M2 macrophages mediate fibrotic scar formation in the early stages after cerebral ischemia in rats
Jia-Gui Huang, Jiang-Xia Ren, Yue Chen, Ming-Fen Tian, Li Zhou, Jun Wen, Xiao-Song Song, You-Lin Wu, Qing-Huan Yang,Pei-Ran Jiang, Jia-Ni Wang, Qin Yang
Abstract
Key Words:central nervous system; extracellular matrix; fibronectin; fibrotic scar; macrophage; interleukin 4; ischemic cerebral injury; neurological function;Sonic hedgehog; transforming growth factor β1
Introduction
Ischemia in the brain or spinal cord induces the formation of an inner fibrotic scar as well as an outer glial scar (Dorrier et al., 2021; Shao et al., 2021; Yan et al., 2022).After injury, reactive astrocytes form the glial scar to surround the lesion area, and this process has been extensively studied.A few days following injury, fibroblasts gradually infiltrate, and extracellular matrix (ECM)proteins are deposited at the core of the injury, where they form the fibrotic scar adjacent to the medial side of the glial scar.Little is known about the formation and role of fibrotic scar after brain injury or spinal cord injury (SCI).After organ injury, the tissue undergoes three stages of healing: inflammation,new tissue formation, and remodeling (D’Ambrosi and Apolloni, 2020).Excessive invasion of inflammatory cells can lead to the development of fibrosis in injured peripheral organs.In an experimental autoimmune encephalomyelitis mouse model, inflammatory cell infiltration drove fibrotic scar formation and persistence in the central nervous system (Dorrier et al., 2021).Macrophages, which are a type of inflammatory cell, exert a vital function in initiating, maintaining, and resolving tissue damage, and are a key regulator of tissue fibrosis (Wynn and Vannella, 2016; Henderson et al., 2020).Conventionally, macrophages are categorized as M1 (pro-inflammatory) or M2 (anti-inflammatory).M2 macrophages are involved in regulating fibrotic scar formation, promoting tissue repair, and alleviating damage after injury to peripheral tissues, such as the heart, lung, and kidneys (Liu et al., 2018b;Ueshima et al., 2019; Hou et al., 2021).After SCI, M2 macrophages promote migration and ECM protein secretion of platelet derived growth factor receptor β positive (PDGFRβ+) pericytes (Li et al., 2021a).However, whether macrophage recruitment causes fibrotic scar formation and the signaling mechanisms that regulate this process after ischemic cerebral stroke remain unclear.
Interleukin 4 (IL4) is mostly produced by activated T cells and plays a vital role in M2 polarization of macrophages.IL4-induced M2 macrophages can engulf cell debris, secrete anti-inflammatory factors, reduce neuronal damage,improve long-term neurological function (Liu et al., 2016; Kolosowska et al.,2019; Xu et al., 2020; Chen et al., 2022), and regulate fibrosis of the heart,kidney, skin, liver, and lung (Knipper et al., 2015; Liang et al., 2017; Weng et al., 2018).Macrophage-specific knockout of IL4 receptor α diminishes pancreatic fibrosis in chronic pancreatitis (Xue et al., 2015).However, it remains to be clarified whether and how IL4-induced M2 macrophages regulate fibrotic scar formation after ischemic cerebral injury.
The sonic hedgehog (Shh) pathway comprises the Shh ligand, Gli transcription factor, and Smoothened (Smo) and Patched-1 receptors.Shh signaling is widely involved in repairing and reconstructing tissues post-ischemic brain injury (Chen et al., 2017; Yu et al., 2017; Liu et al., 2018a) and in regulating fibrotic scar generation following kidney, lung, and liver injury and glial scar generation post-SCI or post-brain injury (Chung et al., 2016; Honsa et al., 2016; Kim et al., 2017; Hou et al., 2021).However, it remains to be investigated whether Shh signaling is involved in IL4-induced M2 macrophage regulation of fibrotic scar formation after ischemic cerebral stroke.We hypothesized that IL4-induced M2 macrophages regulate fibrotic scar formation via the Shh/transforming growth factor beta 1 (TGFβ1) axis in the early stage (within 2 weeks) of ischemic brain stroke in rats, and validated this hypothesis in this study.
Methods
Animals
In the present study, only male rats were used to avoid the influence of estrogen on ischemic injury and functional prognosis (Koellhoffer and McCullough, 2013; Chauhan et al., 2017).Two hundred eighty-eight 2-monthold male Sprague-Dawley rats (200–250 g) (forin vivomodeling), twenty 4-week-old male Sprague-Dawley rats (for bone marrow-derived macrophage[BMDM] culture), and 20 1-day-old Sprague-Dawley rats (for primary fibroblast culture) were bought from the Department of Animal Experiments,Chongqing Medical University (license No.SCXK (Yu) 2018-0003).The rats were housed under specific pathogen-free conditions at 24°C, with a 12/12-hour light/dark cycle and 60% humidity.The animals were allowed ad libitum access to water and food.All animal experiments were carried out in accordance with the related laws and relevant regulations of the Animal Experimental Committee of Chongqing Medical University and were approved by the Animal Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (approval No.2022-K314) on June 29, 2022.The animalrelated procedures were conducted following the Ischaemia Models:Procedural Refinements Ofin VivoExperiments (IMPROVE) guidelines (Percie du Sert et al., 2017).The protocols and details of the study complied with the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines(Percie du Sert et al., 2020).The number of animals in each group was predetermined based on published studies (Chen et al., 2017; Rajan et al., 2019)or our previous experiments.Outcomes were measured by researchers who were blinded to the experimental conditions.All rats were randomly assigned to groups for each experiment.The experimental groups, animal numbers,and sample detection methods are shown in Additional file 1.
Middle cerebral artery occlusion/reperfusion model
Intraluminal middle cerebral artery occlusion/reperfusion (MCAO/R) was performed to induce focal cerebral ischemia, as we described previously (Yu et al., 2021).Briefly, male rats were sedated before surgery via intraperitoneal injection of 3.5% chloral hydrate (1 mL/100 g, Chengdu Kelong Chemical Co.,Ltd., Qionglai, Sichuan, China) and subsequently received buprenorphine for pain relief by subcutaneous injection (0.05 mg/kg, twice a day for 2 consecutive days).Throughout surgery, body temperature was maintained at 37 ± 0.5°C with a thermostatically controlled heating pad and monitored by rectal temperature.After isolation of the right common carotid artery, right external carotid artery, and right internal carotid artery, a poly-L-lysine-coated surgical filament (Beijing Cinontech Co.Ltd., Beijing, China) was inserted from the right external carotid artery and advanced about 18 mm to occlude the origin of the right middle cerebral artery.After 2 hours of ischemia, the filament was withdrawn to allow reperfusion.Sham-operated rats underwent the same anesthesia and surgical procedures without filament insertion.Animals were closely monitored until they recovered from anesthesia.Only animals with a Longa score (Yu et al., 2017) of 1–3 points were included for further studies.Rats with a Longa score of 0 or 4 points, rats that exhibited subarachnoid hemorrhage and rats that died during or immediately after surgery were excluded from further experiments (Additional Table 1).
Intracerebroventricular catheterization and injection
Intracerebroventricular catheterization was performed as previously described (Chen et al., 2017).In brief, rats were anesthetized, and a 23 G guiding cannula (RWD Life Science, Shenzhen, China) was inserted in the right lateral ventricle (bregma-based coordinates: 1.5 mm lateral, 0.9 mm posterior, 3.6 mm ventral).Thereafter, the guide cannula was secured with dental cement and sealed with a catheter cap.After 7 days, MCAO/R was induced.For intracerebroventricular injection, the drug was injected using a 10-μL Hamilton microsyringe-connected 27 G injection cannula.The Hamilton micro-syringe was mounted on a variable-speed micro-infusion pump (model KDS LEGATO 130, RWD Life Science).Rats in the sham operation group underwent the identical operation with no MACO induction or cerebroventricular injection.
