Multi-faceted small molecule for Alzheimer’s disease
Min Hee Park, Hee Kyung Jin, Jae-sung Bae
The main pathologies of Alzheimer’s disease (AD)are the accumulation of amyloid-β (Aβ) and tau tangles, and these two fundamental pathologies accompany the various neuropathological features, such as neuroinflammation, loss of synapse and neurogenesis, disruption of the blood-brain barrier (BBB), autophagy dysfunction,and cognitive impairment (Lee et al., 2014;Sweeney et al., 2018; Kashyap et al., 2019; Park et al., 2022).AD is thus considered a multifactorial disease in which each of these complex neuropathological features somehow interrelates,eventually leading to neuronal and functional loss in the nervous system.Although researchers have focused on Aβ and tau tangles for the development of therapeutics for AD, the clinical efficacy of drugs targeting these major pathologies remains controversial.Moreover, other currently available drugs that are not effective in treating AD solely provide symptomatic relief to patients with AD.For this reason, researchers are on the onlook for new strategies to treat AD and emphasize the importance of multi-target directed drugs.
Sphingolipids are known to play various cellular and tissue homeostatic roles, and their alterations are increasingly being recognized as contributors to the pathological processes in neurodegenerative diseases, including AD (Kornhuber et al., 2015;Hannun et al., 2018).Acid sphingomyelinase (ASM)is one of the significant sphingolipid-metabolizing enzymes which normally catalyze the conversion of sphingomyelin to ceramide.ASM thereon works alongside other sphingolipid metabolites for proper cellular function (Kornhuber et al., 2015).However, increased activity of this enzyme is found in the blood and/or brain during neurodegenerative diseases, which contributes to a range of pathological features, including inflammation, apoptosis, neurogenesis loss,defective autophagy, and leakage of the BBB (Lee et al., 2014; Kornhuber et al., 2015; Park et al.,2018, 2020).Based on its pathological roles, ASM has been considered a potential and promising therapeutic target for treating neurodegenerative diseases.
ASM inhibition has been resulted with excellent therapeutic effects in AD and the aged brain.In the AD brain, neurons were the main contributor cells of ASM activity elevation.Increased activity of neuronal ASM inhibited autophagic protein degradation by reducing the function of the autophagic-lysosome pathway and lysosome biogenesis, leading to enhanced Aβ deposits (Lee et al., 2014).In contrast, in the aged brain, the ASM increase was mainly derived from endothelial cells, constituting the BBB.Elevated ASM activity in endothelial cells caused an increase in apoptotic endothelial cells and BBB permeability, leading to the reduction of neuronal cells and severe memory impairment in aged mice (Park et al., 2018).The genetic or pharmacological inhibition of ASM in AD mouse models or aged mice improved Aβ accumulation, BBB disruption, neuronal cell death,and memory impairment (Lee et al., 2014; Park et al., 2018).Therefore, these reports supported the potential of ASM inhibitors for the therapy of AD along with other neurodegenerative diseases in which ASM activity is increased.
Based on these previous findings and with the aim of developing therapeutic agents for AD,our research group sought compounds that could inhibit ASM activity.We found five small compounds, called the KARI series compounds,of < 500 Da of molecular weight using cell-based ASM activity assays and further optimization.These compounds could directly inhibit ASM activity without an effect on other sphingolipid metabolites and enzymes.Moreover, one of these compounds, KARI 201, showed the best pharmacokinetic properties with regard to high systemic bioavailability, stability, brain distribution,and absence of drug-drug interaction effects.Furthermore, KARI 201 had no substantial offtarget effects on 430 kinases and 38 additional enzymes.The mode of action of KARI 201 on ASM inhibition was revealed by molecular docking calculations and competitive inhibition assay.KARI 201 competitively bound to the active site domain of ASM, which is the sphingomyelin binding site,indicating the drug to be a competitive inhibitor of ASM (Park et al., 2022).To date, most ASM inhibitors were identified as functional inhibitors,which primarily had an antidepressant action and were cationic amphiphilic compounds that indirectly inhibited ASM activity.The functional mechanism of a representative functional ASM inhibitor, amitriptyline (AMI), is the alteration of the electrostatic properties of the ASM-anchored inner lysosomal membrane, which leads to the detachment of ASM from the membrane.As a result, ASM freed in the lysosome gets degraded and its expression reduces; owing to this indirect ASM inhibitory effect, functional ASM inhibitors lack specificity and have the potential for offtarget effects.In addition, although a few ASM direct inhibitors have been discovered, the exact mode of action and druggability remains unclear.Therefore, we believe that KARI 201 is possibly a first-in-class direct, selective and competitive ASM inhibitor with excellent brain distribution and druggability.
The therapeutic effects of KARI 201 were evaluated in an AD mouse model and compared with that of AMI.Orally treated KARI 201 reduced ASM activity and brought it to the normal range in the plasma, cortex and hippocampus.This ASM inhibitory effect of KARI 201 was considerably greater than that of AMI, despite its low dose and shorter dosing period.Moreover, KARI 201 reduced Aβ accumulation and improved learning and memory in the AD mouse model compared with AMI, demonstrating the excellent therapeutic effects of direct inhibition of ASM activity by KARI 201.One of the therapeutic mechanisms of KARI 201 was revealed to be the improvement of autophagy dysfunction by ASM inhibition in the neurons of the AD mouse model and patients with AD.The restoration of neuronal function by the autophagic amelioration by KARI 201 led to the promotion of the Aβ phagocytic function of microglia, and the further decrease of Aβ accumulation and mitigation of neuroinflammation and synapse loss (Figure 1).The ASM activity inhibition by KARI 201 resulted in a reduction of ceramide which is involved in amyloidopathy.This observation indicated that the effects on the decrease of Aβ deposits by KARI 201 could be combined effects of ASM activity inhibition and ceramide reduction.The other therapeutic mechanism of KARI 201 was its role as a ghrelin receptor (GHSR1α) agonist.This unexpected role of KARI 201 was found through a G-protein coupled receptor screening based on RNA-seq analysis in the brain of the KARI 201-treated AD mouse model.KARI 201, as a ghrelin receptor agonist, induced Ca2+influx and activated AMPactivated protein kinase which is one of the downstream targets of ghrelin/GHSR1α signaling.Since the expression of the ghrelin receptor is more abundant in the hippocampus than in other brain regions, the effects of KARI 201 as a ghrelin receptor agonist are more dominant in the hippocampus.Moreover, AMP-activated protein kinase activation in the hippocampus is involved in the improvement of hippocampal neurogenesis and synapse plasticity by increasing transcription of pro-neurogenesis and neurotrophic factors such asBdnf(brain-derived neurotrophic factor),Map2(microtubule-associated protein 2), andTuj1(neuron-specific class III beta-tubulin).As results, KARI 201 promoted hippocampal neurogenesis and synaptic plasticity in Aβ-treated human and mouse hippocampal neurons and AD mouse model, contributing to the improvement of learning and memory impairment in mice (Park et al., 2022; Figure 1).These results are supported by several previous studies that demonstrated the therapeutic significance of hippocampal ghrelin/GHSR1α signaling in learning and memory in AD (Jeon et al., 2019; Reich et al., 2020).Taken together, these findings suggest that KARI 201,as a direct ASM inhibitor and GHSR1α agonist,has outstanding therapeutic effects on various neuropathological features, including autophagy dysfunction, neuroinflammation, synaptic loss, Aβ deposition, decreased hippocampal neurogenesis,synaptic plasticity, and learning and memory impairment, in AD mouse models.
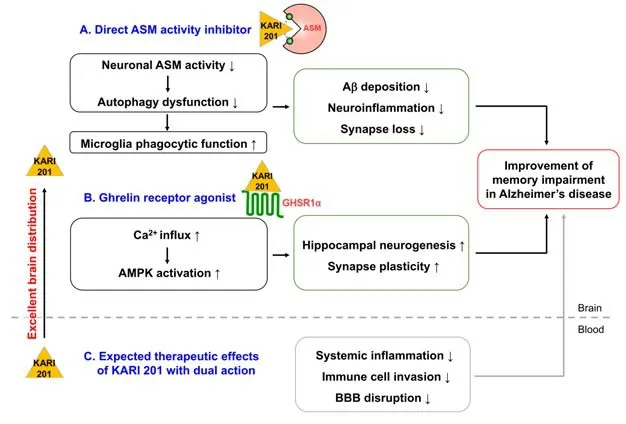
Figure 1|Therapeutic effects of KARI 201 on neuropathological features in AD.
Recently, systemic pathologies such as peripheral inflammation are being considered as important pathological features in AD.For instance,circulating pathogenic factors stimulate a peripheral pro-inflammatory response by immune cells, and it further causes BBB disruption and induces the efficient entry of blood-derived immune cells into the brain.The blood-derived immune cells infiltrating into the brain contribute to neuroinflammation and Aβ accumulation(Tahmasebinia et al., 2017).Thus, specific circulating factors may be a promising therapeutic target for the treatment of AD.In this regard,increased ASM activity in the blood of patients and mice with AD can negatively regulate peripheral immune cell functions (Bai et al., 2017).For example, ASM could induce the differentiation of T helper 17 (Th17) cells from CD4+T cells,promoting inflammation.Recently, an increase of Th17 cells has been observed in the blood of AD patients compared to control subjects, and there was a positive association found between the level of Th17 cells and amyloidopathy/inflammation.These observations are related to results showing an increase in ASM activity in the blood of AD patients.In the contrast, the activation of the ghrelin receptor in immune cells has a positive effect on the suppression of systemic immune responses (Jeon et al., 2019; Reich et al., 2020).Although the investigation of further therapeutic effects by KARI 201 in AD is needed, we speculate that the dual action of this small molecule may improve systemic pathological features (Figure 1).Collectively, we believe that KARI 201 might be an innovative multi-faceted agent for the treatment of AD owing to its excellent pharmacological properties and therapeutic effects.
As per the recently updated AD drug development pipeline, the most common agents on clinical trials currently are disease-modifying therapies,which are used for treatments intended to change the biology of AD by intervening in the processes leading to cell death.Among them,66.4% are small molecules taken orally and are< 500 Da in molecular weight.Their primary mechanistic targets are amyloid or tau proteins,neuroinflammation, synaptic plasticity, or neuroprotection (Cummings et al., 2022).From our perspective, KARI 201 has the potential as a new promising disease-modifying therapy that targets various neuropathological features of AD.Therefore, our future research will be focused on the preclinical and clinical studies of this promising multi-faceted small molecule.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by theKorea government (MSIT) 2018M3C7A1056513,2020R1A2C3006734 (to HKJ), 2020R1A2C3006875(to JSB).This research was also supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare and MSIT, Republic of Korea(HU20C0345) (to JSB).
Min Hee Park, Hee Kyung Jin*,Jae-sung Bae*KNU Alzheimer’s Disease Research Institute,Department of Physiology, School of Medicine,Kyungpook National University, Daegu, South Korea (Park MH, Bae JS)KNU Alzheimer’s Disease Research Institute,Department of Laboratory Animal Medicine,College of Veterinary Medicine, Kyungpook National University, Daegu, South Korea (Jin HK)
*Correspondence to:Jae-sung Bae, DVM, PhD,jsbae@knu.ac.kr; Hee Kyung Jin, DVM, PhD,hkjin@knu.ac.kr.https://orcid.org/0000-0002-8270-0072(Jae-sung Bae)https://orcid.org/0000-0001-6403-008X(Hee Kyung Jin)
Date of submission:November 9, 2022
Date of decision:December 20, 2022
Date of acceptance:January 11, 2023
Date of web publication:March 3, 2023
https://doi.org/10.4103/1673-5374.369110
How to cite this article:Park MH, Jin HK, Bae JS(2023) Multi-faceted small molecule for Alzheimer’s disease.Neural Regen Res 18(10):2198-2199.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and buildupon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Boopathi Subramanian,Universidad Nacional Autonoma de Mexico Instituto de ciencia physica, Mexico.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain

