Adipose mesenchymal stem cell-derived extracellular vesicles reduce glutamate-induced excitotoxicity in the retina
Tian-Qi Duan, Zhao-Lin Gao, Ai-Xiang Luo, Dan Chen, Jian-Bin Tong, Ju-Fang Huang,*
AbstractAdipose mesenchymal stem cells (ADSCs) have protective effects against glutamate-induced excitotoxicity, but ADSCs are limited in use for treatment of optic nerve injury.Studies have shown that the extracellular vesicles (EVs) secreted by ADSCs (ADSC-EVs) not only have the function of ADSCs, but also have unique advantages including non-immunogenicity, low probability of abnormal growth, and easy access to target cells.In the present study, we showed that intravitreal injection of ADSC-EVs substantially reduced glutamate-induced damage to retinal morphology and electroretinography.In addition, R28 cell pretreatment with ADSC-EVs before injury inhibited glutamate-induced overload of intracellular calcium, downregulation of α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptor (AMPAR) subunit GluA2, and phosphorylation of GluA2 and protein kinase C alphain vitro.A protein kinase C alpha agonist,12-O-tetradecanoylphorbol 13-acetate, inhibited the neuroprotective effects of ADSC-EVs on glutamate-induced R28 cells.These findings suggest that ADSCEVs ameliorate glutamate-induced excitotoxicity in the retina through inhibiting protein kinase C alpha activation.
Key Words:adipose mesenchymal stem cells; calcium overload; electroretinography; excitotoxicity; extracellular vesicles; GluA2; glutamate; protein kinase C alpha; R28 cells; retina; retinal ganglion cell
Introduction
Excitotoxicity is now recognized as a crucial pathophysiological mechanism in a multitude of retinal diseases, including glaucoma (Vernazza et al.,2021), diabetic retinopathy (Zaitone et al., 2020; Alomar et al., 2021), and retinal vein occlusion (Wasilewa et al., 1976; Dionysopoulou et al., 2020).Memantine, an N-methyl-D-aspartic acid receptor (NMDAR) inhibitor, is the primary strategy for alleviating excitotoxicity in the clinic (Liu et al., 2020).However, oral memantine may result in side effects, including wooziness,headaches, constipation, and diarrhea (Kuns et al., 2022).Therefore, the clinical anti-excitotoxic treatment of retinal diseases remains an important issue (Chen and Kukley, 2020).
In recent years, mesenchymal stem cell (MSC)-based cell transplantation therapy has become a promising approach for treating retinal disorders.This therapeutic strategy is attributed to the ability of MSCs to transdifferentiate into neurons, their self-renewal abilities, pro-proliferative properties, and neuroprotective effects (Harrell et al., 2019, 2022).Previous studies have identified a protective role for adipose mesenchymal stem cells (ADSCs)in glutamate-induced excitotoxic injury, but the mechanism is not clear(Zhao et al., 2009; Hao et al., 2014).Further, there are still some limitations of MSC therapy in the eye, including few cells integrated into the retina,abnormal growth, and immune rejection (Yu et al., 2020).It has been found that intravitreal injection of MSC-derived extracellular vesicles (EVs) reduces apoptosis of retinal ganglion cells (RGCs) in a rat model of acute intraocular hypertension (Mathew et al., 2019).Thus, it may be possible to avoid the limitations of ADSCs by using EVs in the treatment of retinal diseases.
Excessive activation of ion-associated glutamate receptors is critical in the pathogenesis of glutamate-induced retinal damage (Connaughton, 1995; Xu et al., 2017).Ion-associated glutamate receptors include NMDARs, kainate receptors, and α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptors (AMPARs) (Dingledine et al., 1999).In contrast to NMDARs, AMPARs are almost impermeable to calcium ions under physiological conditions (Bowie and Mayer, 1995; Jacobi and von Engelhardt, 2017).AMPARs are tetrameric membrane proteins consisting of four different subunits, GluA1–4.Of all the subunit combinations, the GluA2-lacking AMPARs are permeable to calcium ions (Cull-Candy and Farrant, 2021).Furthermore, an increased expression of GluA2-lacking AMPARs was found in rat models of retinal disease (Dong et al., 2015).It has been reported that the GluA2 deletion mechanism is associated with GluA2 phosphorylation (S880).Additionally, activation of the protein kinase C (PKC) pathway may be a key GluA2 stimulator (Purkey and Dell’Acqua, 2020; Guo and Ma, 2021).Hence, upregulating GluA2 expression and inhibiting its phosphorylation may be a reliable approach to ameliorate glutamate-induced retinal damage.In the present study, we investigated whether ADSC-EVs inhibit glutamateinduced retinal excitotoxic damagein vivoandin vitro, and their effects on GluA2 and PKC.