Cell culture
RAW264.7 cells (Cat# TIB-71, RRID: CVCL_0493) are a cell line of mouse monocyte macrophages, were purchased from American Type Culture Collection (Rockefeller, MD, USA), and their identify was confirmed by DNA profiling (using the short tandem repeat profiling method).The cells were maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM;Gibco, Beijing, China) that contained 1% penicillin/streptomycin as well as 10% fetal bovine serum (FBS; Gibco, Gaithersburg, MD, USA).
BMDMs were extracted as described previously (Mounier et al., 2013; Zhou et al., 2018).Briefly, rats were euthanized by carbon dioxide overexposure(a flow rate of 30% to 70% of the chamber volume per minute from a compressed carbon dioxide cylinder).Bone marrow was harvested from the femurs and tibias by flushing the bone cavities with DMEM.The collected fluid was subjected to erythrocyte lysis and filtered through a 70-μm cell strainer to isolate bone marrow cells, which were then cultured in macrophage differentiation media (DMEM containing 1% penicillin/streptomycin, 20 ng/mL recombinant rat macophage colony stimulating factor (PeproTech, Rocky Hill, NJ, USA, Cat# 400-28-10), and 10% FBS).The next day, non-adhering cells were collected, followed by 1 week of culture to generate BMDMs.By day 7, the adhering cells had developed into mature macrophages.F4/80 immunofluorescence labeling was used to identify macrophages.
Rat meningeal-derived fibroblasts were isolated from newborn rats according to Abe et al.’s approach (Abe et al., 2019) with minor modifications.In brief, rats were euthanized by carbon dioxide overexposure, sterilized in 75% ethanol, and decapitated using sharp scissors.The meninges were harvested, diced, and incubated with 0.125% trypsin for 5 minutes at 37°C.Then, DMEM containing type IV collagenase (1 mg/mL) was added and the cells were incubated at 37°C for another 15 minutes.After eliminating large particles, the resulting cell suspension was cultured in DMEM/F12 (Gibco,Beijing, China) containing 10% FBS at 37°C in a 5% CO2atmosphere overnight.Afterwards, non-adhering cells were discarded, whereas the adhering cells were further cultured to passage 4/5, and the primary fibroblasts were collected for use in later assays.Vimentin immunofluorescence labeling was used to identify fibroblasts.
To examine the effect of macrophages on fibroblasts, fibroblasts were grown in macrophage-conditioned medium (MCM).To do this, BMDMs and RAW264.7 cells were starved for 24 hours by incubation with DMEM containing 2% FBS and subsequently stimulated with or without recombinant rat IL4 (20 ng/mL,Beyotime Institute of Biotechnology, Nantong, Jiangsu, China, Cat# P6267) for 2 days.The culture supernatants were gathered and centrifuged at 12,000 ×gfor 10 minutes to remove cell debris.Next, the fibroblasts were incubated with DMEM/F12 containing 2% FBS for 24 hours, and then plated in a 1:1 ratio of fresh DMEM/F12 to MCM containing 2% FBS and robotnikinin (5 μM,an inhibitor of Shh; Medchemexpress, Newark, NJ, USA, Cat# HY-100515) for 2 days.Fresh DMEM/F12 with DMEM containing 20 ng/mL recombinant rat IL4 and robotnikinin (1:1 ratio) was used as the control.
Adenovirus vector transfection
SHH-shRNA (Ad-shSHH), SHH (Ad-SHH), and negative control (Ad-NC)adenoviruses were generated by GenePharma (Shanghai, China).The adenoviruses were delivered via intracerebroventricular injection (5 μL/rat) 4 days prior to MCAO/R, and Shh knockdown or overexpression was confirmed by assessing green fluorescent protein intensity within cerebral tissue sections as well as western blotting of homolateral ischemic tissue samples (Additional
Figure 1).
For adenovirus transfection, RAW264.7 cells were seeded into six-well plates,grown to 70–80% confluency, and infected with adenovirus at a multiplicity of infection of 30 in the presence of polybrene (5 μg/mL, GenePharma).After infection for 72 hours, the medium was replaced with DMEM containing 10%FBS, followed by another 48 hours incubation.Finally, fluorescence detection and western blotting were performed to assess the transfection efficiency(Additional Figure 2).
Drug administration
For thein vivoexperiments, Sprague-Dawley rats, before or after MCAO/R,were treated with phosphate-buffered saline (PBS), 10 μL dimethyl sulfoxide,recombinant rat IL4 (800 ng/d in 10 μL PBS), or 20 μM cyclopamine (Cyc,Medchemexpress, Cat# HY-17024) by intracerebroventricular injection.A constant infusion rate of 0.5 μL/h for 7 or 14 days was controlled by a microinfusion pump, starting from 2 hours after MCAO/R.PBS-filled liposomes or clodronate liposomes (Clo, 2 mL per 200 g body weight, Liposoma B.V.,Amsterdam, Netherlands, Cat# 40337ES10) were administered by vein injection, 24 hours after MCAO/R and then on days 4 and 10.
For thein vitroexperiments, BMDMs, RAW264.7 cells, and fibroblasts were treated with PBS, MCM, 20 ng/mL recombinant rat IL4, 10 μM recombinant mouse Shh (Cat# HY-P7409, Medchemexpress), or 10 μM Cyc for 48 hours.
5-Ethynyl-2'-deoxyuridine assay
Cell proliferation was assessed using a 5-ethynyl-2′-deoxyuridine (EdU)kit (Beyotime Institute of Biotechnology, Cat# C0071S).Briefly, cells were incubated with EdU (10 μM) for 4 hours, fixed with 4% paraformaldehyde for 15 minutes, and permeated with 0.3% Triton X-100 for another 15 minutes,and the EdU signal was then visualized according to the manufacturer’s instructions.4,6-Diamidino-2-phenylindole was used for nuclear staining.The cells were visualized at a magnification of 100× using a fluorescence microscope (Olympus, Tokyo, Japan).
Neurological function score
The modified neurological severity score was used to assess neurological dysfunction 7 days post-MCAO/R (Yu et al., 2017).The modified neurological severity score includes motor tests (score 0 to 6), sensory tests (score 0 to 2), balance beam tests (score 0 to 6), and assessment for absent reflexes and abnormal movements (score 0 to 4).The higher the score, the more severe the injury.The neurological function was evaluated by an independent researcher who was blinded to the experimental conditions.
Determination of cerebral infarct volume
For 2,3,5-triphenyltetrazolium chloride staining (TTC) staining, rats were euthanized by carbon dioxide overexposure, and brain tissues were rapidly removed on ice, frozen for 20 minutes at –20°C, and sliced into 2-mm coronal sections.The sections were then incubated with 2% TTC solution (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China, Cat# G3005) for 15 minutes and fixed in 4% paraformaldehyde for 2 hours at 4°C.TTC-stained sections were photographed, and the infarct area was extracted from the brain slices and stored immediately in a –80°C freezer for future use.ImageJ software (version 1.8.0, National Institutes of Health, Bethesda, MD, USA)(Schneider et al., 2012) was used for measuring the infarct area, and the following formula was used to determine infarct percentage: (contralateral area – ipsilateral noninfarct area)/contralateral area × 100.The infarct areas were calculated by a single researcher blinded to the experimental design.
Histology staining and immunostaining
For the preparation of paraffin sections, rats were anesthetized with chloral hydrate and buprenorphine and subjected to cardiac perfusion with 250 mL of cold saline followed by 250 mL of 4% paraformaldehyde.Next, the brains were harvested and placed in 4% paraformaldehyde overnight, dehydrated,and paraffin-embedded, after which sequential coronal sections (4-μm thickness) were taken.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)staining was performed using a TUNEL Cell Apoptosis Detection Kit (Servicebio,Wuhan, China, Cat# G1502-50T).In brief, sections were treated with proteinase K solution (20 μg/mL) for 20 minutes and equilibration buffer for 10 minutes.Thereafter, sections were incubated with TUNEL reaction mixture for 1 hour at 37°C, followed by 4,6-diamidino-2-phenylindole (Beyotime Institute of Biotechnology, Cat# C1005) counter-staining.A Zeiss LSM 800 confocal microscope (Zeiss, Braunschweig, Germany) was used for imaging.Apoptotic cell percentage was measured by dividing the TUNEL-positive cell count by the overall cell number.
