Regulation of cytohesins by their interactors in the nervous system
Yuqi Zhai, King To Leung, Kwok-Fai Lau
Adenosine diphosphate ribosylation factors(ARFs) are small GTP-binding proteins of the Ras superfamily which are involved in membrane trafficking and actin cytoskeleton remodeling.Like other small GTPases, their functions are attributed to the cycling of ARF between GTP(active) and GDP (inactive) states through the actions of guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins,respectively.Cytohesins are a GEF subfamily of ARFs in mammals with four members, namely,cytohesin-1, -2, -3, and -4.
The functions of cytohesins in the nervous system:Increasing evidence suggests that cytohesins play important roles in the development and functioning of the nervous system by regulating ARFs.Cytohesin-2 has been shown to activate ARF6 signaling to stimulate axonal elongation and branching during neuronal development in both hippocampal neurons and embryonic cortical neurons.Axon pathfinding is activated in commissural neurons by cytohesin-2 through the suppression of Slit-mediated repulsive axon guidance (Ito et al., 2022).In addition,cytohesin-2-ARF6 signaling is also implicated in the regulation of dendritic development.For example,dendritic elongation is suppressed in hippocampal neurons through the interaction of cytohesin-2 with pallidin (Ito et al., 2018).Moreover,cytohesin-2 inhibits the dendritic branching of hippocampal neurons by activating two independent pathways associated with ARF6 and Rac1 (Hernandez-Deviez et al., 2002).Cytohesin-1 also stimulates ARF6 to trigger the migration of hippocampal neurons.Evidence suggests that cytohesins have roles in the synaptic functions of mature neurons.For instance, the recruitment of cytohesin-1 to the presynaptic active zone promotes the generation of readily releasable vesicles and subsequent neurotransmitter release (Neeb et al., 1999).At the post-synaptic terminal, cytohesin-2 regulates the distribution of group 1 metabotropic glutamate receptors on the cell surface through endocytic trafficking,demonstrating the role of cytohesins in synaptic plasticity (Kitano et al., 2002).
In addition to their physiological roles, the cytohesin-ARF pathway has been implicated in various neurodegenerative disorders,including Alzheimer’s disease (AD) which is characterized by the aggregation of amyloid-β and hyperphosphorylated tau in the brain.Notably,the ARF6-dependent endocytic pathway has been shown to regulate the accumulation of amyloid β by modulating the trafficking of amyloid precursor protein and β-secretase (Ito et al.,2022).Moreover, FRMD4A, which is associated with the risk of late-onset AD, has been shown to activate cytohesin-ARF6 signaling and stimulate tau secretion (Yan et al., 2016).The cytohesin-ARF pathway has also been implicated in amyotrophic lateral sclerosis in which upper and lower motor neurons exhibit progressive degeneration.The pathogenesis of amyotrophic lateral sclerosis is associated with the mutation of proteins, including superoxide dismutase 1 and TAR DNA-binding protein 43.Notably, the treatment of neurons with SecinH3, a pan-cytohesin inhibitor, ameliorates the endoplasmic reticulum stress caused by promoting the autophagic clearance of the mutant proteins(Zhai et al., 2015).
Emerging roles of cytohesin interactors in regulating the GEFs:The above-mentioned findings underscore the significance of cytohesins in neurodevelopment and neuronal disorders.Hence, tight regulation of the functions of the GEFs is crucial.Notably, emerging evidence suggests the roles of cytohesin interactors in modulating these GEFs (Table 1).Interacting proteins, identified thus far, include mostly scaffold proteins, G-protein signaling regulators, and cytoskeleton-interacting proteins.