Methods
Ethics statement
All adipose tissue collections were approved by the Medical Ethics Committee of Central South University (IRB No.202111001) on November 9, 2021,and all volunteers signed informed consent.All animal experiments were approved by the Animal Ethics Committee of Central South University (No.CSU-2022-0088) on February 22, 2022.All animal experiments were reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE)guidelines (Percie du Sert et al., 2020).
Experimental design
In vivo experiments
In total, 38 specific-pathogen-free male Sprague-Dawley rats, weighing 200 g and aged 6–8 weeks, were purchased from the Hunan SJA Laboratory Animal Co., Ltd.(Changsha, Hunan, China) (license No.SYXK (Xiang) 2020-0019).The animals were housed in a specific-pathogen-free environment, under a 12-hour light/dark cycle, 60 ± 5% humidity, and a temperature of 23 ± 1°C.They were randomly divided into six groups with the following treatments:Con group: intravitreal injection of 4 μL phosphate buffered saline (PBS) (n= 8); Glu group: intravitreal injection of 4 μL 100 mM glutamate (Aladdin,Shanghai, China) (n= 8); Glu + EVs group: intravitreal injection of 3 μL of 1 ×109 particles/mL ADSC-EVs, followed by intravitreal injection of 4 μL of 100 mM glutamate 1 hour later (n= 8); Glu + PBS group: intravitreal injection of 3 μL PBS, followed by intravitreal injection of 4 μL of 100 mM glutamate 1 hour later (n= 8); PKH26-Control group: intravitreal injection of 3 μL PBS (n= 3);and PKH26-labeled group: intravitreal injection of 3 μL of 1 × 109particles/mL PKH26-labeled ADSC-EVs (n= 3).Intravitreal injection was performed by inserting the needle 1 mm outside the limbus using a micro syringe (Hamilton,Reno, NV, USA) after the pupil was dilated with compound tropicamide(Bausch & Lomb, Jinan, Shandong, China).Animals were killed 24 hours after intravitreal injection (Sisk and Kuwabara, 1985) (Figure 1).

Figure 1|Schematic of the experimental timeline of in vitro and in vivo modeling.
In vitro experiments
Rat retinal precursor (R28) cells were provided by Central South University.This cell line had been characterized by short tandem repeat analysis and confirmed according to the ICLAC Database of Cross-Contaminated or Misidentified Cell Lines.In previous studies (Lee et al., 2020; Huang et al.,2021; Yao et al., 2022; Yan et al., 2023), the R28 cell line has been widely used to explore the neuroprotection and pathological mechanism of RGCsin vitro.To determine the optimal time point for glutamate damage on R28 cells, we treated cells with 34 mM glutamate for 4, 6, 8, 10, and 12 hours.To determine the most effective concentration of ADSC-EVs to protect R28 cells,we pretreated cells with 5 or 25 μg/mL ADSC-EVs for 1 hour.We pretreated cells with 10, 25, 50, 75, and 100 ng/mL 12-O-tetradecanoylphorbol 13-acetate (TPA; Beyotime, Shanghai, China) for 1 hour to determine the optimal concentration of TPA, which activates the PKC pathway.In vitroexperiments included in five groups with the following treatments: Con group: cells were cultured under normal conditions; Glu group: cells were treated with 34 mM glutamate; Glu + EVs group: cells were pretreated with ADSC-EVs for 1 hour before the addition of glutamate; Glu + PBS group: cells were pretreated with PBS before the addition of glutamate; and Glu + TPA +EVs group: cells were pretreated with TPA and ADSC-EVs for 1 hour before the addition of glutamate.We cultured the cells at least three times, and three dishes were used for each group during each culture (Figure 1).
Culture and identification of ADSCs
Human-derived ADSCs were isolated and cultured using the methods described in a previous study (Ahmadian Kia et al., 2011).Briefly, adipose tissue of healthy adults aged 18–50 years old collected from the Third Xiangya Hospital of Central South University was mechanically chopped before centrifugation at 450 ×gfor 5 minutes.Afterward, we collected the middle layer of the three-layer fraction, which is the stromal vascular fraction(Hearnden et al., 2021).Then the stromal vascular fraction was digested with 0.1% collagenase I (Servicebio, Wuhan, China; GC305013) in a 37°C incubator with 225 r/min shaking for 30–60 minutes.We filtered the digested tissue solution with a 40-μm filter and centrifuged it at 450 ×gfor 10 minutes.The pellet was resuspended in a low glucose Dulbecco’s modified Eagle’s medium (Hyclone, Logan, UT, USA; Cytiva SH30021.01) containing 10% fetal bovine serum (ExCell Bio, Suzhou, China).The cells were stored in a sterile moist environment of 37°C and 5% carbon dioxide.After reaching 80–100%confluence, we passaged the cells with 0.25% trypsin (Gibco, Grand Island,NY, USA).
After the third generation of cell transmission, we identified the characteristics and differentiation ability of the cells.Initially, cell morphology was observed by light microscopy, and then cells were digested with trypsin (Gibco),resuspended in culture medium, and transported at 4°C for flow cytometry(Changsha Golden Medical Clinical Laboratory Center, Changsha, China).In addition, we also performed lipogenic and osteogenic differentiation assays on ADSCs (Cyagen Biosciences, Santa Clara, CA, USA; HUXMD-90031,HUXMD-90021).Briefly, we first inoculated the cells in a 6-well plate coated with 1% gelatin, and when the cell fusion reached 70%, 2 mL of the prepared medium for differentiation induction was added to the wells.After 2–4 weeks,the cells were stained with Alizarin Red and Oil Red O (Cyagen Biosciences) to observe the morphological changes and growth.
Extraction and identification of ADSC-EVs
In accordance with previous methods (Dou et al., 2020; Yang et al., 2020),we obtained EVs by ultrafiltration combined with a polymer precipitation strategy.Briefly, cells were cultured for more than 48 hours in a serum-free medium (Hyclone; Cytiva SH30021.01).Then we removed cells and debris by centrifugation (200 ×g, 10 minutes, 4°C).The supernatant was concentrated using a 0.45-μm filter and a 100-kDa ultrafiltration tube (Millipore, Burlington,MA, USA; UFC9100, Amicon Ultra-15).Thereafter, EVs were pelleted by low-speed centrifugation (1500–3000 ×g) after overnight incubation with polyethylene glycol at 4°C.Then, precipitates were resuspended in sterile PBS and EVs were obtained for further applications.Finally, we performed nanoparticle tracking analysis (Particle Metrix, Munich, Germany; ZetaView),electron microscopy, and western blot to identify the concentration, size,shape, and surface markers of ADSC-EVs.
ADSC-EVs uptake by rat retina and R28 cells
EVs were labeled according to the instructions of the PKH26 Red Fluorescent Cell Ligation Kit (Umibio, Shanghai, China; UR52302).Briefly, EVs were suspended in 10 μL diluted PKH26 solution (1 μL PKH26 and 9 μL PBS) for 10 minutes.After that, we washed the mixture in 10 mL 1× PBS and concentrated it in a 100-kDa ultrafiltration tube (3000 ×g, 20 minutes, 4°C).The pellets were resuspended and added to the R28 cells at 25 μg/mL.After incubating with PKH-labeled EVs for 8 hours, the cells were stained with 4′,6-diamidino-2-phenylindole (DAPI, Solarbio, Beijing, China) and observed by fluorescence microscope (Zeiss, Jena, Germany, AXIO Vert.A1).
For thein vivoexperiments, 24 hours after the intravitreal injection of 3 μL 1 × 109particles/mL PKH26-labeled EVs, the rats were anesthetized with an intraperitoneal injection of 2% sodium pentobarbital (40 mg/kg; FWD Chemical Company, Shanghai, China) and perfused with 4% paraformaldehyde via myocardium.Then, the eyeballs were removed and fixed in ocular fixative (Servicebio, G1109) for more than 1 day, dehydrated in 30% sucrose,embedded with optimal cutting temperature compound (Sakura, Tokyo,Japan; 4583), and then stored at –80°C.The eyeballs were sectioned sagittally with a cryostat (Leica, CM1520) at a thickness of 8 μm.After DAPI staining,the sections were observed using a fluorescence microscope.
Hematoxylin and eosin staining
After 2% sodium pentobarbital anesthesia and 4% paraformaldehyde cardiac perfusion as above, bilateral eyeballs were removed with forceps and then fixed in an ocular fixative for more than 1 day.Subsequently, we embedded the eyeballs in paraffin and then obtained continuous sagittal sections (4 μm thick), including the optic nerve head.After staining with hematoxylin and eosin (Servicebio, G1003), we observed the morphology of retinal layers using a light microscope (Toupcam, Hangzhou, China) analyzed by ImageJ software and Adobe Illustrator 26.0 (San Jose, CA, USA) software.The number of RGCs,and thickness of the inner plexiform layer and inner nuclear layer in one paraffin section per eye were recorded at 1000-μm intervals beginning at the optic papilla.
Electroretinography
Electroretinography (ERG) waveforms were recorded 24 hours after modeling by an eye electrophysiological detector (Roland, Brandenburg, Germany;RETI port/scan 21).Before dark-adapted ERG assays, rats were maintained in a dark environment for 24 hours.After anesthetization with 1% sodium pentobarbital (50 mg/kg), ERG data were collected by full field stimulation.The original data of scotopic 3.0 and 3.0 cd s/m2oscillatory potential ERG were recorded and analyzed by Microsoft Excel 2016 (Redmond, WA, USA)software.Next, we measured the a-wave amplitude from the prestimulated baseline to the wave trough.The b-wave amplitude was measured from the trough of the a-wave to the peak of the waveform.The analysis and description of the scotopic 3.0 oscillatory potentials (SOPs) were mainly based on the peaks of the three main oscillatory waveforms in the signal (McCulloch et al., 2015).
Propidium iodide staining
Positive propidium iodide (PI) staining is regarded as a marker of necrotic cells (Crowley et al., 2016).After washing twice with PBS, the R28 cells were incubated in PI-dye solution (10 μg/mL; Sigma, St.Louis, MO, USA,Cat# P4170) for 15 minutes.Subsequently, the cells were fixed with 4%paraformaldehyde for 20 minutes and nuclei were stained with DAPI for 5 minutes.Finally, the ratio of necrotic cells was calculated as PI-positive cells/DAPI-positive cells under a microscope.
Calcium measurement
After washing three times with calcium-free Hank’s balanced salt solution(Solarbio), the cells were labeled by 4 μM fluorescent probe (Fluo-4 AM, Solarbio) for 30 minutes at 37°C.Then, the cells were fixed with 4%paraformaldehyde and stained with DAPI.The Fluo-4 AM signal was observed using a fluorescence microscope.The fluorescence signal intensity values of Fluo-4 AM were obtained by dividing the integrated density by the number of cells, as described in a previous study (Wang et al., 2019).ImageJ v.1.5.1(National Institutes of Health, Baltimore, MD, USA) software (Schneider et al.,2012) was used for integrated density measurement and cell counting.
Western blot analysis
The R28 cells were lysed for 30 minutes in radioimmunoprecipitation assay lysis buffer (CWBIO, Beijing, China) with the addition of protease and phosphatase inhibitors (Roche, Basel, Switzerland).After performing electrophoresis and electroblotting, we blocked the nitrocellulose membranes(Pall, New York, NY, USA, Cat# 66485) in 5% nonfat milk (MengNiu, Hohhot,Inner Mongolia, China) or QuickBlockTMBlocking Buffer (Beyotime, P0252)at room temperature (approximately 25°C) for 1–2 hours.The samples were incubated with the following primary antibodies overnight at 4°C: 1)Extracellular vesicles and MSC surface markers: polyclonal rabbit anti-CD81(1:1000; GeneTex, Irvine, CA, USA, Cat# GTX101766, RRID: AB_10618892)and anti-CD63 (1:1000; Wanleibio, Shenyang, Liaoning Province, China,Cat# WL02549, RRID: AB_2910631); 2) Key regulatory proteins of AMPAR:polyclonal rabbit anti-GluA2 (1:1000; Abclonal, Wuhan, China, Cat# A0111;RRID: AB_2756954), polyclonal rabbit anti-phospho-GluA2 (Ser 880; 1:500;Affinity Bioscience, Beijing, China, Cat# AF3307, RRID: AB_2834726),polyclonal rabbit anti-PKC alpha (1:1000; Wanleibio, Cat# WL02234, RRID:AB_2925211), and monoclonal rabbit anti-phospho-PKC alpha (1:10,000;Abcam, Cambridge, UK, Cat# ab32502, RRID: AB_777295); 3) Apoptosisrelated proteins: polyclonal rabbit anti-cleaved caspase 3 (1:1000; Cell Signaling Technology, Danvers, MA, USA, Cat## 9661, RRID: AB_2341188);and monoclonal rabbit anti-β-tubulin (1:10,000; Proteintech, Wuhan, China,Cat# 66240-1-Ig, RRID: AB_2881629).After washing in PBS-Tween 20 (0.05%),the membranes were incubated with goat anti-rabbit IgG (1:10,000; Abbkine,Wuhan, China, Cat# A25222, RRID: AB_2922982) at room temperature for 2 hours.The immunoreactive band was then visualized using a high sensitivity chemiluminescent reagent (CWBIO).The grayscale intensities quantified with ImageJ software were normalized to the control and β-tubulin.
Immunofluorescence staining
R28 cells were fixed for 20 minutes with 4% paraformaldehyde.Then, the cells were washed three times with 0.01 M PBS.The cells were blocked for 1 hour in 5% bovine serum albumin, according to a previous study (Dong et al., 2015).Then, R28 cells were incubated with polyclonal rabbit anti-GluA2(1:50; ABclonal, Cat# A0111, RRID: WX924262) overnight at 4°C and Alexa Fluor 594-labeled goat anti-rabbit IgG (1:200; Abcam, Cat# ab150160, RRID:AB_2756445) for 2 hours at room temperature.After staining with DAPI for 5 minutes, images were acquired with a laser-scanning microscope and analyzed by ImageJ software.
Statistical analysis
No statistical methods were used to predetermine sample sizes; however, our sample sizes were similar to those reported in previous publications (Charles-Messance et al., 2020; Fang et al., 2023).No animals or data points were excluded from the analysis.All numerical data were recorded by at least two researchers and were analyzed via one-way analysis of variance followed by Brown-Forsythe test using SPSS 22.0 statistical software (IBM, Armonk,NY, USA).P< 0.05 was set as the significance threshold.GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA, www.graphpad.com) was used to create the bar graphs.
Results
Identification of ADSCs and ADSC-EVs
ADSCs are characterized by adherent growth, the positive expression of CD105, CD73, and CD90, the negative expression of CD45, CD34, and CD19,and adipogenic and osteogenic abilities (Dominici et al., 2006).We used primary ADSCs from human adipose tissue, and found that 3rdgeneration ADSCs presented a spindle-shaped morphology (Figure 2A).More than 95%of cells expressed the positive markers (CD73, CD105, and CD90), and less than 5% of cells expressed the negative markers (CD34, CD45, and CD19)(Figure 2D).The cells had adipogenic and osteogenic abilities (Figure 2B and C).In addition, EVs were enriched from the medium supernatant of ADSCs.These EVs had a cup shape, with a size distribution peak at 136.87 ± 4.52 nm, and were positive for surface markers CD81 and CD63 (Figure 2E–G),consistent with the characteristics of exosomes (Phinney and Pittenger, 2017).These results confirmed the successful isolation of ADSCs and their EVs.
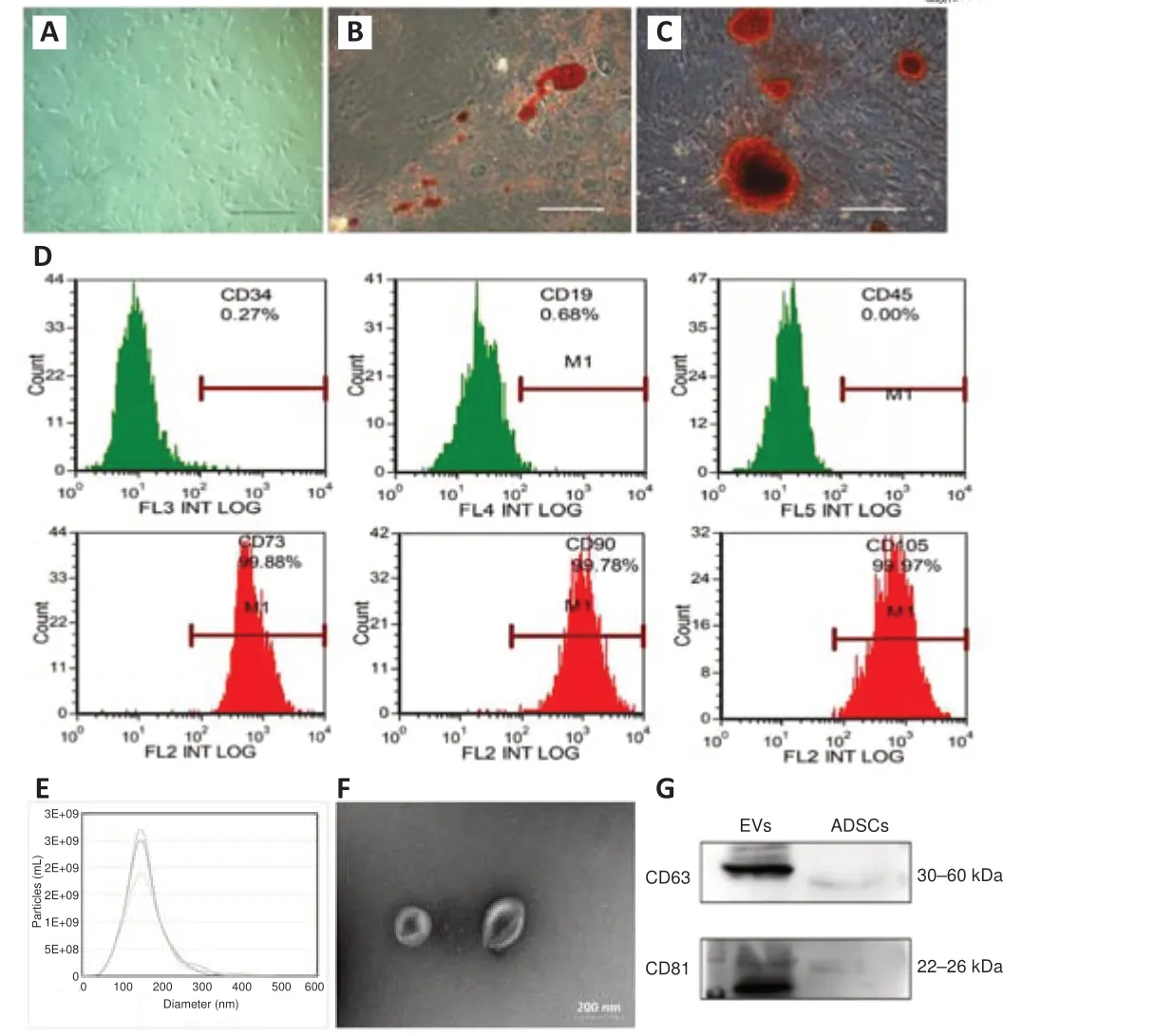
Figure 2|Identification of ADSCs and ADSC-EVs.
ADCS-EVs ameliorate the glutamate-induced damage of the rat retina
To test the effects of ADSC-EVs on glutamate-induced retinal damage, we injected ADSC-EVs and glutamate into the vitreous sequentially with a 1-hour interval.We found that PKH-26-labeled ADSC-EVs were primarily distributed in RGCs (Figure 3A).Compared with those in the Con group, the number of RGCs (P< 0.001; Figure 3B and C), inner plexiform layer thickness (P< 0.001,Figure 3B and D), inner nuclear layer thickness (P< 0.001; Figure 3B and E),and the amplitudes of the a- (P< 0.001), b- (P< 0.001), and SOP-waves (P<0.001; Figure 3F–J) in ERG were decreased significantly in the Glu group; these alterations were markedly ameliorated in the Glu + EVs group.These findings suggest that ADSC-EVs decreased glutamate-induced inner retinal damage.
ADCS-EVs reduce intracellular calcium concentration and GluA2 expression in the glutamate damage model
Increased intracellular calcium concentration plays a vital role in glutamate damage (Hartwick et al., 2008).To assess the effects of ADSC-EVs on the elevated intracellular calcium concentration, R28 cells were treated with EVs for 1 hour before the glutamate supplement.PKH-26-labeled ADSC-EVs were distributed mostly in the cytoplasm of R28 cells (Figure 4A).After application of 34 mM glutamate for 8 hours, more than 50% of R28 cells were necrotic (P< 0.001; Additional Figure 1A and B) and intracellular calcium concentration was increased (P< 0.001) compared with control group; these changes were considerably ameliorated in the Glu + EVs group (Additional Figure 1C–F and
Figure 4B and C).
Additionally, we observed the effect of ADSC-EVs on AMPAR subunit GluA2 expression and phosphorylation in the vitro model of glutamate damage.Compared with the Con group, glutamate damage led to decreased surface GluA2 expression (Figure 4D) and increased GluA2 phosphorylation in R28 cells (P< 0.001; Figure 4E and F); the changes were significantly ameliorated in ADSC-EV-treated R28 cells (P< 0.001; Figure 4D–F) compared with Glu group.The results suggested that ADSC-EVs reduced the intracellular calcium concentration, upregulated GluA2 expression, and inhibited GluA2 phosphorylation in thein vitroglutamate damage model.
PKC-α activation reduces the effects of ADSC-EVs on GluA2 phosphorylation in glutamate-treated R28 cells
Previous research has indicated that PKC-α plays a crucial role in regulating GluA2 phosphorylation (Kou et al., 2019).To investigate the mechanism by which ADSC-EVs regulate GluA2 phosphorylation, we activated PKC-α with PKC agonist TPA (100 ng/mL, 1 hour) in R28 cells (Figure 5A and B).ADSCEVs inhibited the phosphorylation of PKC-α and GluA2 in the Glu + EVs group compared with Glu group (bothP< 0.001); these changes were significantly reversed in the Glu + TPA + EVs group compared with Glu+EVs group (PKC-α:P< 0.01, GluA2:P< 0.001; Figure 5C–E).The results indicated that ADSC-EVs inhibited PKC-α activation and modulated GluA2 phosphorylation.