Forin vitroimmunofluorescence staining, cells were fixed for 30 minutes in 4% paraformaldehyde.To stain tissue sections, each slide was deparaffinized and rehydrated according to routine procedures.Then, the cells or tissues were permeabilized for 20 minutes using 0.3% Triton X-100, treated with Trisethylene diamine tetraacetic acid solution (pH 9.0, Beijing Solarbio Science &Technology Co., Ltd.) for antigen retrieval, and blocked for 1 hour with 10%normal goat serum (Beyotime Institute of Biotechnology).Next, they were incubated overnight at 4°C with the primary antibodies shown in Additional Table 2, followed by further incubation with Dylight-conjugated IgGs (1:200,all from Abbkine, Wuhan, China) for 1 hour at 37°C, including goat anti-mouse IgG, Dylight 488 (Cat# A23210, RRID: AB_2923050), goat anti-rabbit IgG,Dylight 594 (Cat# A23420), goat anti-rabbit IgG, Dylight 488 (Cat# A23220,RRID: AB_2737289), and goat anti-mouse IgG, Dylight 594 (Cat# A23410).Nuclei were stained with 4,6-diamidino-2-phenylindole for 10 minutes.Finally,a Zeiss LSM 800 confocal microscope or Dragonfly 200 high speed confocal microscope (Andor, Oxford, UK) was employed to observe cells/tissues.
For immunohistochemistry staining, each slide was deparaffinized and rehydrated according to routine procedures, treated with 3% H2O2for 10 minutes and Tris-ethylene diamine tetraacetic acid solution (pH 9.0) for 20 minutes, incubated with bovine serum albumin (Beyotime Institute of Biotechnology) for 15 minutes and the primary antibodies shown in Additional Table 2 overnight at 4°C, followed by another 20 minutes of incubation at 37°C using poly-horseradish peroxidase and polymer Helper goat anti-rabbit IgG (ready to use, Zhongshan Goldenbridge Biotechnology,Beijing, China, Cat# PV-9001, RRID: AB_2868452).3,3-Diaminobenzidine and hematoxylin were used as the chromogen and nuclear counter-stain,respectively.The immunostained sections were examined using a light microscope (Zeiss).
Image analysis
Three non-continuous brain sections (at least 60 μm apart) per rat or sixin vitrocell climbing sheets were randomly selected.Three regions of interest per brain section in the ischemic core in the cortex and striatum or six microscopic fields (200×) per cell climbing sheets were randomly selected and imaged using the same imaging parameters.Cell counting and assessment of fluorescence intensity, microvascular density, and integrated optical density in immunostaining images were performed using ImageJ software.The results from each section were averaged, with 3–6 samples per group.All quantitative analyses were performed by an investigator blinded to the experimental groups.
Western blot assay
Following euthanasia, brain tissues were harvested and lysed in radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology)containing protease/phosphatase inhibitors (Beyotime Institute of Biotechnology).Bicinchoninic acid assay (Beyotime Institute of Biotechnology)was used to measure protein content.Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes.Afterwards, the polyvinylidene fluoride membranes were blocked with 5% defatted milk for 1 hour and incubated with the primary antibodies shown in Additional Table 2 overnight at 4°C, followed by incubation with one of the following horseradish peroxidase-conjugated secondary antibodies (1:5000, all from Proteintech,Wuhan, China) at 27°C for 1 hour: goat anti-rabbit (Cat# SA00001-2, RRID:AB_2722564) or goat anti-mouse (Cat# SA00001-1, RRID: AB_2722565).A luminescent image analyzer (Bio-Rad, Hercules, CA, USA) was used for quantifying protein bands.Images were analyzed using ImageJ software.The protein expression level was determined by the mean signal level on a grayscale image, and glyceraldehyde phosphate dehydrogenase was used as the reference.
Enzyme-linked immunosorbent assay
To examine IL4/6/10 and interferon γ (IFNγ) levels, brain tissues were homogenized in extraction buffer, the homogenate was centrifuged, and the supernatants were collected.Thereafter, cytokine levels in supernatants were determined using commercially available quantitative enzyme-linked immunosorbent assay kits (Multi Sciences, Hangzhou, China) according to the manufacturer’s instructions.Each assay was conducted in triplicate.
Statistical analysis
The Shapiro-Wilk test was used to determine whether data were normally distributed.The two-tailed Student’st-test was used to evaluate differences between two groups.One-way analysis of variance followed by Tukey’s multiple comparisons test was used to compare several groups.Correlations among the groups were determined by Pearson’s correlation analysis.The results are shown as mean ± standard deviation (SD).P< 0.05 was considered statistically significant.GraphPad Prism software (version 8.0.2; GraphPad Software; San Diego, CA, USA; www.graphpad.com) was used to perform the statistical analyses.The detailed statistical reports are shown in Additional Table 3.
Results
MCAO/R injury induces marked fibrosis within the ischemic core in rats
The fibrotic scar that forms after tissue injury mostly consists of myofibroblasts and excess ECM, primarily containing collagen (Col), fibronectin (FN), and laminin (Li et al., 2021b).Therefore, we first evaluated whether MCAO/R injury induced the formation of fibrotic scar within the ischemic core in rats.Immunohistochemistry staining showed that Col1 (Figure 1A–I)deposition in the ischemic core was increased at 7 and 14 days following MCAO/R compared with the Sham group.Moreover, western blot analysis demonstrated that FN and Col1 expression were higher in the MCAO/R group than in the Norm and Sham groups (Figure 1J–L), peaked at 14 days, and then gradually decreased.Taken together, these results show that MCAO/R injury induces significant fibrosis within the ischemic core in rats.
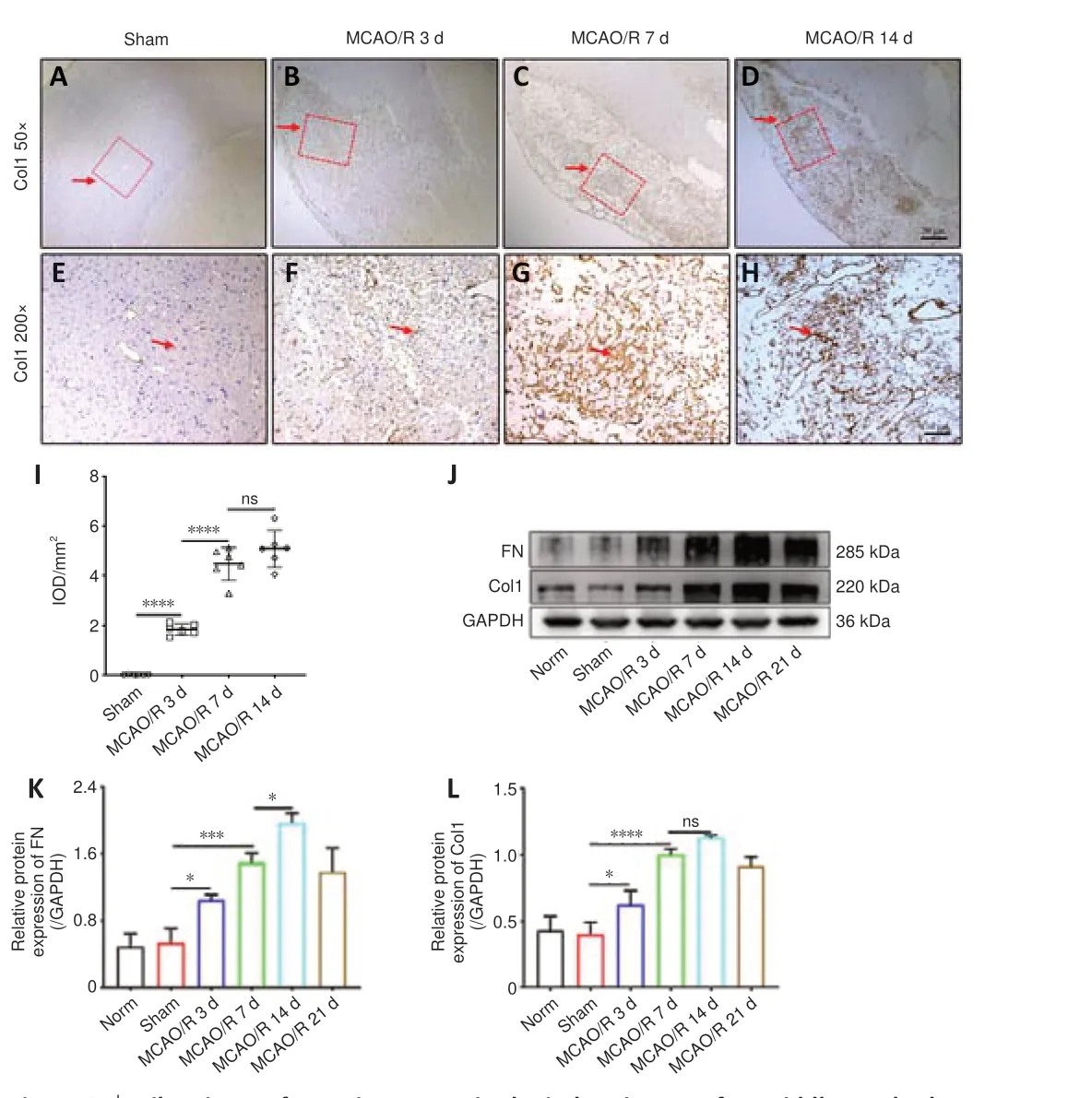
Figure 1|Fibrotic scar formation occurs in the ischemic core after middle cerebral artery occlusion/reperfusion (MCAO/R) in rats.
BMDMs mediate fibrogenesis in the ischemic core that forms after MCAO/R in rats
F4/80 is a monocyte-derived macrophage marker that has relatively high specificity (Wang et al., 2020).We first determined the temporal and dynamic response of macrophages after MCAO/R injury.As revealed by immunofluorescence staining, excessive F4/80-positive cell infiltration was observed in the ischemic core 3 days after MCAO/R, which was not seen in the contralateral hemisphere or in the Sham group (Figure 2A–D).In addition,the number of F4/80-positive cells was notably higher in the ischemic core at 7 and 14 days than that at 3 days after MCAO/R, and peaked at 14 days (Figure 2E–P).F4/80-positive cells had a uniform round shape without apparent processes.Immunofluorescence double staining demonstrated that FNpositive fibers and F4/80-positive macrophages were co-localized, and this interaction became more pronounced over the time course of injury (Figure 2E–R).Pearson’s correlation analysis suggested a robust positive association between the number of F4/80-positive macrophages and FN fluorescence intensity (Figure 2S).
Next, circulating monocyte/macrophages were transiently depleted with clodronate-filled liposomes (Figure 3A), which mediate macrophage“suicide”(Miró-Mur et al., 2016).After Clo treatment for 7 days following cerebral ischemia in rats, the number of F4/80-positive cells in the ischemic core was two-fold lower than that seen in mice that received vehicle-filled liposomes(Veh) (Figure 3B–I).At the same time, The number of FN-positive fibers and Col1-positive fibers decreased by 65.3% and 41.3%, respectively, in the Clo group compared with the Veh group at 7 days post-MCAO/R in rats (Figure 3J–O).In addition, western blot analysis showed that FN and Col1 expression was reduced in the Clo group compared with the Veh group at 7 days post-MCAO/R in rats (Figure 3P and Q).These results confirm that blood monocyte-derived macrophages contribute to the development of fibrotic scar in the early stage of ischemic core formation after MCAO/R in rats.
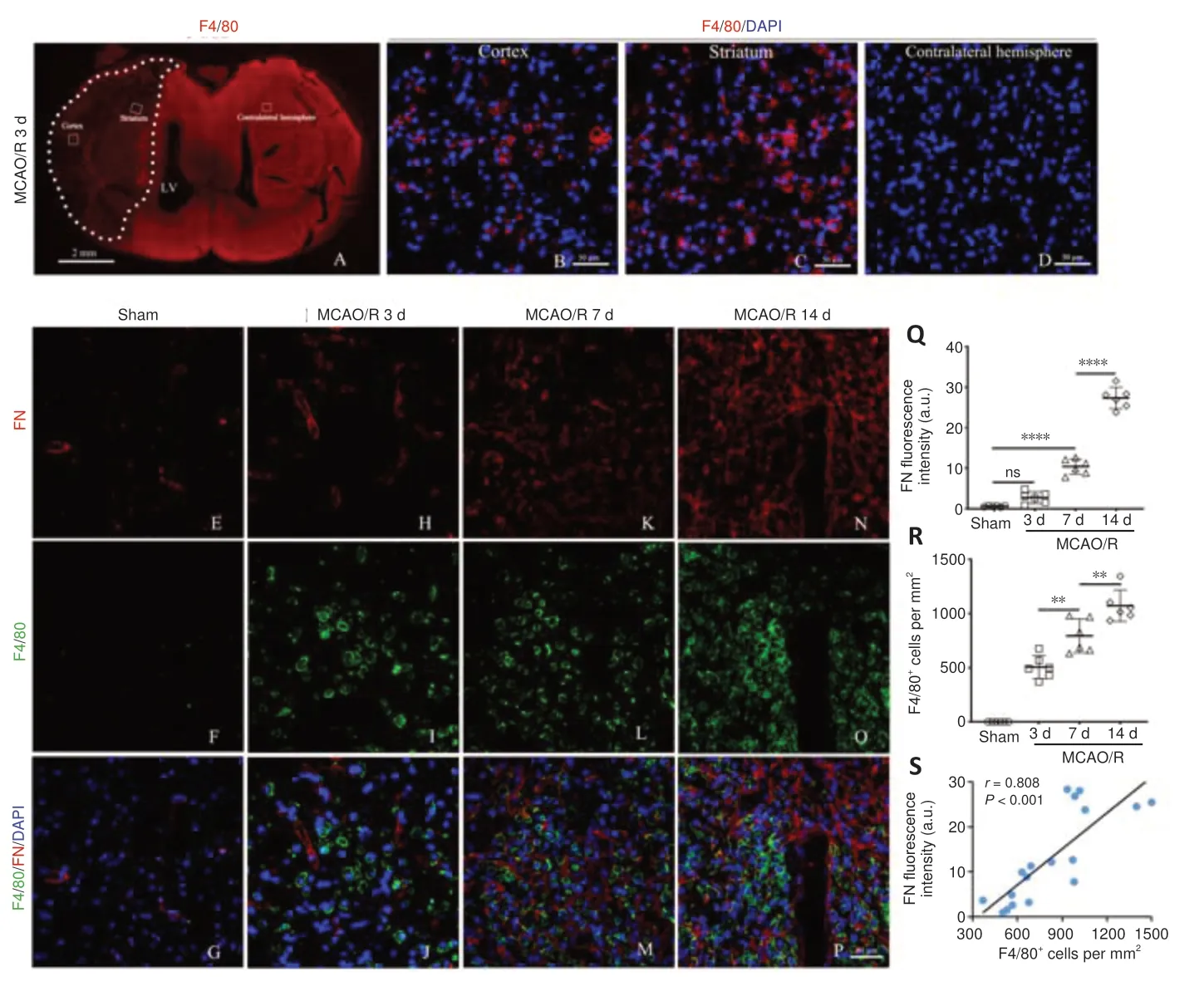
Figure 2|Macrophages infiltrate into the ischemic area and may be positively correlated with fibrotic scar formation.
