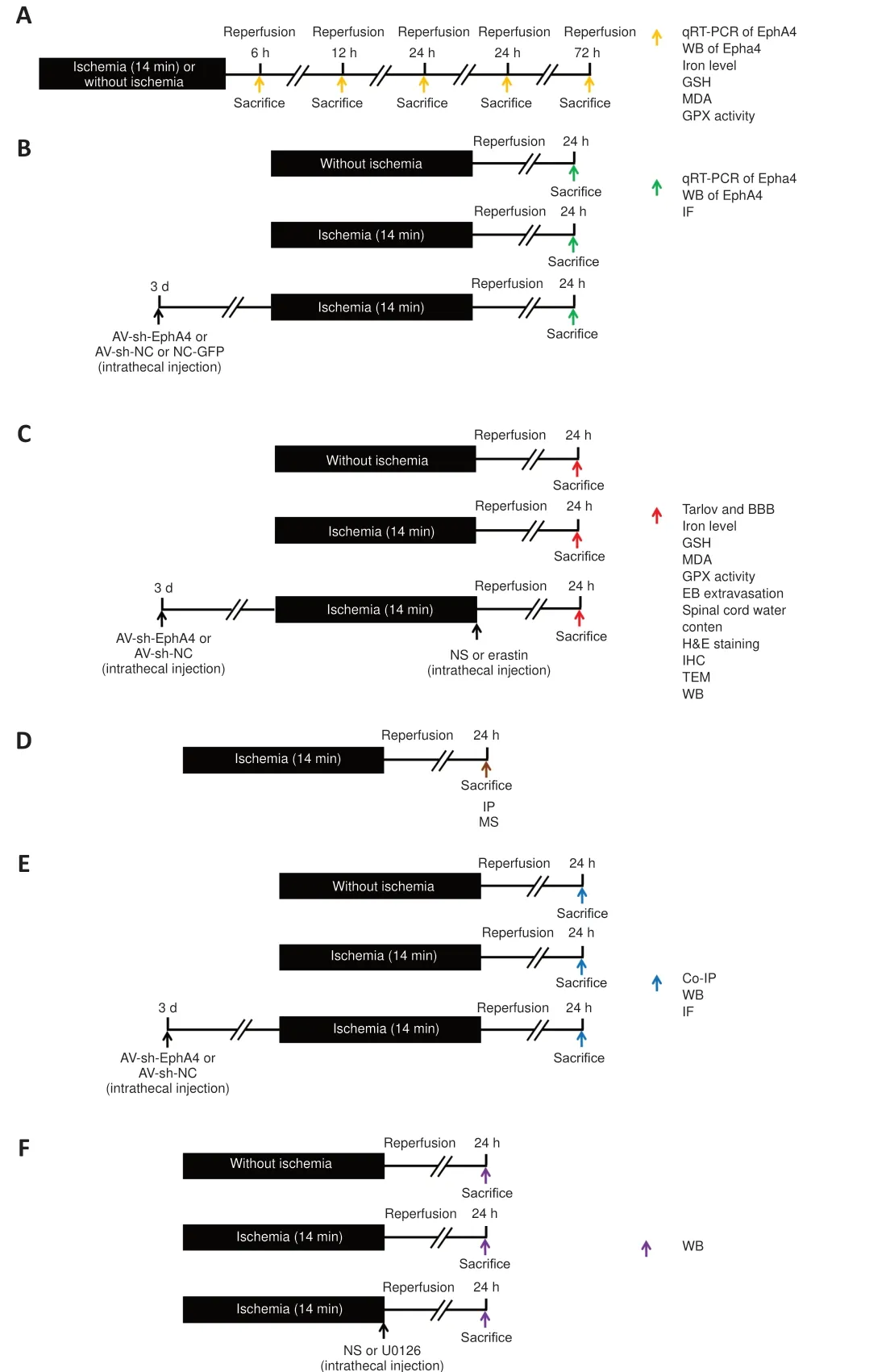
Figure 1|Experimental design.
Rats were sacrificed with an overdose of anesthesia (100 mg/kg, 4%pentobarbital sodium), the spinal cords were immediately harvested on ice,and the pia matter was stripped from the cord.Then, the L4–L6 anterior horn region (n= 5) was dissected out and weighed.Total RNA was extracted with Trizol reagent (Takara, Otsu, Japan).The spinal concentration and purity of each RNA specimen was detected using a Nano Drop spectrophotometer(Thermo Fisher, Waltham, MA, USA).Next, 1000 ng RNA was reverse transcribed using a PrimeScriptTMRT Reagent Kit with gDNA Eraser (Takara,RR047) in a volume of 20 μL.Subsequently, quantitative reverse transcriptionpolymerase chain reaction (qRT-PCR) was performed with TB Green®Premix Ex TaqTMII (Takara, RR820) on an ABI 7500 PCR instrument (Applied Biosystems, Waltham, MA, USA) with the following conditions: 95°C (30 seconds), followed by 40 cycles at 95°C for 5 seconds and 60°C for 34 seconds.Relative EphA4 mRNA expression was assessed by the 2–∆∆Ctmethod (Livak and Schmittgen, 2001), with glyceraldehyde-3-phosphate dehydrogenase (GAPDH)as the internal reference.The primers used (provided by Sangon, Shanghai,China) were as follows: GAPDH: forward: 5′-GGG GCT CTC TGC TCC TCC CTG-3′, reverse: 5′-AGG CGT CCG ATC GGC CAA A-3′; EphA4: forward: 5′-CTG GGC AGT GAA TGG CGT GTC-3′, reverse: 5′-TGG TGC TGC TTG GTT TGT C-3′.
Hematoxylin and eosin staining
The rats (n= 3 per group) were deeply anesthetized by intraperitoneal injection of 4% pentobarbital sodium (100 mg/kg) 24 hours after reperfusion.Then, cardiac perfusion was performed with normal saline and 4%buffered paraformaldehyde according to a previously reported method(Li et al., 2019b).The spinal cord was then removed and immersed in 4%paraformaldehyde for 24 hours, after which the L4–L6 segments were collected and embedded in paraffin.The paraffin-embedded specimens were then cut into 4-μm-thick slices, and the slices were stained with hematoxylin and eosin (Servicebio, Wuhan, China, G1005) and observed under an optical microscope (Nikon Eclipse E100, Tokyo, Japan).
Immunohistochemical staining
The 4-μm-thick paraffin-embedded L4–L6 sections were dewaxed and rehydrated, then treated with citric acid for antigen retrieval.The sections were placed in 3% H2O2for 25 minutes and blocked with 10% bovine serum albumin (Solarbio, Beijing, China) for 30 minutes.The sections were incubated at 4°C overnight with a choline acetyltransferase (ChAT) primary antibody(rabbit, 1:200, Bimake, Houston, TX, USA, Cat# A5303, RRID: AB_2766115),and then incubated with a goat anti-rabbit secondary antibody (1:200,Servicebio, Cat# GB23303, RRID: AB_2811189) for 30 minutes at 37°C.Finally, 3,3′-diaminobenzidine was used for color rendering, and the nuclei were stained with hematoxylin.The sections were dehydrated, sealed, and observed under an optical microscope.Positive neurons in the anterior horn were counted by two blinded investigators for three slices for each rat (n= 3).ChAT-positive neurons indicated motor neurons (Liang et al., 2021).
Spinal cord water content evaluation
Spinal cord edema was evaluated using the wet and dry method (Li et al., 2014).The fresh L4–L6 anterior horn segment (n= 5) was collected at 24 hours after SCIRI, weighed to determine the wet weight, and dried at 110°C for 24 hours to determine the dry weight.The spinal cord water content was calculated using the following formula: (wet weight – dry weight)/wet weight × 100%.
Evans blue extravasation
Evans blue (EB) extravasation was used to evaluate blood-spinal cord barrier(BSCB) permeability (Li et al., 2014).EB (Solarbio) solution (30 g/L, 45 mg/kg)was administered via the tail vein.After 1 hour, the rats were received an overdose of intraperitoneal anesthesia with 4% pentobarbital sodium(100 mg/kg) and were perfused transcardially.Next, L4–L6 segments were harvested, embedded in optimal cutting temperature compound, frozen, and sliced into coronal frozen sections (10 μm) using a microtome cryostat (Thermo Fisher) at –20°C.Slices were observed under a fluorescence microscope(Nikon).Three slices for each rat (n= 3) were assessed for red fluorescence intensity.
Western blotting
The proteins from fresh L4–L6 anterior horn tissue were extracted using radioimmunoprecipitation assay buffer (Beyotime Biotechnology),supplemented with protease inhibitors (Roche, Basel, Switzerland, Cat#5892791001) and phosphatase inhibitors (Roche, Cat# 4906837001).The BCA method (Li et al., 2014) was used to measure protein concentration with an assay kit (Beyotime Biotechnology).Sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed with equal amounts of protein for each sample.The isolated proteins were electro-transferred onto polyvinylidene fluoride membranes (Millipore, Boston, MA, USA).They were then blocked with 5% non-fat milk for 2 hours and incubated at 4°C overnight with the following primary antibodies: anti-EphA4 (rabbit, 1:1000, Abcam, Cambridge,MA, USA, Cat# ab5396, RRID: AB_304857), anti-glial fibrillary acidic protein(GFAP, goat, 1:5000, Abcam, Cat# ab53554, RRID: AB_880202), antiionized calcium binding adapter molecule 1 (Iba1, goat, 1:500, Abcam, Cat#ab5076, RRID: AB_2224402), anti-extracellular regulated protein kinases 1/2 (Erk1/2, rabbit, 1:1000, Affinity, Liyang, Jiangsu, China, Cat# AF0155,RRID: AB_2833336), anti-p-Erk1/2 (Thr202/Tyr204) (rabbit, 1:1000, Affinity,Cat# AF1015, RRID: AB_2834432), anti-myosin-like BCL2 interacting protein(Beclin1, rabbit, 1:1000, Cell Signaling Technology, Boston, MA, USA, Cat#3495, RRID: AB_1903911), anti-p-Beclin1 (Ser90/93/96) (rabbit, 1:1000,Affinity, Cat# AF7386, RRID: AB_2843826), anti-v-myc avian myelocytomatosis viral oncogene homolog (c-Myc, rabbit, 1:1000, Affinity, Cat# AF6054, RRID:AB_2834973), anti-transferrin receptor 1 (TFR1, rabbit, 1:1000, Affinity, Cat#AF5343, RRID: AB_2837828), anti-amino acid transport system Xc- (XCT, rabbit,1:1000, Affinity, Cat# DF12509, RRID: AB_2845314), and anti-GAPDH (mouse,1:10,000, ProteinTech, Wuhan, China, Cat# 60004-1, RRID: AB_2107436).Next,the membrane was incubated for 2 hours at 37°C with the following secondary antibodies: goat anti-rabbit (1:10,000, ProteinTech, Cat# SA00001-2, RRID:AB_2722564), goat anti-mouse (1:10,000, ProteinTech, Cat# SA00001-1, RRID:AB_2722565), or rabbit anti-goat (1:10,000, ProteinTech, Cat# SA00001-4,RRID: AB_2864335).The optical densities of the protein bands were detected using an enhanced chemiluminescence kit (Bio-Rad, Philadelphia, PA, USA)and a gel imager system (Bio-Rad) with Image Lab software version 6.0.1.Relative protein expression was normalized to GAPDH.ImageJ 2.0 (National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012) was utilized to determine the optical density of the western blot bands.
Immunofluorescence staining
After paraformaldehyde fixation and sugar dehydration, the frozen spinal cord was sectioned into 4-μm-thick coronal slices.The slices were blocked and then incubated overnight at 4°C with the following primary antibodies: anti-EphA4(1:500), anti-neuronal nuclei (NeuN, mouse, 1:500, Abcam, Cat# ab104224,RRID: AB_10711040), anti-GFAP (1:500), anti-Iba1 (1:500), anti-endothelial cell (Reca-1, mouse, 1:500, Abcam, Cat# ab9774, RRID: AB_296613),anti-myelin basic protein (MBP, mouse, 1:500, Cell Signaling Technology,Cat# 83683, RRID: AB_2140060), anti-EphA4 (mouse, 1:100, Santa Cruz Biotechnology, Dallas, TX, USA, Cat# sc-365503, RRID: AB_10843811), anti-p-ERK1/2 (Thr202/Tyr204) (1:200), and anti-p-Beclin1 (Ser90/93/96) (1:200).Then the sections were incubated for 2 hours in the dark at room temperature with the following secondary fluorescent antibodies: Cy3-conjugated donkey anti-rabbit IgG (1:200, Servicebio, Cat# GB21403, RRID: AB_2818951), Alexa Fluor 488-conjugated donkey anti-mouse IgG (1:200, Abcam, Cat# ab150105,RRID: AB_2732856), and fluorescein isothiocyanate-conjugated donkey anti-goat IgG (1:200, ProteinTech, Cat# SA00003-3, RRID: AB_2857365).Finally,images were obtained using a fluorescence microscope.The images were used to analyze cell types, numbers, and fluorescence intensity of co-labeling for the target proteins.ImageJ 2.0 was utilized to determine optical density.
Immunoprecipitation and coimmunoprecipitation assay
The fresh L4–L6 anterior horn of rats (n= 5) was lysed with immunoprecipitation (IP) lysis buffer (Servicebio), and the protein concentration was measured (Li et al., 2019a).Lysate solutions containing equivalent amounts of protein were mixed with 1.0 μg mouse IgG(ProteinTech, B900620) and 20 μL protein A/G-beads (Millipore, IP05) and incubated at 4°C for 1 hour.Then, the supernatant was incubated with 10 μL anti-EphA4 (1:50) or anti-Beclin1 (mouse, 1:50, Santa Cruz biotechnology,Cat# sc-48341, RRID: AB_626745) antibody or mouse IgG overnight at 4°C, followed by incubation with 80 μL of protein A/G beads for 2 hours at 4°C.The precipitate was collected, and 80 μL loading buffer (Beyotime Biotechnology) was added.After boiling, the supernatant was collected for mass spectrometry and western blotting.
Transmission electron microscopy
Fresh spinal cord tissue from the L4–L6 anterior horn (1 mm3) was immediately harvested after reperfusion.After fixation, the specimen was dehydrated in ethyl alcohol and acetone and embedded in 812 epoxy resin (SPI, West Chester, PA, USA, 90529-77-4).Then, 60-nm sections were obtained with an ultramicrotome (Leica, Wetzlar, Germany).Sections were double-stained with a solution of 2% saturated uranyl acetate in alcohol(Yingxin, Shanghai, China, Cat# 6159-44-0) and a lead citrate (Yingxin, Cat#512-26-5) solution for 15 minutes each, then dried overnight (Ge et al., 2021).Finally, the slices were viewed by transmission electron microscope (Hitachi,Tokyo, Japan).Three slices for each rat (n= 3) were assessed.
Mass spectrometry
Mass spectrometry was performed using a Thermo Q ExactiveTMliquid mass spectrometry system (Ma et al., 2021).Mass spectrometry data were retrieved by Protein Discover (V2.2) using the database retrieval algorithm Percolator.The database used for retrieval was the Uniprot rat proteome reference database (UniProt_rat_20180405.fasta).The search results were screened with a card value of ≥ 0.05.The retrieved items and contaminating proteins in the reverse database were deleted.PubMed was searched, using“ferroptosis”as the search term, and a list was made of proteins involved in ferroptosis.Ferroptosis-related proteins were detected by mass spectrometry.
Detection of iron, malondialdehyde, glutathione, and glutathione peroxidase activity
Fresh L4–L6 anterior horn tissue from rats (n= 5) was accurately weighed.Normal saline was added to the spinal cord tissue at a ratio of 9:1.After homogenization and centrifugation, the protein concentration in supernatant was measured.The iron level, malondialdehyde (MDA) content, glutathione(GSH) amount, and glutathione peroxidase (GPX) activity were determined using an iron assay kit (Jiancheng, Nanjing, China, A039-2-1), an MDA assay kit(Jiancheng, A003-1-2), GSH assay kit (Jiancheng, A006-2-1), and a GPX activity assay kit (Jiancheng, A005-1-2).
Molecular docking
The protein-protein docking method in ClusPro server (https://cluspro.org)was used to predict the binding mode between intracellular EphA4 and Beclin1 and between intracellular EphA4 and Mapk3/Mapk1.One thousand conformations were clustered by a greedy clustering algorithm (Sun et al.,2021).Then, the binding patterns were screened manually to obtain the greatest number of possible conformations.
Statistical analysis
SPSS 25.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8.0.1 (GraphPad Software Inc., La Jolla, CA, USA, www.graphpad.com) were used for statistical analysis and mapping.Results are expressed as the mean ± standard error of measurement (SEM).For normally distributed data, one-way analysis of variance was used for comparison between multiple groups with the Tukey’spost hoctest.Krukal-Wallis test was used for non-normal distribution data.P< 0.05 was considered statistically significant.
Results
SCIRI triggers changes in ferroptosis-related factors and abnormal expression of EphA4 in the L4–L6 anterior horn
Ferroptosis is accompanied by substantial iron accumulation, production of lipid peroxides, a decrease in glutathione levels, and a decline in GPX4 activity(Dixon et al., 2012).Thus, we measured the iron level, lipid peroxidation product MDA content, GSH amount, and GPX4 activity in the L4–L6 anterior horn at different time points (6, 12, 24, 48, and 72 hours) after reperfusion.We found that the iron level was significantly increased at 6 hours, reached its highest level at 24 hours, and then fell back to its normal level at 48 and 72 hours.The MDA content was noticeably increased at all time points studied and reached a maximum at 24 hours.The GSH level was low at all time points studied, and reached a minimum at 24 hours.GPX activity began to decrease at 12 hours and reached its lowest level at 24 hours.Taken together,all of these ferroptosis-related indices were clearly altered at 24 hours after reperfusion (Figure 2A).EphA4 mRNA expression was significantly increased at 24 and 48 hours.EphA4 protein expression was significantly elevated at 12 and 24 hours (Figure 2B).Therefore, we chose 24 hours after reperfusion as the appropriate timepoint for investigating the role and mechanism of EphA4 in SCIRI-induced ferroptosis in the subsequent experiments.
Intrathecal pretreatment with AV-sh-EphA4 reduces the increase in EphA4 expression induced by SCIRI in rat anterior horn neurons
An adenoviral vector containing a fluorescence gene (NC-GFP) was delivered to the subarachnoid space 3 days before SCIRI injury.A large area of fluorescence was observed in the anterior horn that co-localized with cell nuclei (Figure 3A).Furthermore, intrathecal injection of adenovirus encoding sh-EphA4 significantly reduced EphA4 mRNA and protein expression levels at 24 hours after reperfusion compared with the AV-sh-NC and I/R groups(Figure 3B).To determine the cell type in which EphA4 was expressed in the anterior horn of the spinal cord, immunofluorescence double labeling was performed for EphA4 and NeuN (neuronal marker) (Zhang et al., 2022),GFAP (astrocyte marker) (Zhang et al., 2022), Iba1 (microglial marker) (Zhang et al., 2022), Reca-1 (endothelial cell marker) (Saenno et al., 2022), or MBP(oligodendrocyte marker) (Wang et al., 2022a).The results showed that EphA4 expression mainly overlapped with neuronal markers in the anterior horn.Compared with the AV-sh-NC and I/R groups, intrathecal injection of AVsh-EphA4 significantly reduced the fluorescence intensity of the EphA4 signal in neurons of the anterior horn (Figure 3C).
EphA4 knockdown ameliorates SCIRI-induced neurobehavioral deficits,histological changes, decrease in motor neuron number, increase in BSCB permeability, spinal cord edema, and neuroinflammation by inhibiting ferroptosis
To evaluate whether EphA4 was involved in the regulation of SCIRI relatedferroptosis, we performed a rescue experiment using Erastin.First of all, we examined the effect of knocking down EphA4 on ferroptosis-related factors.The iron level and MDA content were significantly lower, and the GSH and GPX activities were remarkably higher, in theAV-sh-EphA4 group compared with the I/R and AV-sh-NC groups.Administration of Erastin reversed these effects(Figure 4A).Furthermore, we employed transmission electron microscopy to assess neuronal structure in the anterior horn and found that SCIRI induced neuronal damage.Injured neurons were irregular in shape and exhibited complete or local rupture of the cell membrane.The nuclear membranes were intact, locally sunken, wrinkled, or serrated.The mitochondria were shrunken, the internal cristae were dilated and broken, and the membrane density was increased, all of which are characteristic morphologic features of ferroptotic mitochondria.Mitochondria with ferroptosis-related morphological features also appeared in the axons.Injection of AV-sh-EphA4 markedly reduced the number of mitochondria exhibiting ferroptosis-related features, while Erastin administration reversed this effect (Figure 4B).The degree of damage to the BSCB was assessed by detecting EB leakage into the spinal cord tissue.The EB fluorescence intensity increased markedly after SCIRI, whereas inhibiting EphA4 expression substantially reduced EB extravasation.Moreover, intrathecal injection of Erastin counteracted the protective effect that intrathecal injection of AV-sh-EphA4 had on the BSCB(Figure 4C).Hematoxylin and eosin staining showed that the structure of the anterior horn was extremely disordered after SCIRI, the morphology of some neurons was irregular, and multipolarity disappeared.Neurons varied in their appearance, being shrunken, blunt, lightly stained, or diffusely swollen with eosinophilic cytoplasm, and the nuclei were blurred or pyknotic.In some tissue slices, a large area of cavitation was evident.Individual nerve fibers could not be distinguished.In the sham group, the neurons in the anterior horn were intact and exhibited fine, granular cytoplasm and Nissl substance.SCIRI decreased the number of intact neurons (Figure 4D).Immunohistochemical staining showed that the number of motor neurons in the anterior horn decreased markedly after SCIRI.In the AV-sh-EphA4 group,the number of motor neurons was markedly higher than that in the I/R group and AV-sh-NC group.Intrathecal injection of Erastin reversed the increase in motor neuron numbers in the anterior horn induced by intrathecal injection of AV-sh-EphA4 (Figure 4E and F).The degree of spinal cord edema showed a similar trend (Figure 4G).Next, we used Tarlov and BBB scores to assess the motor function of the lower extremities.Compared with the sham group,SCIRI substantially decreased motor function scores, while the AV-sh-EphA4 group showed markedly higher scores than the I/R and AV-sh-NC groups.Rats injected with AV-sh-EphA4 and Erastin had lower scores than rats injected with AV-sh-EphA4 alone (Figure 4H).Next, we performed western blotting for astrocyte and microglia markers to investigate the degree of proliferation and inflammatory state.SCIRI induced substantial increases in GFAP and Iba1 protein expression in the anterior horn.Inhibiting EphA4 expression markedly reduced GFAP and Iba1 expression, and Erastin reversed this effect.Taken together, these findings suggest that knocking down EphA4 suppresses inflammation by inhibiting ferroptosis (Figure 4I).
Screening for proteins that interact with EphA4 to regulate ferroptosis
EphA4 was used as a bait protein for immunoprecipitation, and the immunoprecipitated proteins were detected by mass spectrometry (Figure 5A).A total of 863 proteins were detected (Additional Table 1).When uncharacterized proteins were excluded, 829 proteins remained.Searching PubMed with“ferroptosis”as the search term retrieved 134 reviews, and a list of 118 proteins involved in ferroptosis was generated from these papers(Additional Table 2).There were 13 proteins in common between these two groups: GPX4, Beclin1, Acsf2, Nfs1, Gclm, Gstp1, Vdac2, Vdac3, Pcbp2,Mapk3, Mapk1, Hspb1, and Prkaa1 (Figure 5B).Eight of these proteins are known to be involved in IRI: GPX4, Beclin1, Gclm, Gstp1, Vdac2, Mapk3,Mapk1, and Hspb1.Three of these proteins co-localized with EphA4, can be modified by phosphorylation, and positively regulate ferroptosis: Beclin1,Mapk3 and Mapk1 (Erk1/2).EphA4 binding modes to Beclin1 and Erk1/2 was analyzed by molecular docking simulation (Figure 5C).According to the docking analysis, the amino acid residues bind to each other by hydrogen bonds and hydrophobic interactions.The specific interaction sites are shown in Additional files 1–3.These results indicate that EphA4 most likely regulates ferroptosis through these proteins and their associated signaling pathways.
EphA4 knockdown suppresses ferroptosis by downregulating p-Beclin1 and p-Erk1/2, weakening Beclin1-XCT complex formation, and reducing TFR1 protein expression in the L4–L6 anterior horn
To ve r i f y t h e m a s s s p e c t ro m e t r y re s u l t s, we ca r r i e d o u t coimmunoprecipitation (Co-IP) of spinal cord anterior horn lysates using anti-EphA4 antibody followed by western blotting for Beclin1 and Erk1/2.The Co-IP results showed that SCIRI increased binding to Beclin1, while inhibiting EphA4 expression decreased EphA4 binding to Beclin1 (Figure 6A and B).SCIRI did not cause any change in Erk1/2 expression.However, SCIRI increased p-Erk1/2 binding to EphA4, while knocking down EphA4 decreased p-Erk1/2 binding to EphA4 (Figure 6A and B).Next, Co-IP was performed using Beclin1 as the bait protein to detect Beclin1-XCT complex formation.Inhibiting EphA4 expression decreased XCT binding to Beclin1 (Figure 6C and D).Analyzing p-Erk1/2, Erk1/2, c-Myc, and TFR1 expression levels in the spinal cord anterior horn showed that SCIRI induced substantial increases in p-Erk1/2, c-Myc, and TFR1 expression, while knocking down EphA4 substantially reduced p-Erk1/2,c-Myc, and TFR1 expression.We further investigated the effect of Erk1/2 activation on the increased expression of c-Myc and TFR1 by intrathecal injection of U0126.U0126 had similar effects on c-Myc and TFR1 expression(Figure 6E and F).Simultaneously knocking down EphA4 diminished the SCIRIinduced increase in p-Beclin1 (Figure 6G).Fluorescence double-labeling showed that EphA4 co-localized with p-Beclin1 and p-Erk1/2, and that this co-localization was increased by SCIRI.However, inhibiting EphA4 expression decreased co-labeling.These finding support the Co-IP and western blotting results (Figure 7A and B).