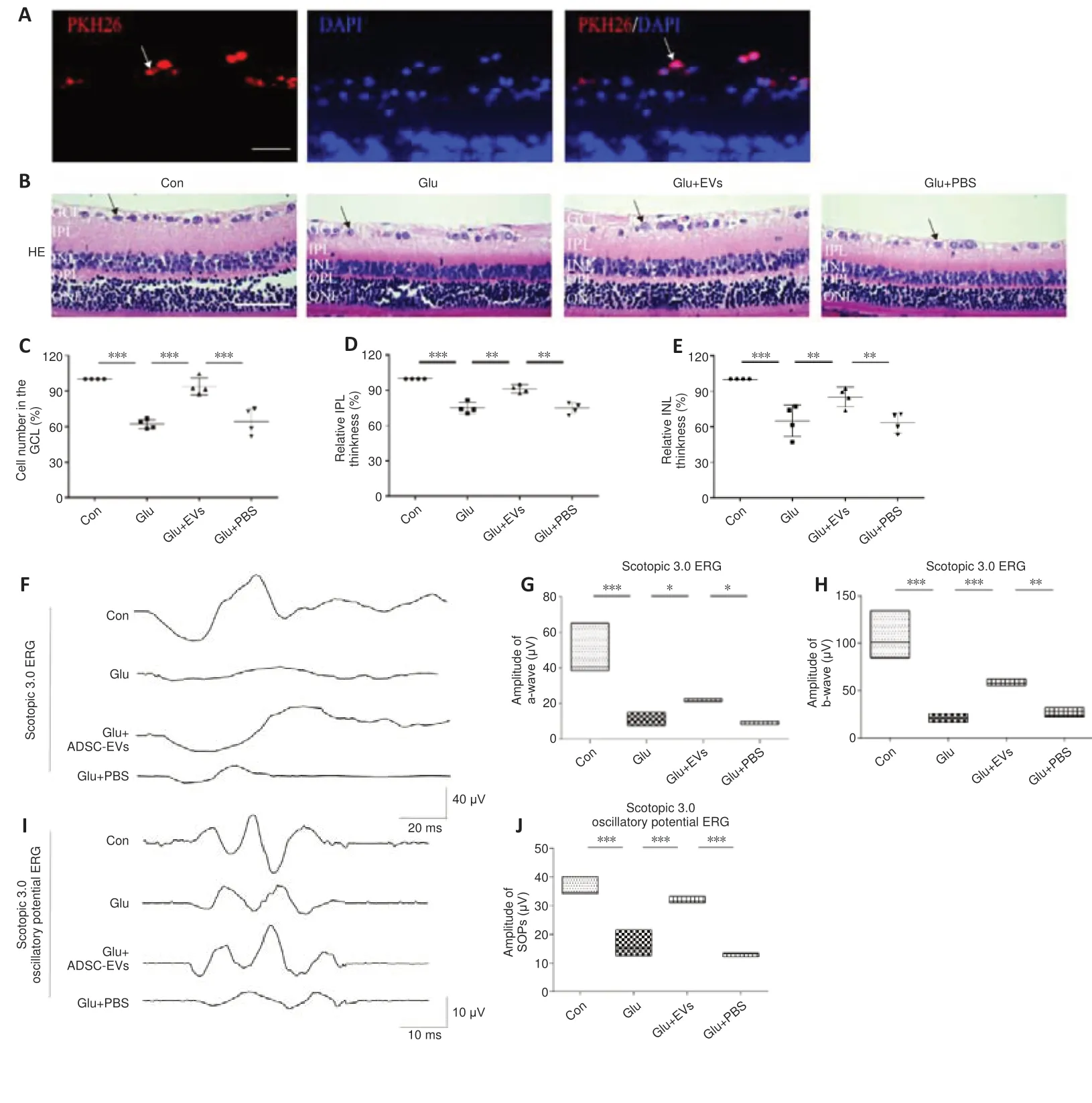
Figure 3|ADCS-EVs reduce glutamateinduced morphological and functional damage in rat retina.

Figure 4|ADSC-EVs reduce intracellular calcium concentration by regulating GluA2 expression in vitro.
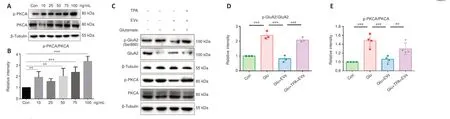
Figure 5|PKC-α activation reduces the effects of ADSC-EVs on GluA2 phosphorylation in glutamate-treated R28 cells.
Discussion
Excitotoxicity, a common secondary injury after retinal disease, is significantly associated with the prognosis of retinal structure and function (Dionysopoulou et al., 2020; Vernazza et al., 2021).Our study aimed to explore whether intravitreal injection of ADSC-EVs decreased glutamate-induced retinal damage.The results indicated that intravitreal-injected ADSC-EVs were taken up by RGCs within 24 hours, and the glutamate-induced morphological and functional abnormalities in the inner retinal layers were significantly ameliorated.This is consistent with previous studies (Papazian et al., 2018;Zhuang et al., 2022) on the effects of MSC transplantation on excitotoxicity in a rat controlled cortical impact-induced TBI model and an NMDA-induced mouse cortical neuron damage model.A recent review indicated that intravitreal injection of MSCs in ocular diseases may result in undesirable differentiation and proliferative vitreoretinopathy (Yu et al., 2020).These side effects were not observed in our study.These findings suggest that, as an alternative to stem cell therapy, intravitreal injection of ADSC-EVs is a simple and effective intervention for ameliorating retinal excitotoxicity.
Previous studies have shown that MSC-EVs carry various bioactive cargos such as proteins, microRNAs, lipids, and mRNAs (Bang and Thum, 2012).When MSC-EVs are taken up by targeted cells, these components modulate targeted cells through a variety of pathways.The reported effects include NLRP3 inflammasome inhibition (Zhang et al., 2022), regulation of microglial polarization (Xin et al., 2020), anti-apoptosis (Cui et al., 2022), and promotion of regeneration (Demyanenko et al., 2022).However, the molecular mechanism of glutamate-induced excitotoxicity remains elusive.Here, we found that ADSC-EVs exhibited anti-excitotoxic and neuroprotective effects on glutamate-induced retinal damage.Intravitreal injection of ADSC-EVs decreased the intracellular calcium concentration and upregulated surface GluA2 expression, suggesting that ADSC-EVs inhibited the calcium overload by upregulating the GluA2-containing AMPARs.GluA2 phosphorylation is an essential event that decreases the expression of surface GluA2 (Guo and Ma, 2021).Furthermore, GluA2 phosphorylation induced by PKC-α activation was inhibited by ADSC-EVs.These findings support that the regulatory role of ADSC-EVs on the PKC-GluA2 signaling pathway may be an essential mechanism in its anti-excitotoxic effect.
In recent years, matrix-bound nanoparticles, localized in the bioscaffolds of the extracellular matrix, have also been considered as an effective EV-based therapeutic.The extracellular matrix provides the structural basis for cell growth and repair (Swinehart and Badylak, 2016; Mathivanan, 2017), and matrix-bound nanoparticles carry bioactive factors that inhibit inflammatory responses (Huleihel et al., 2017) and promote regeneration (van der Merwe et al., 2017).A recent study found that matrix-bound nanoparticles modulated ischemia-induced RGC injury by intravitreal injection (van der Merwe et al.,2019).Our study indicated that ADSC-EVs regulate the PKC-GluA2 signaling pathway and inhibit retinal excitotoxic injury.Future studies should investigate whether the effectiveness of ADSC-EVs could be improved by modifying extracellular matrix bioscaffolds with ADSC-EVs (Hao et al., 2020a, b).
This study used only male rats in thein vivoexperiments.Although most previous studies have been performed in male experimental animals (Beery and Zucker, 2011), a recent study reported that testosterone injection can cause open-angle glaucoma and ischemic retinal disease (Dahshan et al.,2022).To avoid bias in the results due to sex and to analyze the damage in the glutamate model and the ADSC-EV therapeutic effects more comprehensively,it is necessary to include female rats in future studies.In addition, only 24 hours were observed afterin vivointervention with ADSC-EVs.An extended observation period is needed to further investigate the efficacy and side effects of ADSC-EVs.This study did not investigate which components of ADSC-EVs modulate the PKC-GluA2 pathway, which should be investigated in future studies.In conclusion, our results indicated that ADSC-EVs ameliorated glutamateinduced retinal damage by inhibiting the phosphorylation of GluA2 and PKC-α.These findings suggest the potential of ADSC-EVs for use in the clinical therapy of retinal diseases.
Acknowledgments:We thanked Dr.Kun Xiong (Department of Human Anatomy and Neurobiology, School of Basic Medical Science, Central South University, Changsha, Hunan Province, China) for providing the rat retinal precursor (R28) cells.
Author contributions:Experimental design: JFH and DC; experiments implementation: TQD, ZLG and AXL; data collection and analysis: DC, JFH and TQD; manuscript drafting: TQD, JBT and JFH.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:All data relevant to the study are included in the article and its Additional files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:Additional Figure 1:Glutamate-induced damage decreased by ADSC-EVs in R28 cells.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain