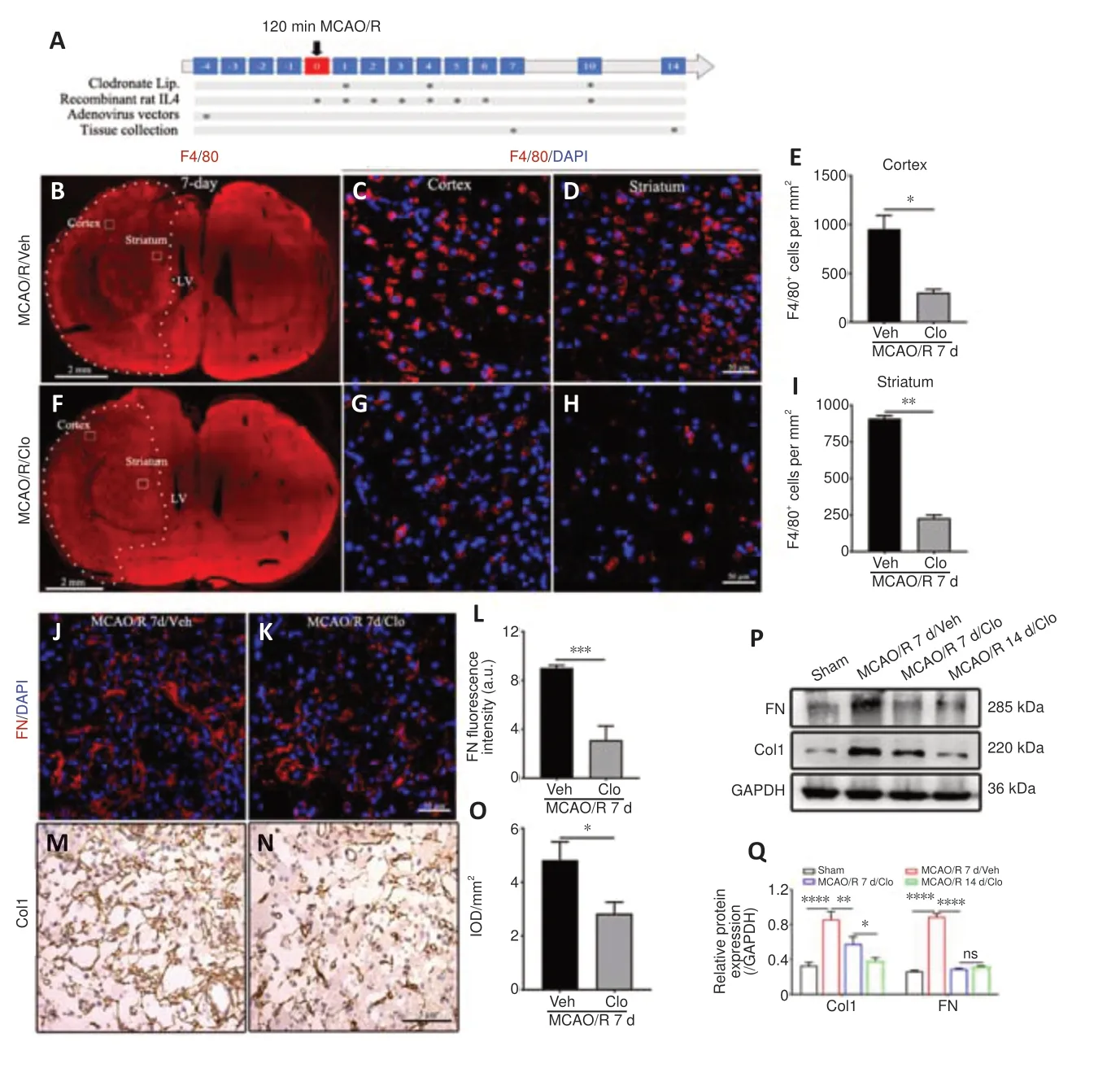
Figure 3|Macrophage depletion prevents fibrotic scar formation in the lesion core after MCAO/R in rats.
IL4-induced M2 macrophages participate in fibrogenesis after MCAO/R
Macrophages have two main polarization statuses, including classicallyactivated type 1 (M1) and alternatively-activated type 2 (M2) (Liu et al., 2016).M1 macrophage polarization can be triggered by IFNγ or lipopolysaccharides,and activated M1 macrophages secrete pro-inflammatory cytokines such as tumor necrosis factor-α, IL1, and IL6.IL4/10/13, Toll-like receptors, and immune complexes can induce M2 macrophage polarization.Given that circulating monocyte-derived macrophages appeared to mediate fibrotic scar formation in the ischemic core after MCAO/R in rats, next we used IL4 to induce M2 macrophage polarization and investigate whether M2 macrophages play a role in fibrotic scar formation after MCAO/R.
First, we determined the concentrations of IL4/6/10 and IFNγ in the ischemic core post-MCAO/R.We found that IL4 expression increased within 3 days following cerebral ischemia, remained steady for at least 14 days, and then decreased dramatically at 21 days (Figure 4A), while IL10 expression increased later, at 14 days post-MCAO/R (Figure 4B).Expression of the pro-inflammatory cytokines IFNγ and IL6 increased shortly and slightly after MCAO/R (Figure 4C and D).In addition, greater numbers of F4/80-positive and CD206-positive(an M2 macrophage marker) cells were found in the ischemic core in the IL4-treated rats compared with the PBS-treated rats (Figure 4E–K) at 7 days after MCAO/R.Furthermore, stimulating RAW264.7 cells (Figure 4L and M) or BMDMs (Figure 4N and O) with recombinant rat IL4 for 2 daysin vitroresulted in a clear increase in the number of CD206-positive cells.
Fibroblasts are vital to ECM remodeling and deposition.Macrophages form a stable association with fibroblasts and exert reciprocal control of cell survival and proliferation (Zhou et al., 2018).To investigate whether fibroblasts are directly related to macrophages, we analyzed how the MCM or RAW264.7-conditioned medium (RCM) affected cultivated fibroblasts (Figure 5A).According to the EdU cell proliferation assay, immunofluorescence staining,and western blot assay results, MCM/DMEM increased the number of EdUpositive proliferating fibroblasts (Figure 5B–E), α-smooth muscle actinpositive myofibroblasts (Figure 5F–I), and Col1-positive fibroblasts (Figure 5J–M), as well as the FN fluorescence intensity of fibroblasts (Figure 5N–W) and the production of Col1 and FN (Figure 5X and Y) compared with the control group, and MCM/IL4 further enhanced these effects.Moreover,immunofluorescence staining showed that RCM/IL4 increased Col1 and FN production to a greater extent than RCM/DMEM (Figure 6).
Next, we investigated the effects of IL4 on fibrogenesis and macrophage M2 polarization after MCAO/Rin vivo.Based on western blot assay and immunofluorescence or immunohistochemistry staining, both Col1 and FN deposition and the number of CD206-positive macrophages were clearly elevated in the IL4-treated groups than in the PBS-treated groups at 7 days after MCAO/R (Figure 7A–H).Pearson’s correlation analysis demonstrated a robust positive correlation between the number of CD206-positive macrophages and FN fluorescence intensity (Figure 7I).Furthermore, western blotting showed that Col1 and FN protein levels in the ischemic core were higher in the IL4-treated groups at 14 days after MCAO/R than at 7 days after MCAO/R (Figure 7J–O).These results suggest that IL4-induced M2 macrophages participate in fibrogenesis after MCAO/R.
IL4-induced M2 macrophages promote fibrogenesis by secreting Shh
Shh signaling plays a vital role in the pathogenesis of lung, kidney, skin, and liver fibrosis (Feng et al., 2019; Hou et al., 2021).Given that M2 macrophages appeared to mediate fibrosis within the ischemic core after MCAO/R, next we investigated whether IL4-induced M2 macrophages mediate fibrosis after MCAO/R by secreting Shh.
First, we investigated whether the Shh signaling pathway was activated during IL4-induced M2 macrophage polarization in BMDMs and RAW264.7 cellsin vitroand in thein vivoMCAO/R model.Shh, Smo, and Gli1 are vital constituents of the Shh pathway.When the Shh pathway is activated, Gli1,Shh, and Smo levels are increased (Huang et al., 2014).
In BMDM cells, IL4 treatment enhanced Smo and Gli1 expression, and treatment with the Smo receptor inhibitor Cyc inhibited Smo and Gli1 expression (Figure 8A).However, treatment of RAW264.7 cells with Shh alone had little effect on Gli1 and Smo expression levels.Combined treatment with IL4 and Shh markedly elevated Gli1 and Smo expression levels compared with treatment with Shh alone or IL4 alone, and combined treatment with IL4,Shh, and Cyc markedly reduced Gli1 and Smo expression levels (Figure 8B).Additionally, knockdown of Shh in Raw264.7 cells abrogated the effects of IL4 on Gli1, Shh, and Smo expression levels (Figure 8C).
Following cerebral ischemia, clear immunoreactivity for Shh could be seen in F4/80-positive macrophages at 7 days, and the Shh fluorescence signal gradually decreased until day 14 (Figure 8D).Shh expression in the ischemic core was markedly increased as early as day 3, peaked on day 7, and then declined, as determined by western blot assay (Figure 8E).Further, Smo and Gli1 expression levels in the ischemic core were notably increased at 7 days after ischemia in rats treated with recombinant rat IL4 by intracerebroventricular injection compared with the Sham and Veh groups;and this effect was abrogated by combined treatment with IL4 and Cyc (Figure 8F).Shh and Smo expression levels in the ischemic core following cerebral ischemia were notably reduced in rats treated with Clo for 7 days compared with the Veh group, and were further lowered by treatment with Clo for 14 days (Figure 8G).These results suggest that IL4-induced M2 macrophages maybe secrete Shh and activate the Shh pathwayin vivoandin vitro.Next, we explored whether the Shh signaling pathway affected the production of FN and Col1 in fibroblasts treated with MCMin vitroand in the MCAO/R modelin vivo.Western blot analysis demonstrated that FN and Col1 expression in fibroblasts were higher in the MCM/IL4 group than in the MCM/DMEM, Con, or DMEM/IL4/Cyc groups, and that their expression was abolished by MCM/IL4/Cyc (Figure 9A).In vivo, Shh knockdown decreased FN and Col1 expression levels in the ischemic core at 7 days after MCAO/R (Figure 9B and C) and abrogated the effects of IL4 on FN and Col1 expression (Figure 9C).Furthermore, Shh overexpression had the opposite effect on FN and Col1 expression (Figure 9D and E).These results suggest that Shh signaling regulates FN and Col1 expressionin vitroandin vivo.
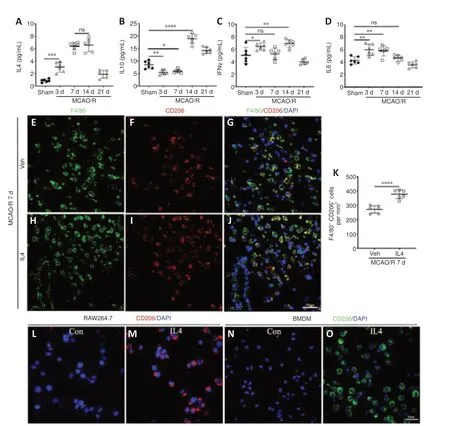
Figure 4|Macrophages undergo M2 polarization in the ischemic core and in vitro after IL4 treatment.

Figure 5|MCM promotes fibroblast proliferation and activation.
Taken together, these findings indicate that IL4-induced M2 macrophages mediate fibrosis after MCAO/R by secreting Shh.
IL4-mediated Shh signaling regulates TGFβ1 and matrix metalloproteinase 9 production in M2 macrophages in vitroand in the MCAO/R modelin vivo
Tissue fibrosis is regulated by a variety of signals and related proteins.As a primary regulator of liver, kidney, lung, skin, and heart fibrosis, TGFβ1 expression is immediately increased following stroke or other central nervous system injuries, and regulates glial and fibrotic scar formation (Frangogiannis,2020).Matrix metalloproteinase 9 (MMP9) is also extensively involved in the pathogenesis of fibrosis (Li et al., 2019).We next asked whether the Shh signaling modulates TGFβ1 and MMP9 expression in M2 macrophagesin vitroand in the MCAO/R modelin vivo.
Stimulating BMDMs with recombinant rat IL4 for 2 days markedly increased MMP9 and TGFβ1 expression levels compared with the control group (Figure 10A), and MMP9 and TGFβ1 expression in RAW264.7 cells was clearly inhibited by treatment with the Smo receptor inhibitor Cyc or both IL4 and Cyc (Figure 10B).
TGFβ1 and MMP9 expression in rats in the ischemic core increased as early as day 3 after MCAO/R, peaked on day 7, and then declined; furthermore,the expression of both proteins was notably reduced by Shh knockdown and enhanced by Shh overexpression on day 7 (Figure 10C–F).Moreover, Shh knockdown and overexpression respectively abrogated and enhanced the ability of IL4 to upregulate of TGFβ1 and MMP9 expression (Figure 10E and F).These results suggest that IL4-mediated Shh signaling regulates TGFβ1 and MMP9 expression in M2 macrophagesin vitroand in the MCAO/R modelin vivo.
Fibrotic scar formation is positively associated with the angiogenesis and neuroprotection induced by IL4-mediated Shh signaling in the early stages of recovery from MCAO/R
Excessive fibrosis hinders functional tissue reconstruction, but completely inhibiting fibrotic scar formation leads to cavity formation, which is also not compatible with regeneration (Gӧritz et al., 2011).Therefore, we next asked whether fibrotic scar formation affects angiogenesis and decreases injury in the early stage after MCAO/R in rats.
Immunofluorescence staining showed that IL4 treatment increased the expression of CD31, an angiogenesis-related marker, in the ischemic core compared with the Control and Sham groups at 7 days after MCAO/R, and that this effect was blunted by treatment with Cyc or with both IL4 and Cyc.Moreover, the increase in CD31 expression was minimal in rats treated with Cyc alone (Figure 11A).Further, immunofluorescence double labeling demonstrated that FN fluorescence intensity was positively correlated with microvessel density (Figure 11B).In addition, TUNEL staining (Figure 12A–Q), TTC staining (Figure 12R and S), and modified neurological severity score(Figure 12T) indicated that IL4 treatment clearly decreased apoptosis and infarct volume and alleviated the neurological deficits in comparison with the control group, while these effects were abolished by treatment with Cyc or combined treatment with IL4 and Cyc, and were minimal in rats treated with Cyc alone.These results show that IL4-mediated Shh signaling promotes angiogenesis and decreases injury in the early stage of recovery after MCAO/R and may be associated with fibrotic scar formation.