All four cytohesins share a conserved domain structure, with an N-terminal coiled-coil (CC)domain, a central SEC7 catalytic domain,a pleckstrin homology (PH) domain, and a C-terminal helix/polybasic (PB) region(Additional Figure 1).These domains play significant roles in regulating the functions of cytohesins.Akin to many GEF proteins,cytohesins are subjected to autoinhibition by the intramolecular interaction between the Sec7-PH linker and the PB domain.Cytohesininteracting molecules have been reported to release such autoinhibitory conformation.For instance, the neuronal adaptor FE65 has been shown to interact with both cytohesin-2 and ARF6,which brings them into proximity.Subsequently,FE65 disrupts the intramolecular interaction of cytohesin-2.Moreover, FE65 promotes the targeting of cytohesin-2 to the endocytic recycling compartments which regulate cargo recycling to the plasma membrane (Zhai et al., 2022).Thus,the cytohesin-2-FE65 interaction potentiates ARF6-mediated neurite outgrowth.Notably, it has been reported that ARF6-GTP allosterically activates cytohesin-2 and -3 by interacting with their PH domains, resulting in their conformational rearrangement (Malaby et al., 2013).
Additionally, the functions of cytohesins may be regulated by homo-dimerization through their CC domains (Das et al., 2019).Cytohesin homodimers are then recruited to the membrane,where a conformational change is induced,priming them for allosteric activation by ARF-GTP.Intriguingly, cytohesins have also been shown to be stimulated via heterodimerization with other CC-domain-containing proteins, such as CCDC120 and FRMD4A.CCDC120 has been shown to mediate ARF6 activation and neurite outgrowth by their CC domain interaction.Furthermore, The FRMD4A-cytohesin-1 heterodimer binds to Par-3, an upstream signaling component of ARF6,to facilitate ARF6 activation during junctional remodeling and epithelial polarisation (Ikenouchi and Umeda, 2010).FRMD4A bridges cytohesin-1 and its upstream signaling component, Par3, by recruiting cytohesin-1 to Par3-containing adherens junctions.This facilitates ARF6 activation during junctional remodeling and epithelial polarisation(Ikenouchi and Umeda, 2010).
Kinases interact with their substrates to induce functional changes to the target via phosphorylation.Of note, several kinases have been reported to alter the functions of cytohesins.Protein kinase C has been shown to phosphorylate cytohesin-2 on serine 392 within the PB domain,which reduces the affinity of cytohesin-2 for membranes where it activates ARFs (Santy et al.,1999).Furthermore, the association between cytohesin-2 and the membrane is hindered by the intramolecular interaction between the CC domain and the rest of the protein.Akt potentiates the membrane association of cytohesin-2 by phosphorylating threonine 276 in its PH domain,which triggers the relief of the autoinhibitory structure (Hiester and Santy, 2013).
Concluding remarks:Cytohesins play significant roles in neurodevelopment by activating ARFs.Dysregulation of cytohesin-ARF signaling is observed in several neurodegenerative diseases.Although small-molecule inhibitors of cytohesins have been developed, their global inhibitory effects may lead to significant side effects.Mounting evidence suggests that cytohesin interactors affect the functions of cytohesins through different mechanisms.Small molecules or peptides that target these interactions may provide novel routes for specifically altering the functions of cytohesins.Understanding the entire interaction networks of various cytohesins and the mechanisms by which these interactions affect cytohesins will provide further insights into the physiological control of GEFs and facilitate the development of specific therapeutic strategies to target cytohesin-related neuronal diseases.