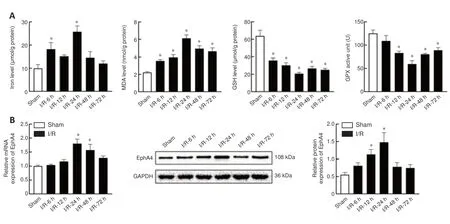
Figure 2|Ferroptosis-related indices were clearly altered at 24 hours after reperfusion,accompanied by a marked increase in EphA4 expression.
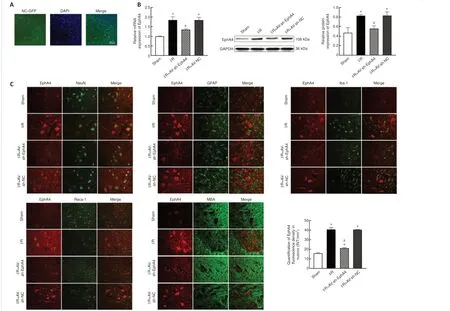
Figure 3|AV-sh-EphA4 decreases EphA4 expression in rat anterior horn neurons.
Discussion
In physiological states, iron plays a crucial role in the maintenance of normal nervous system function (Zhao et al., 2022), but excess iron deposition in cells can produce a large amount of ROS through Fenton and Haber-Weiss reactions (Li et al., 2021a).In addition, iron is indispensable for the function of the enzymes that catalyze ROS production (Nakamura et al., 2019).Iron overload was found in the heart, brain, kidney, and liver after IRI (Li et al.,2021b).Deferoxamine can effectively alleviate the cell damage caused by IRI (Parra-Flores et al., 2019).In cerebral IRI, regulating the expression of iron metabolism-related proteins can effectively reduce the infarct size (Groenendaal et al., 2000).This suggests that iron overload plays an important role in IRI.SCIRI induced a substantial increase in iron levels.In intracerebral hemorrhage, hemoglobin was taken up by microglia, and iron was transported from microglia to neurons by transferrin, causing neuronal iron overload (Li et al., 2017, 2021c; Wan et al., 2019).In a model of cerebral IRI, hemoglobin levels increased in brain tissue because of blood-brain barrier destruction (Yang et al., 2020).In SCIRI, BSCB destruction can lead to leakage of erythrocytes and hemoglobin into the spinal cord.All of these mechanisms create iron overload in the neurons of the anterior horn, leading to ferroptosis.A large amount of ROS is generated during ischemia/reperfusion.ROS attack polyunsaturated fatty acids in biofilms and cause lipid peroxidation(Li et al., 2021b).We detected MDA, a representative indicator of lipid peroxidation that indirectly reflects the degree of nerve cell injury, in the anterior horn (Su et al., 2020).After IRI, the level of GSH gradually decreased as it removed the massive amount of ROS that had been produced, resulting in an overall decrease in antioxidant capacity (Li et al., 2021a).We observed that the GSH decreased substantially and the antioxidant capacity decreased to the its point at 24 hours after reperfusion.GPX activity barely remained normal at 6 hours after reperfusion, and began to decrease markedly at 12 hours, indicating that the catalytic activity of GPX may be related to GSH consumption, and therefore may not inhibited until the body’s antioxidant capacity decreases to a certain level (Yang et al., 2014b).In our SCIRI model,the ferroptosis-related indices changed substantially, but the time course of change of each index was not completely identical.This suggests that ferroptosis may be a sequential process that occurs because of a gradually increasing imbalance between oxidation and antioxidation.
EphA4 and its signaling pathway are associated with the regulation of cerebral and intestinal IRI and cellular oxygen-glucose deprivation/reperfusion(Woodruff et al., 2016; Chen et al., 2018; Wei et al., 2019).These studies mainly focused on EphA4 leading to apoptosis and inflammation (Li et al.,2012; Wei et al., 2019).However, EphA4 overexpression in pluripotent stem cell-derived motor neurons from patients with motor neuron disease directly led to an increase in motor neuron death (Zhao et al., 2021).Inhibiting EphA4 expression protects motor neurons in amyotrophic lateral sclerosis(Van Hoecke et al., 2012; Zhao et al., 2018).Although different results were obtained in mice, this may be because of model differences, the differenttime window of lowering EphA4 expression, and the degree to which EphA4 expression was lowered (Ling et al., 2018; Poppe et al., 2019; Rué et al.,2019; Dominguez et al., 2020).A recent study found that ferroptosis is a major type of motor neuron cell death in motor neuron disease (Wang et al., 2022b).In our study, we found that EphA4 is involved in SCIRI regulation.We also found that EphA4 is expressed in anterior horn neurons.Inhibition of EphA4 expression effectively increased motor neuron survival and improved indicators related to ferroptosis, and these effects were reversed by administration of a ferroptosis inducer, indicating that EphA4 is involved in the regulation of motor neuron ferroptosis in SCIRI.

Figure 4|Intrathecal administration of AV-sh-EphA4 ameliorates the SCIRI-induced increase in BSCB permeability, histologic changes, decrease in the number of motor neurons, decrease in neurological function score, spinal cord edema, and neuroinflammation by inhibiting ferroptosis.
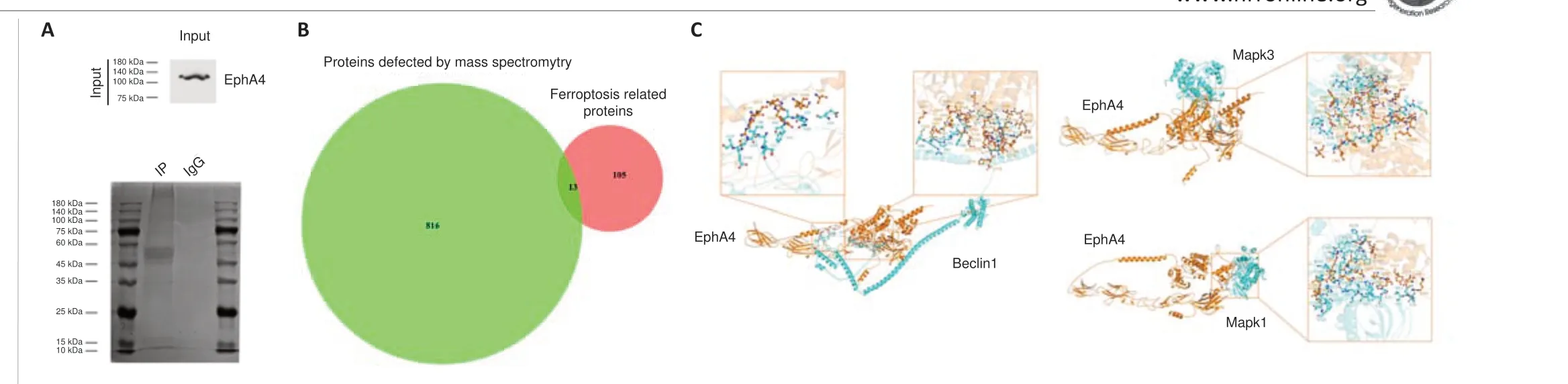
Figure 5|Screening for EphA4-interacting proteins involved in ferroptosis.
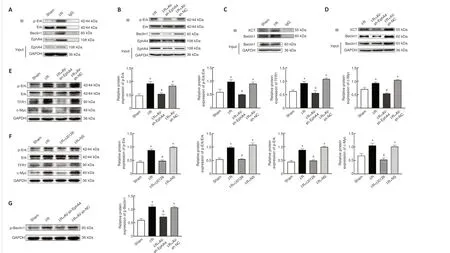
Figure 6|EphA4 contributes to ferroptosis by upregulating p-Beclin1 and p-Erk, promoting Beclin1-XCT complex formation, and increasing TFR1 protein expression.
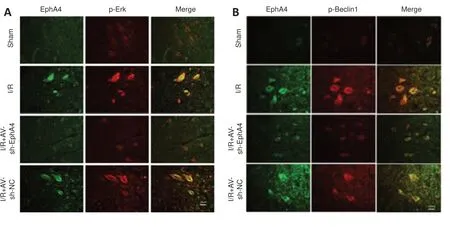
Figure 7|Immunofluorescence double-labeling of EphA4 and p-Erk1/2 or p-Beclin1 in the rat L4–L6 spinal cord anterior horn.
Previous studies have indicated that the EphA4 signaling pathway plays a variety of roles by phosphorylating downstream proteins and activating downstream pathways through cascade reactions (Yang et al., 2018; Zhao et al., 2021).We identified Beclin1 and Erk1/2 as the main proteins affected by EphA4 and analyzed their phosphorylation levels and downstream pathways.Beclin1 is a key protein involved in autophagy that forms autophagosomes in the early stage of autophagy induction (Tran et al., 2021).Reports have shown that phosphorylated Beclin1 can promote cell ferroptosis.In cellular ferroptosis induced by Erastin, AMPK increased Beclin1 phosphorylation,promoting formation of the Beclin1-XCT complex, decreasing system Xc–activity, preventing cystine from entering into cells, and ultimately leading to ferroptosis (Song et al., 2018).Ferroptosis induced in the neuroblastoma cell line SH-SY5Y by a high concentration of isoflurane was also mediated by increasing the phosphorylation of Beclin1 and promoting the formation of Beclin1-XCT complex (Liu et al., 2019).Similarly, suppressing Beclin1 expression and reducing Beclin1 phosphorylation relieved the inhibition of system Xc–, reduced neuronal ferroptosis, and improved neurological function after subarachnoid hemorrhage (Guo et al., 2019).In our study,we showed that SCIRI-induced ferroptosis of motor neurons is regulated by EphA4 activation via the same mechanism.In addition, Erk1/2 participates in the regulation of a variety of physiological and pathological processes(Kong et al., 2019).U0126 can effectively reduce motor neuron apoptosis by inhibiting SCIRI-induced Erk1/2 activation (Lu et al., 2010).Previous studies have shown that Erk1/2 is an important signaling factor downstream of EphA4 activation (Shu et al., 2016; Lim et al., 2019).In our study we found that EphA4 directly binds Erk1/2 and promotes Erk1/2 phosphorylation.The most classical pathway activated by Erk1/2 is the rat sarcoma-rapidly accelerated fibrosarcoma-mitogen-activated protein-ERK (Yagoda et al., 2007) pathway,which is considered to vital to ferroptosis (Chen et al., 2021).This suggests that Erk1/2 signaling pathway activation plays a key role in ferroptosis.ERK1/2 activation has been reported to lead to c-Myc phosphorylation at S62, which increases c-Myc stability and c-Myc expression levels (Sears et al., 2000).c-Myc then acts as a transcription factor to bind to its typical response element (E-boxes, CANNTG) and promote the transcription of downstream genes (O’Donnell et al., 2006; Ning et al., 2011).In a cerebral IRI model, c-Myc protein expression increased substantially in the brain (Zhang et al., 2020).Furthermore, inhibiting c-Myc expression reduced the level of inflammation and promoted neuronal survival in SCIRI (Zhang et al., 2022).Up-regulated c-Myc expression in tumor cells mediates tumor proliferation and tumorigenesis by increasing TFR1 expression (O’Donnell et al., 2006).TFR1 expression is upregulated in tumor tissues and some pathological conditions such as neurodegenerative diseases (Du et al., 2015), IRI (Tang et al., 2021), myocarditis (Xu et al., 2015), and nephropathy (Berthelot and Monteiro, 2013).TFR1, an iron receptor, causes cellular iron overload and mediates ferroptosis (Tang et al., 2021).Consequently, TFR1 is considered to be a specific marker of ferroptosis (Feng et al., 2020).Knocking down TFR1 can inhibit cardiomyocyte ferroptosis induced by myocardial IRI (Tang et al.,2021).After intracerebral hemorrhage, downregulation of TFR1 expression regulates iron metabolism and promotes neuronal survival (Yang et al., 2021).In our model, SCIRI activated Erk1/2 and increased c-Myc and TFR1 protein levels.Inhibiting EphA4 expression reduced the expression of downstream proteins c-Myc and TFR1 by suppressing Erk1/2 activation.This suggests that EphA4 regulates ferroptosis of motor neurons in the anterior horn through the Erk1/2/c-Myc/TFR1 signaling pathway.
In addition, we found that inhibiting neuronal ferroptosis affected inflammatory levels and BSCB permeability in SCIRI.Ferroptosis differs from apoptosis in that it causes cell membranes to break down and cell contents to flow out, which activates the immune system, inducing inflammatory cell infiltration and proliferation (Chen et al., 2015; Proneth and Conrad,2019).Thus, inhibiting ferroptosis in neurons can alleviate the inflammatory response.In addition, the BSCB may be damaged by inflammatory mediators secreted by inflammatory cells (Ren et al., 2015).Therefore, inhibiting neuronal ferroptosis can reduce BSCB leakage and spinal cord edema.
There were some limitations to our study.First, we did not perform anyin vitroexperiments.Second, we cannot exclude the possibility that SCIRI causes ferroptosis in other cells in the spinal cord, such as glial cells and endothelial cells.Finally, given that EphA4 can transmit information from neurons to other cells, thereby affecting protein expression and signaling pathways in other cells (Carmona et al., 2009; Yang et al., 2014a), we cannot rule out the potential effect EphA4 inhibition on other cells in the spinal cord.
In conclusion, we found that EphA4 participates in the regulation of ferroptosis of spinal anterior horn motor neurons by promoting formation of the Beclin1-XCT complex and activating the Erk1/2/c-Myc/TFR1 axis in SCIRI.Inhibition of EphA4 expression in the spinal cord improved neurological function and reduced neuroinflammation and BSCB leakage by inhibiting neuronal ferroptosis.In short, ferroptosis is an essential form of neuronal death that occurs during SCIRI.Future studies should investigate the mechanisms and signaling pathway that regulate cell death in SCIRI.Our findings could provide new treatment targets for increasing cell survival after SCIRI.In particular, EphA4 could be targeted to inhibit neuronal ferroptosis,although the development of small molecular inhibitors of EphA4 might be a challenging task (Tognolini et al., 2014).
Author contributions:YD, HM designed the experiments; YD, YC, DZ, SL,XT, CA, and ZZ carried out experiments and achieved the data; YD and YC performed the statistical analysis; YD, DZ and ZZ made the figures; YD, CA,and SL drafted the manuscript.All authors approved the final version of thismanuscript.
Conflicts of interest:The authors report no conflicts of interest and are solely responsible for the content and writing of this manuscript.
Data availability statement:All relevant data are within the paper and its Additional files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Table 1:EphA4 is used as a bait protein for immunoprecipitation and the detected proteins list by mass spectrometry.
Additional Table 2:118 ferroptosis related proteins list were obtained through searching PubMed with ferroptosis.
Additional file 1:The specific interaction sites of EphA4 with Beclin1 by the protein-protein docking in ClusPro server.
Additional file 2:The specific interaction sites of EphA4 with Mapk3 by the protein-protein docking in ClusPro server.
Additional file 3:The specific interaction sites of EphA4 with Mapk1 by the protein-protein docking in ClusPro server.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain