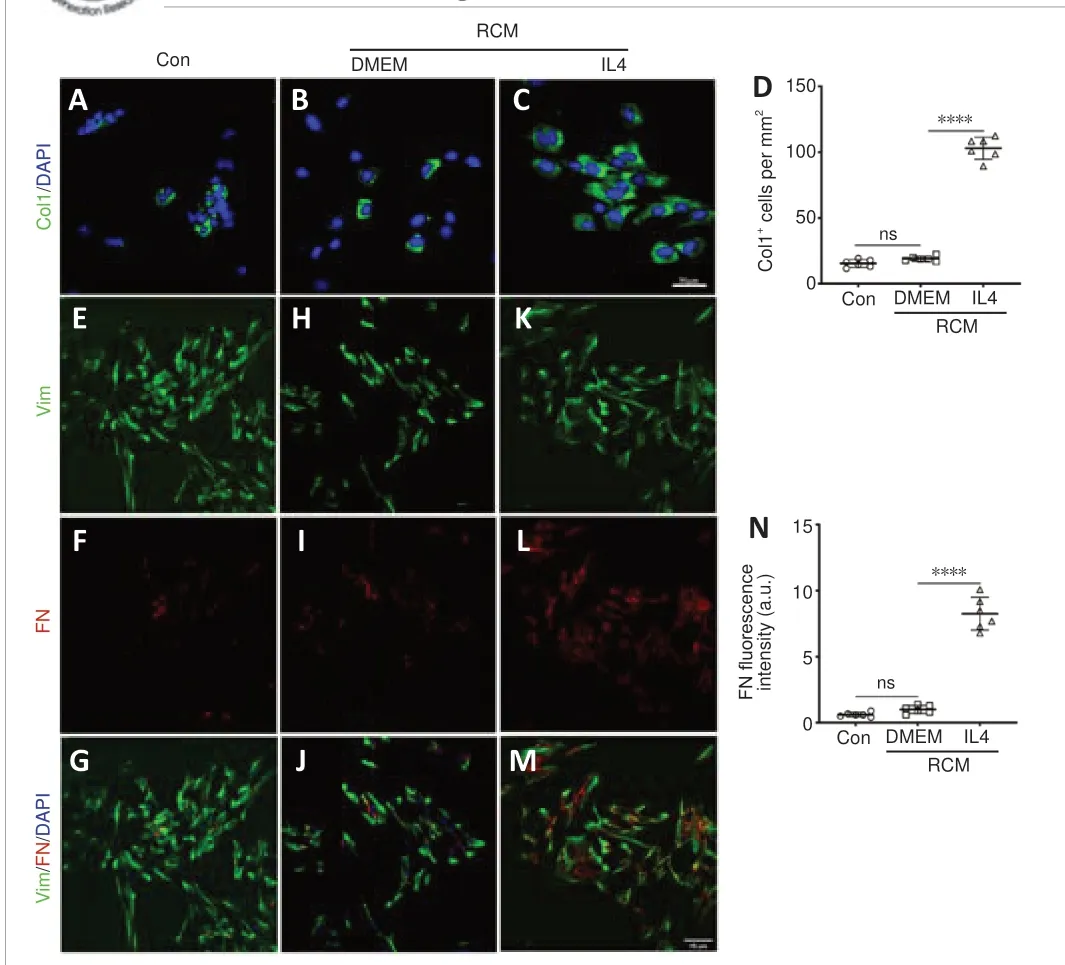
Figure 6|RCM induces extracellular matrix production by fibroblasts.
Discussion
In the present study we found that cerebral ischemia resulted in persistent fibrosis, massive macrophage infiltration, and upregulation of IL4, Shh, Smo,Gli1, TGFβ1, and MMP9 in the ischemic core in rats.Depletion of circulating monocyte-derived macrophages attenuated fibrotic scar formation.IL4-induced M2 macrophage polarization promoted fibrotic scar formation in the ischemic core in rats.Using the Smo receptor inhibitor Cyc, or Shh knockdown or overexpression via adenovirus infection, we showed that Shh expressed by IL4-induced M2 macrophages regulates fibrogenesisin vitroandin vivo,promotes secretion of the fibrosis-associated regulatory proteins TGFβ1 and MMP9, enhances angiogenesis, inhibits cell apoptosis, and reduces infarct volume in the early stages of recovery after MCAO/R.
Monocyte-derived macrophages and resident microglia are the most abundant innate immune cells in the brain after stroke, but there are clear differences in their distribution and cell morphology.The former exhibits a uniform round shape and are confined to the ischemic core, while the latter display a ramified morphology with many processes and are mainly distributed in the perilesional area (Rajan et al., 2019; Shibahara et al., 2020).F4/80 is a relatively specific marker for monocyte-derived macrophages (Chen et al., 2017; Shibahara et al., 2020).Here we found that a large numbers of blood monocyte-derived macrophages were present in the cerebral ischemic core following cerebral ischemia, which extends previous findings.Based on previously published studies (Ueshima et al., 2019; Hou et al., 2021; Li et al.,2021a), we continued to investigate the effect of M2 macrophages on fibrotic scar formation in MCAO/R rats.IL4 is a robust promoter of M2 polarization in macrophages.In ischemic and hemorrhagic brain injury models, IL4 induces macrophage polarization to the M2 phenotype, thereby improving functional recovery (Liu et al., 2016; Li et al., 2020; Xu et al., 2020).Here we found that IL4-induced M2 macrophage polarization regulates fibrotic scar formation in the ischemic core in rats.Our findings, taken together with findings from previous studies, indicate that IL4-induced M2 macrophages mediate fibrosis in a range of injured tissues.
Fibroblast-macrophage crosstalk is related to homeostasis maintenance in healthy tissues and during fibrosis (Zhou et al., 2018; Li et al., 2021b).Macrophages have been suggested to be a source of ligands that promote fibrotic activation within fibroblasts.Typically, TGFβ1 has been considered a master regulator of fibrosis (Han et al., 2019; Buechler et al., 2021).Macrophage-specificTgfb1deletion markedly reduces the total fibrosis score and lung collagen level in the context of bleomycin-mediated fibrosis(Young et al., 2016).In line with this, we observed dynamic changes in TGFβ1 expression in the ischemic core after MCAO/R, implying that it may directly stimulate fibroblasts.Macrophages have a critical effect on ECM remodeling through promoting ECM degradation (Franklin, 2021).As an example, macrophages promote MMP9 production upon stimulation with inflammatory factors (Murray and Wynn, 2011).We also found that IL4-induced macrophages expressed MMP9 in the ischemic core after MCAO/Rin vivoas well asin vitro.These results show that IL4-induced M2 macrophages secrete TGFβ1 and MMP9, which regulate fibrogenesis through interaction with fibroblasts in the ischemic core after MCAO/R.
The Shh pathway can be activated during chronic or acute injury to the central nervous system, such as stroke (Yu et al., 2021).SHHis expressed in human macrophages as well as monocytes (Schumacher et al., 2012; Effendi and Nagano, 2021).Schistosome antigens stimulate the production of biologically active Shh ligands via macrophages (Pereira et al., 2013).Here we found that IL4 treatment activated Shh signaling by macrophagesin vivoandin vitro,which is consistent with previous findings (Hou et al., 2021).Activation of fibroblasts and differentiation of myofibroblasts represent two critical fibrosisrelated pathogenic mechanisms.The Shh pathway has been previously suggested to mediate the differentiation of myofibroblasts in the presence(Sun et al., 2021) or absence (Cigna et al., 2012) of TGFβ1.The current study confirmed TGFβ1 expression by macrophages in response to IL4 treatment.Additionally, Shh knockdown prevented fibrogenesis in the ischemic core after MCAO/R.Thus, it is likely that the IL4/Shh/TGFβ1 axis in macrophages indirectly regulates fibroblast activation in the context of fibrosis via paracrine signaling.
Eliminating proliferating fibroblasts can reduce scar formation and decrease motor disability (Dorrier et al., 2021).On the contrary, central nervous system scarring is thought to protect tissues (Anderson et al., 2016).In fact, the functional consequences of fibrotic scar are often context-dependent (Dias and Gӧritz, 2018).Overall, the function of fibrotic scar in ischemic stroke remains largely unclear.We observed that a large numbers of cell types,not only macrophages, accumulated in the center of the fibrotic scar.Thus,the main role of secreted ECM proteins may be the creation of a scaffold structure in the areas that are extensively damaged by MCAO/R that is crucial for brain injury repair because of the very low regenerative capacity of brain tissue.Chemokine C-C motif receptor 2–positive monocytes promote deadly cerebral edema in the acute stage post-cerebrovascular injury, but they are also needed at later stages to promote angiogenesis (Mastorakos et al., 2021).Similarly, in our study we observed extensive neovascularization within the ischemic core at an early stage after MCAO/R in rats treated with IL4, which is consistent with fibrosis.Angiogenesis plays an important role in repairing damaged brain tissues.When MCAO is induced inPdgfrβ+/–mice, the formation of fibrotic scars is decreased, while the total infarct size is increased, compared with wild-type mice subjected to the same injury(Makihara et al., 2015).Clearly, IL4 treatment reduces infarct volume and the number of apoptotic cells within the ischemic core, and alleviates the neurological deficits in the early stage of recovery from MCAO/R in rats.
To have clinical translational value, there are a few aspects of our results that require further investigation.First, we did not evaluate the effects of IL4-induced M2 macrophages in elderly subjects, subjects with systemic comorbidities, or female animals.Second, addressing the therapeutic window of IL4 may also be of value.Third, the mechanism by which the Shh pathway regulates fibrosis-associated regulatory protein production remains to be further elucidated.
The present study is the first to suggest that the IL4-induced M2 macrophages have a critical effect on fibrotic scar formation and functional restoration in the early stage after MCAO/R in rats through the Shh/TGFβ1 axis.Therefore,regulating M2 macrophage polarization to moderate the formation of the fibrotic scar via targeting the Shh/TGFβ1 axis is potentially a promising therapeutic target for cerebral ischemia.
Author contributions:Study conceptualization: JGH, QY; methodology: JGH,LZ, JW, XSS, YLW, PRJ, JNW, QY; validation and writing—original draft: JGH,JXR, YC, MFT, QHY; data curation: JXR, YC, MFT, QHY; visualization: LZ, JW, XSS,YLW; writing—review and editing: JGH; supervision: PRJ, JNW, QY; funding acquisition: QY.All authors have read and approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no competing interests.
Author statement:This paper has been posted as a preprint on Research Square with doi: https://doi.org/10.21203/rs.3.rs-1589056/v1, which is available from: https://assets.researchsquare.com/files/rs-1589056/v1/9f6c5c5c-f484-4dcf-bc0a-da32e9e390b8.pdf?c=1654772372.
Data availability statement:All relevant data are within the paper and its Additional files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
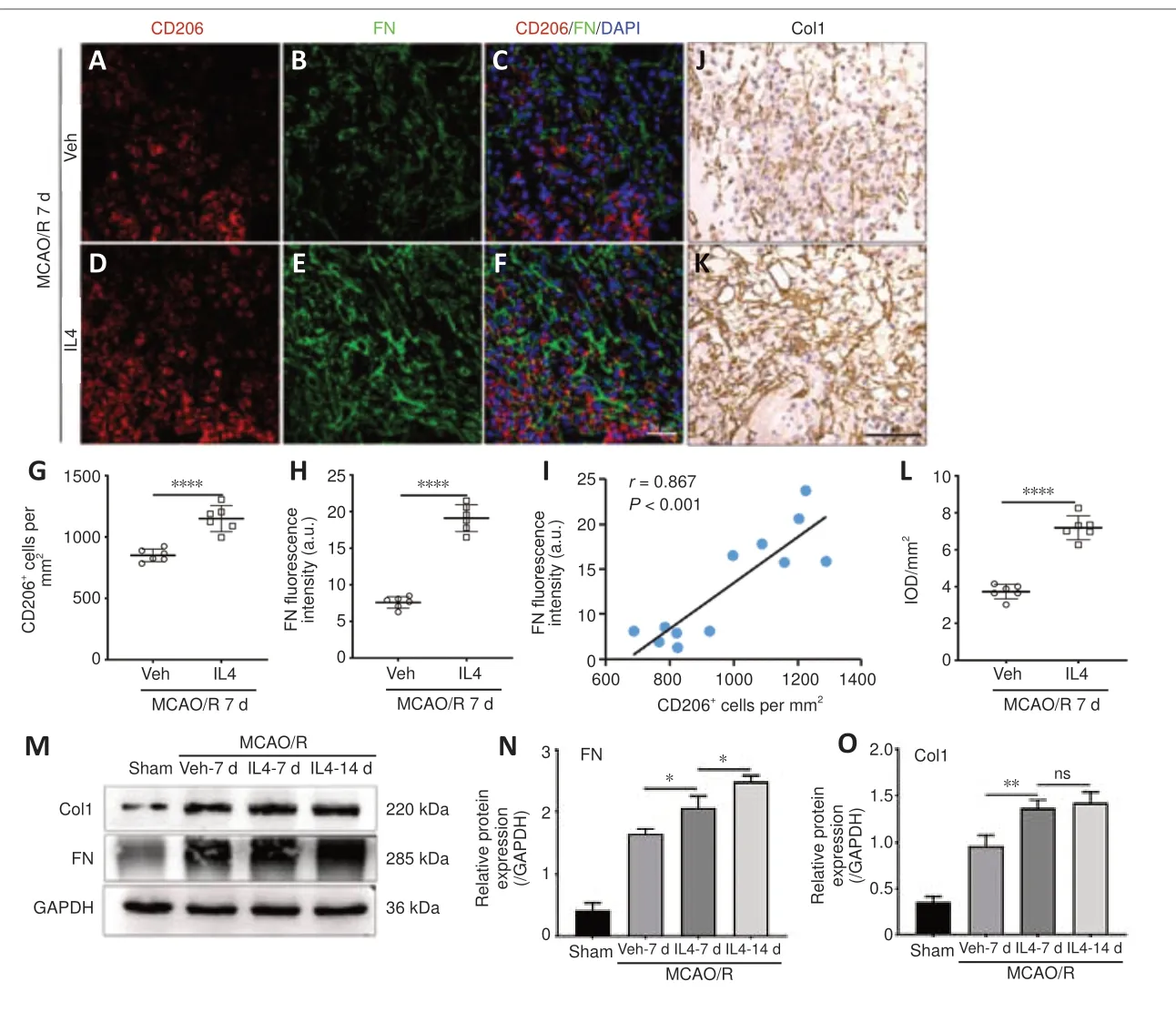
Figure 7|IL4 treatment induces macrophages to undergo M2 polarization and produce extracellular matrix in the ischemic core.
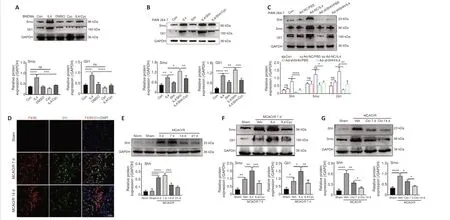
Figure 8|IL4-induced M2 macrophages secrete Shh and activate Shh signaling in the ischemic core and in vitro.
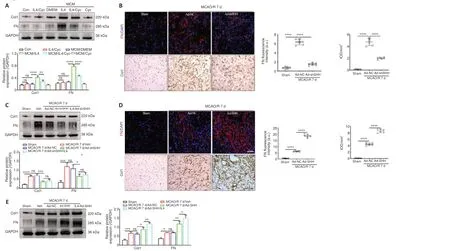
Figure 9|Shh signaling regulates extracellular matrix protein production.

Figure 10|IL4-mediated Shh signaling regulates TGFβ1 and MMP9 expression.
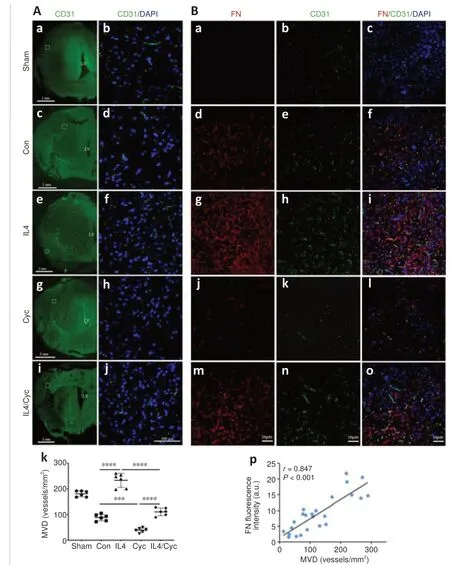
Figure 11|Fibrotic scar formation is positively correlated with neovascularization induced by IL4-mediated Shh signaling.

Figure 12|IL4-mediated Shh signaling decreases infarct volume, apoptosis, and mNSS score after MCAO/R in rats.
Additional files:
Additional file 1:Experimental group design.
Additional Figure 1:Adenovirus delivery efficiency of Ad-NC, Ad-shSHH, or Ad-SHH (1×1010PFU/mL) injected into the right lateral ventricle.
Additional Figure 2:RAW264.7 cell adenovirus infection.
Additional Figure 3:Uncropped images of blots.
Additional Table 1:Summary of experimental groups and mortality rate in the study.
Additional Table 2:Primary antibody used in this study.
Additional Table 3:Statistics reporting.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain