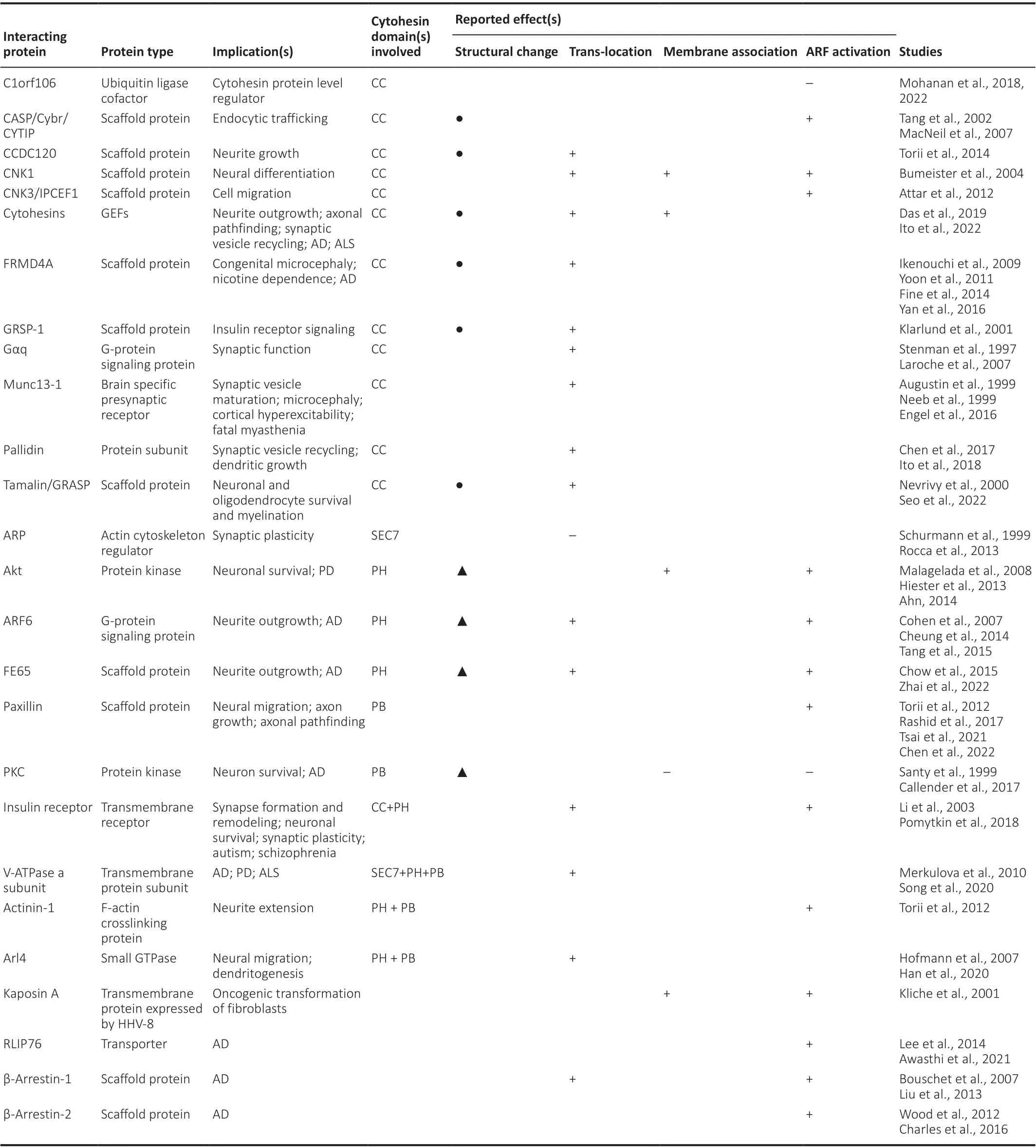
Table 1 |A summary of cytohesin-interacting proteins and their reported effects on cytohesins
This work was supported by funds from Research Grants Council Hong Kong, Health and Medical Research Fund (Hong Kong), CUHK direct grant scheme, United College endowment fund and the TUYF Charitable Trust (to KFL).
Yuqi Zhai, King To Leung, Kwok-Fai Lau*School of Life Sciences, Faculty of Science, The Chinese University of Hong Kong, Hong Kong Special Administrative Region, China
*Correspondence to:Kwok-Fai Lau, PhD,kflau@cuhk.edu.hk.https://orcid.org/0000-0002-0193-1152(Kwok-Fai Lau)
Date of submission:November 19, 2022
Date of decision:December 30, 2022
Date of acceptance:January 12, 2023
Date of web publication:March 3, 2023
https://doi.org/10.4103/1673-5374.369105
How to cite this article:Zhai Y, Leung KT, Lau KF(2023) Regulation of cytohesins by their interactors in the nervous system.Neural Regen Res 18(10):2186-2187.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:Additional Figure 1:The general structure of cytohesins.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain

