Metabotropic glutamate receptor 1 alpha: a unique receptor variant with variable implications for Alzheimer’s disease pathogenesis
Jason HY Yeung, Andrea Kwakowsky
Alzheimer’s disease (AD) is the leading neurodegenerative disorder globally.Despite this,there is minimal effective therapeutics proven to reduce or prevent the progression of this disease.Glutamate is the main excitatory neurotransmitter within the central nervous system and plays a crucial role in neuronal and synaptic functions.As such, the glutamatergic system is finely regulated within normal physiology, with multiple mechanisms to prevent excessive or insufficient glutamatergic receptor activation.This perspective article aims to highlight pertinent findings regarding metabotropic glutamate receptor(mGluR) expression and function in the AD brain,with a particular focus on the mGluR1α variant and its functional significance, concluding with a discussion regarding its potential as a therapeutic target in future AD studies.
Glutamatergic receptors are categorized as ionotropic or metabotropic.Compared to ionotropic receptors, metabotropic receptors mediate slower responses through functional coupling with intracellular second messengers with further downstream cellular alterations(Srivastava et al., 2020).As such, these receptors have the potential to cause longstanding neuronal changes.mGluRs are categorized into three types based on their affinity to specific agonists, with Group I (mGluR1/5) receptors associated with ionotropic receptor activation and upregulation through coupling with excitatory inositol phosphate 3/Ca2+signal transduction (Yeung et al., 2022).Group I mGluRs demonstrate specific expression patterns within the brain, with mGluR1 mainly being expressed in the hippocampus,cerebellum, and substantia nigra (Su et al., 2022).mGluR1/5 have been shown to play a pivotal role in synaptic plasticity through modulation of N-methyl-D-aspartate receptor activity, as well as independently triggering long-term potentiation and depression, cellular processes involved in learning and memory (Srivastava et al., 2020).A few studies have examined the role and therapeutic potential of mGluR1 and 5 in AD,although these studies focused on the examination of mGluR1 expression and function without considering variant-specific changes (Albasanz et al., 2005; Srivastava et al., 2020; Su et al., 2022).mGluR1 variants are derived from alternative splicing of the mGluR1 gene (Grm1), generating four variants with different intracellular-terminal domains (mGluRα, β, γ, and δ) (Naito et al.,2018).mGluR1α is the long variant of mGluR1 and has been shown to have distinct functionality compared to other Group I mGluRs, including regulation of synaptic plasticity and generation of long-term potentiation in certain hippocampal inhibitory neurons (Perez et al., 2001).It has also been demonstrated to be modulated by calcium(Ca2+) fluxes, which suggests possible dysfunction in the context of glutamate excitotoxicity and excessive Ca2+accumulation (Jiang et al., 2014).Even though there is clear differentiation in the function of the mGluR1α variant, there is minimal research into its involvement in disease states.
Dysfunction of the glutamatergic system has previously been implicated in AD, although whether it is a causative or additive factor is currently unknown – likely a mixture of both.Glutamatergic dysfunction in AD appears to be mediated through a variety of mechanisms,including beta-amyloid (Aβ) binding to glutamate receptors, tau aggregates tethering to intrinsic cytoskeletal proteins resulting in overactivation of receptors, and the internalization of glutamate transporters resulting in glutamate accumulation in synaptic and extrasynaptic areas (Wang and Reddy, 2017).Despite clear evidence of the glutamatergic system’s role in neurodegeneration,the pathogenic mechanisms leading to expression alterations of glutamatergic components are yet to be elucidated in AD.In particular, dysfunction in Group I mGluRs appears to be associated with AD pathology, although its role in the prevention or promotion of the progression of AD remains confusingly unclear.mGluR1 dysfunction in AD has been shown to manifest through the obstructed transmission of long-term depression (Figure 1),and this is believed to contribute to early memory and learning deficits in AD patients (Srivastava et al., 2020).mGluR5 has previously been demonstrated to be over-stabilized on exposure to Aβ oligomers, with Aβ acting as a coreceptor and facilitating disruption of normal neuronal signaling and function (Su et al., 2022).Alternatively, Aβ has been shown to occlude mGluR-dependent longterm depression, resulting in reduced functional synaptic activity.Paradoxically, studies have also shown neuroprotective effects of Group I mGluRs, primarily through stimulation of nonamyloidogenic processing of amyloid precursor protein, which will be discussed later (Su et al.,2022).
Recently we reported mGluR1α expression changes within the AD hippocampus compared to controls(Yeung et al., 2022).We found that mGluR1α expression was significantly reduced in specific areas of the hippocampus in AD (Figure 1), in particular within the CA1 region which is critically involved in memory formation, consolidation, and retrieval.Reduced mGluR1α expression was also demonstrated within the subiculum and entorhinal cortex.This can potentially have implications on glutamatergic stability within the hippocampal neuronal network, resulting in excitotoxicity,neuronal dysregulation, and dysfunction.
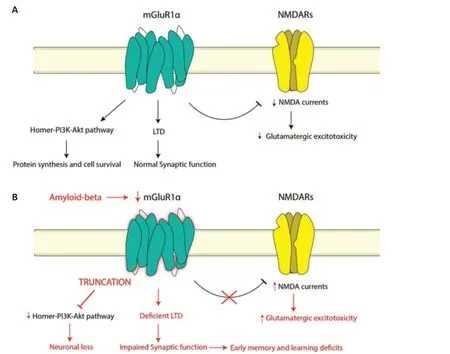
Figure 1|Metabotropic glutamate receptor 1 alpha (mGluR1α) activity in normal physiology and Alzheimer’s disease.
An earlier study that did not differentiate between receptor variants found that mGluR1 expression has also been shown to be altered in AD, with decreased expression in the cerebral cortex appearing to correlate with disease severity(Albasanz et al., 2005).Whether this reduction is a neuroprotective or exacerbating factor in disease progression remains to be seen.Interestingly, no decrease was observed in mGluR5 expression(Albasanz et al., 2005).mGluR1 appears to play a key role in GABAergic inhibition, thus a reduction in mGluR1 activity may be neuroprotective by reducing glutamatergic excitotoxicity characterized in other studies (Wang and Reddy,2017).Alternatively, mGluR1α activation itself has been shown to be potentially therapeutic,with the application of a mGluR1 selective antagonist abolishing a reduction in NMDA currents in rat hippocampal neurons (Blaabjerg et al., 2003).Recent studies utilizing PET imaging have been able to quantify receptor binding within live patients, with comparisons between cognitively normal patients and those with mild cognitive impairment due to AD or mild AD with brain amyloidopathy demonstrating reduced hippocampal mGluR5 binding in early AD (Mecca et al., 2020).Interestingly, a recent PET study examining mGluR1 availability demonstrated nil changes in the early stages of AD (Ishibashi et al.,2019).However, a similar study examining mGluR1 binding activity, and in particular the mGluR1α variant, at more advanced stages would provide significant information and confirmation in regard to upregulation or downregulation of mGluR1 activity in patients, rather than through postmortem examination.Furthermore, whilst previous studies have demonstrated localized expression of mGluR1 and mGluR5 to different brain regions,similar studies quantifying expression patterns in mGluR1 variants would provide more insight into possible variant-specific changes in AD.This may guide future understanding of variant-specific alterations and certain variant susceptibility and dysfunction in AD.
Interestingly, regulation of mGluR1α activity appears to involve an element of a posttranslational modification affecting receptor function.Xu et al.(2007) describe truncation of mGluR1α resulting in interruption of the Homer-PI3K-Akt pathway, which is crucial in preventing neuronal apoptosis, proposing a possible role in neuronal loss (Figure 1).This mechanism also explains how mGluR1α has previously been shown to confer both neuroprotective and excitotoxic effects as described above, as truncation may lead to a transition from a neuroprotective to a neurodegenerative state.These findings add a facet of complexity to the consideration of mGluR1α as a therapeutic target, due to its potential to facilitate both neuroprotective and excitotoxic effects.More nuanced and targeted manipulation of mGluR1α such as specific targeting of its truncated form, or proteins involved in the truncation process, may be a more effective potential intervention through the preservation of the normal physiological form and function.
mGluRs, in particular mGluR5, have previously been explored as possible therapeutic avenues for the treatment and management of neurodegenerative disorders such as AD (Srivastava et al., 2020).Recent studies also describe the dysfunction of group I mGluRs as potentially pivotal in the cognitive decline seen in AD patients(Su et al., 2022).These reviews describe the downregulation and desensitization of Group I mGluRs in AD cases, which indicates both its potential significance in driving AD progression,but also as a potential target for reversing this.As such, modulation of group I mGluRs may present a potential strategy towards alleviating the effects of AD pathology.Discovery of allosteric binding sites on mGluR1α provides a means through which to augment or alter glutamate-evoked cellular activity(Jiang et al., 2014).It has also been demonstrated that group I mGluR activation resulted in increased processing of Aβ by α-secretase, providing a means of potentially reducing the neurotoxic amyloid load and thus ameliorating disease activity (Srivastava et al., 2020).Of course, there is still ongoing contention towards the exact role of Aβ in the pathophysiology of AD, however,these studies demonstrate promise in therapy through augmentation of the glutamatergic system, particularly through the Group I mGluRs.Examination of mGluR1α in other neurological disorders such as spinocerebellar ataxia and status epilepticus also highlights the need for further characterization of this variant in AD,with mGluR1α activity directly affecting cell proliferation and migration through receptor activation, mislocalization, and downstream receptor truncation effects resulting in functional deficits (Armbrust et al., 2014; Friedman et al.,2016).
As such, although most studies currently in the literature are exploratory and preliminary in nature, mGluR1α appears to play an important role in normal physiological functioning.Therefore,derangement or disruption of this receptor’s functioning can have long-reaching implications on the neuronal microenvironment, with potential effects on the progression and worsening of AD.Whilst it has been established there are expression and activation differences in the mGluR1α variant in disease, future studies should aim to further characterize sub-variant specific functional changes and explore the effects of modulation of this and its effect on neuronal activity and cognitive function in animal models.
Jason HY Yeung, Andrea Kwakowsky*Centre for Brain Research, Department of Anatomy and Medical Imaging, Faculty of Medical and Health Sciences, University of Auckland, Auckland,New Zealand (Yeung JHY, Kwakowsky A)Pharmacology and Therapeutics, School of Medicine, Galway Neuroscience Centre, Ollscoil na Gaillimhe - University of Galway, Galway, Ireland(Kwakowsky A)
*Correspondence to:Andrea Kwakowsky, PhD,andrea.kwakowsky@universityofgalway.ie.https://orcid.org/0000-0002-3801-4956(Andrea Kwakowsky)
Date of submission:November 16, 2022
Date of decision:January 10, 2023
Date of acceptance:January 20, 2023
Date of web publication:March 3, 2023
https://doi.org/10.4103/1673-5374.369109
How to cite this article:Yeung JHY, Kwakowsky A(2023) Metabotropic glutamate receptor 1 alpha: a unique receptor variant with variable implications for Alzheimer’s disease pathogenesis.Neural Regen Res 18(10):2196-2197.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain

