Srgap2 suppression ameliorates retinal ganglion cell degeneration in mice
Yi-Jing Gan, Ying Cao, Zu-Hui Zhang, Jing Zhang, Gang Chen, Ling-Qin Dong, Tong Li, Mei-Xiao Shen, Jia Qu,*, Zai-Long Chi,*
AbstractSlit-Robo GTPase-activating protein 2 (SRGAP2) plays important roles in axon guidance, neuronal migration, synapse formation, and nerve regeneration.However, the role of SRGAP2 in neuroretinal degenerative disease remains unclear.In this study, we found that SRGAP2 protein was first expressed in the retina of normal mice at the embryonic stage and was mainly located in the mature retinal ganglion cell layer and the inner nuclear layer.SRGAP2 protein in the retina and optic nerve increased after optic nerve crush.Then, we established a heterozygous knockout (Srgap2+/–) mouse model of optic nerve crush and found thatSrgap2suppression increased retinal ganglion cell survival, lowered intraocular pressure, inhibited glial cell activation, and partially restored retinal function.In vitroexperiments showed that Srgap2suppression activated the mammalian target of rapamycin signaling pathway.RNA sequencing results showed that the expression of small heat shock protein genes (Cryaa, Cryba4, andCrygs) related to optic nerve injury were upregulated in the retina of Srgap2+/–mice.These results suggest that Srgap2suppression reduced the robust activation of glial cells, activated the mammalian target of rapamycin signaling pathway related to nerve protein, increased the expression of small heat shock protein genes, inhibited the degeneration of retinal ganglion cells, and partially restored optic nerve function.
Key Words:astrocyte; crystallins; intraocular hypertension; microglia; mTOR; neuroprotection; optic nerve crush; optic neuropathy; RGC degeneration;Srgap2
Introduction
Glaucoma and other optic neuropathies are characterized by progressive degeneration of retinal ganglion cells (RGCs), resulting in visual field defects and, if not treated, irreversible blindness (Venkataraman et al., 2010; Yu-Wai-Man et al., 2011; Almasieh et al., 2012; Weinreb et al., 2014; Chen et al., 2017; Choi et al., 2017).It has been estimated that millions of people will suffer from glaucoma by 2040 (Tham et al., 2014).However, the mechanism behind glaucoma is not fully understood, and finding the factors that influence RGC survival is of great importance.
The Slit-Robo signaling pathway is involved in many physiologic and pathologic processes, such as organogenesis, neurodegeneration, and angiogenesis.As the downstream signaling molecules, Slit-Robo Rho GTPase-activating proteins(SRGAPs) play important roles in axonal guidance, neuronal migration,spine formation and nerve regeneration (Wong et al., 2001; Whitford et al.,2002; Lucas and Hardin, 2017).SRGAP2encodes a highly conserved protein that is specifically duplicated in human lineage (SRGAP2A,2B,2Cand2D),implying that it may play significant roles in human evolution (Sudmant et al., 2010; Dennis et al., 2012; Sporny et al., 2017).The humanSRGAP2Ahas 98% identity to its mouse ortholog, and inhibition of SRGAP2 during spine maturation induces neoteny (Charrier et al., 2012).Human-specific paralogSRGAP2AandSRGAP2Ccoregulate excitatory and inhibitory synapses in cortical pyramidal neurons (Fossati et al., 2016).Several studies have shown that SRGAP2 promotes neurite initiation and branching, and negatively influences neuronal migration (Soderling et al., 2007; Guerrier et al., 2009).Interestingly, SRGAP2A promotes synaptic maturation and limits synaptic density, and its identified functions were inhibited by SRGAP2C, suggesting that duplication ofSRGAP2might contribute to maintaining the excitation/inhibition balance in humans (Subramanian and Nedivi, 2016).A previous study has also reported an association between aSRGAP2mutation and early infantile epileptic encephalopathy (Saitsu et al., 2012).Slit-Robo signaling plays an important role in regulating axon guidance in the central nervous system and RGCs, implying that Slit-Robo signaling may be involved in optic nerve injury.For this reason, we investigated the function ofSrgap2in optic neuropathic disease models.
The optic nerve crush (ONC) model is frequently used for optic nerve or RGC injury studies (Berkelaar et al., 1994; Fischer et al., 2004; Liu et al., 2014) to investigate the molecular events underlying RGC degeneration in optic nerve injury and traumatic optic neuropathy (Agudo et al., 2008; Agudo-Barriuso et al., 2013; Galindo-Romero et al., 2013; Gramlich et al., 2014; Sánchez-Migallón et al., 2016; Williams et al., 2020; Lucas-Ruiz et al., 2021).Elevated intraocular pressure (IOP) is one of the risk factors for glaucoma (Joos et al.,2010; Templeton and Geisert, 2012; Pang, 2021).A recent study established a chronic ocular hypertension model by silicone oil (SO) injection that causes stable and sustained IOP elevation with glaucomatous neurodegeneration(Zhang et al., 2019).Srgap2inhibition increased the expression of the downstream proteins Ras-related C3 botulinum toxin substrate 1 (Rac1) and cell division cycle 42 (Cdc42) and activated the phosphatidylinositol 3-kinaseserine-protein kinase B-mammalian target of rapamycin (PI3K-Akt-mTOR)signaling pathway.We think that this is the mechanism by whichSrgap2suppression had neuroprotective effects on RGCs and partially restored retinal function.
Alpha-crystallins belong to the superfamily of small heat-shock proteins(sHSPs) in the retina.The sHSPs and related crystallins are strongly induced by a variety of stresses, and act as molecular chaperones resulting in protecting retinal cells from damage (Basha et al., 2012; Kannan et al., 2012; Piri et al.,2016).In this research, we aimed to investigate the expression and function of SRGAP2 after ONC inSrgap2knockdown mice, and explored the relevant mechanismsin vivoandin vitro.
Methods
Animals
All animal studies were conducted according to protocols approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University on March 4, 2022 (approval No.XMSQ (Zhe) 2022-0256) and were in accordance with the Association for Research in Vision and Ophthalmology(ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research(Association for Research in Vision and Ophthalmology, 2021).Srgap2+/–(heterozygote knockout, HET) mice and littermate C57BL/6J mice were used for all experiments.Srgap2gene deficiency mice were generated by Cyagen(Suzhou, China; license No.SCXK (Yue) 2018-0032) using clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) gene-editing technology and were genotyped using the following primers provided by Cyagen: 5′-ATT TCT CAT TTG GGT TCC TGG CC-3′, 5′-GCA CTC ACC TCA ACG TCA AAT TAC CT-3′ and 5′-GTG GTC AGA CAG ACA GCT CTA AAT ATG C-3′.Mice were housed at 21°C and 55% humidity under a 12-hour light/dark cycle (20 lx), provided food and waterad libitumand were under constant supervision from trained staff, with up to six animals in each cage.Sex- and age-matched C57BL/6J mice were used as controls in this experiment.In this study, 164 wild-type (WT) mice and 113Srgap2+/–mice aged 2–4 months (20–26 g) with sexes evenly divided were used.For all experiments, experimenters and observers were blinded to group assignment and outcome assessment.The animals used in each experiment were listed in
Additional Figure 1A.
ONC
Mice were intraperitoneally anesthetized with 30 mg/kg sodium pentobarbital(P3761, Sigma, Darmstadt, Germany) and 5 mg/kg xylazine (Aladdin, Shanghai,China), and topical anesthesia with 0.5% proparacaine hydrochloride (Alcon Laboratories, Fort Worth, TX, USA) was applied before operation.ONC was completed as previously described (Chi et al., 2019).Incision of the superior and lateral conjunctiva was performed to expose the optic nerve,and the optic nerve was crushed 2 mm behind the posterior eye with fine forceps (RWD Life Science, F11020-11) for 10 seconds without damaging the blood supply.During the entire procedure, the animals were maintained at normal body temperature until they woke up.To prevent possible infection,levofloxacin eye drops and ofloxacin oculentum were applied to the treated eye.Animals were harvested at 3, 7 and 14 days following surgery.
SO injection
SO injection on both sides was performed as previously described (Zhang et al., 2019).After anesthetization, eye drops of 0.5% proparacaine hydrochloride (Alcon Laboratories, Fort Worth, TX, USA) were applied once to the mouse cornea.An acupuncture needle was inserted across the sclera near the limbus to reach the anterior chamber without damaging the surrounding tissue.Then, 2 μL of SO (RTSIL-OL5000, Carl Zeiss, Jena, Germany) was slowly injected into the anterior chamber by a sterile glass micropipette.The same volume of normal saline was injected into the anterior chamber of the control group.Artificial tears were applied to the cornea to keep it moist throughout the entire process.After the injection, ofloxacin eye ointment(Sinqi Pharmaceutical Co., Ltd., Shenyang, China) was applied to the ocular surface every day for a week.IOP was measured using a rebound tonometer(SW-500, SUOER, Tianjin, China) in the morning; every measurement was repeated three times and averaged for the final result.The few mice that had complications, such as corneal neovascularization, or those that did not achieve significant ocular hypertension were excluded from the study.
Optical coherence tomography
Mice were anesthetized as described above and one drop of 1% tropicamide ophthalmic solution (Santen Pharmaceutical Co., Ltd., Osaka, Japan) was administered to the eyes before examination.Optical coherence tomography images were captured using Micron IV (Phoenix Research Labs, Pleasanton,CA, USA).Cross-sectional images of the retina in the optic nerve head were then taken.Retinal thickness was measured at the same position of approximately 200 μm from the optic disc.
Quantitative optomotor response
The quantitative optomotor response (qOMR) instrument (PhenoSys qOMR,PhENOSYS GmbH, Berlin, Germany) was made with four screens in a box,and the mice were placed on a platform.The screens displayed moving grids creating virtual cylinders at different frequencies (0.012 to 0.5 cycles/degree).Video-tracking was used to quantify body movements from the top of the box using the center of mass movement speed and the angular body velocity derived from linear regression.Clockwise rotation of the grid and tracking represents the left eye, whereas counterclockwise rotation and tracking represents the right eye.The experiment was carried out between 9 a.m.to 5 p.m.
Immunofluorescence staining
The immunofluorescence staining was performed as previously described(Akopian et al., 2014).Mice were anesthetized as described above and sacrificed by cervical dislocation.After fixation in 4% paraformaldehyde for 30 minutes at 26°C, the eyes were immersed in 30% sucrose overnight and then embedded in optimal cutting temperature compound (Cat# 4583; Sakura,Oakland, CA, USA).The frozen specimens were sectioned at 10 μm thickness on a glass slide.Sections were blocked with 0.5% bovine serum albumin and 0.1% Triton X-100 for 1 hour at 26°C and incubated with primary antibodies overnight at 4°C.After washing, slides were incubated with secondary antibodies conjugated with Alexa Fluor 488/594 for 1 hour at 26°C and 4′,6-diamidino-2-phenylindole (C1002, Beyotime, Shanghai, China) at 26°C for 1 hour.All antibody information was shown in Table 1.After washing, slides were mounted in Vectashield media (P0126, Beyotime).Immunolabeled images were captured using an LSM 880 confocal microscope (Carl Zeiss, Jena,Germany).For RGC counting using anti-RNA-binding protein with multiple splicing (RBPMS), sections were selected across the optic nerve, and RBPMSpositive RGCs were counted from one ora serrata to the other.
Hematoxylin and eosin staining
The eyes were enucleated and fixed with Davidson’s solution (37.5% ethanol,9.3% paraformaldehyde, and 12.5% acetic acid) for 24 hours at 26°C.Fixed eyes were embedded in paraffin.Specimens were cut into 5-mm-thick retinal cross-sections, and all the paraffin tissue sections were dewaxed, rehydrated and stained with hematoxylin and eosin.Sections were photographed using a light microscope (Leica, Wetzlar, Germany).A cell nucleus represents one cell in the ganglion cell layer (GCL).
Transmission electron microscopy
Eyeballs were fixed in 2.5% glutaraldehyde overnight, and then retinal tissue was fixed in 1% osmic acid for 1 hour at 37°C in the dark.After rinsing in 1×phosphate buffered saline three times and in 1% uranyl acetate for 1 hour at 37°C, tissues were dehydrated in a graded series of acetone and embedded in Epon-Araldite.The sections were cut at 4 μm thickness and incubated with 1% toluidine blue.Ultra-thin sections (90 nm) were further cut using an ultramicrotome and examined with a transmission electron microscope(Hitachi-7500, Hitachi, Tokyo, Japan).
Cell culture and transfection
PC12, one of the most commonly used cell lines in neuroscience research(You et al., 2022), was purchased from CHI Scientific (Jiangyin, China;RRID: CVCL_0481).The experiments of PC12 cells were used within 15 passages.Cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco)supplemented with 10% inactivated fetal bovine serum (Gibco), penicillin(100 IU/mL) and streptomycin (100 mg/mL) in a CO2incubator (Thermo Fisher Scientific).Subconfluence cells were digested with 0.25% trypsin and passaged at a ratio of 1:8.Cells at 60% confluency were transfected with 20 nM ofSrgap2small interfering RNA (siRNA) for 4–6 hours using Lipofectamine RNAiMax Reagent (Thermo Fisher Scientific) and harvested at 48 hours and 72 hours after transfection for RNA analysis and protein analysis, respectively.
Quantitative reverse transcription-polymerase chain reaction
Total RNA of retinal tissue and optic nerve or cultured PC12 cells was extracted by E.Z.N.A.® Total RNA Kit II (Omega Bio-Tek, Norcross, GA, USA)and reverse transcribed into complementary DNA (cDNA) using GoScriptTMReverse Transcription System (Promega, Madison, WI, USA) according to the manufacturer’s instructions.The quantitative polymerase chain reaction(qPCR) was conducted on QuantStudio 5 (Applied Biosystems, Foster, CA, USA)with iTaqTMUniversal SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA)and the data were analyzed by 2–∆∆CTmethod (Livak and Schmittgen, 2001).Relative mRNA expression levels were normalized with the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase.Primer sequences were shown in Table 2.
RNA sequencing
Total RNA extracted with E.Z.N.A.® Total RNA Kit II from WT andSrgap2+/–mouse retina was submitted to Novogene (Beijing, China).A Bioanalyzer 2100(Agilent Technologies, Palo Alto, CA, USA) was used to assess RNA integrity using the RNA Nano 6000 Assay Kit (Agilent Technologies).Following the manufacturer’s instructions, 3 g/sample RNA was applied for generating cDNA libraries using NEBNext® UltraTM Directional RNA Library Prep Kit(NEB, Ipswich, MA, USA).The library fragments of 250–300 bp in length were purified with AMPure XP system (Beckman Coulter, Brea, CA, USA).An index of the reference genome was built and paired-end clean reads were aligned to the reference genome using Hisat2 v2.0.5 (University of Texas Southwestern Medical Center, Dallas, TX, USA).A transcript was considered differentially expressed if itsP-adjustment was less than 0.05.
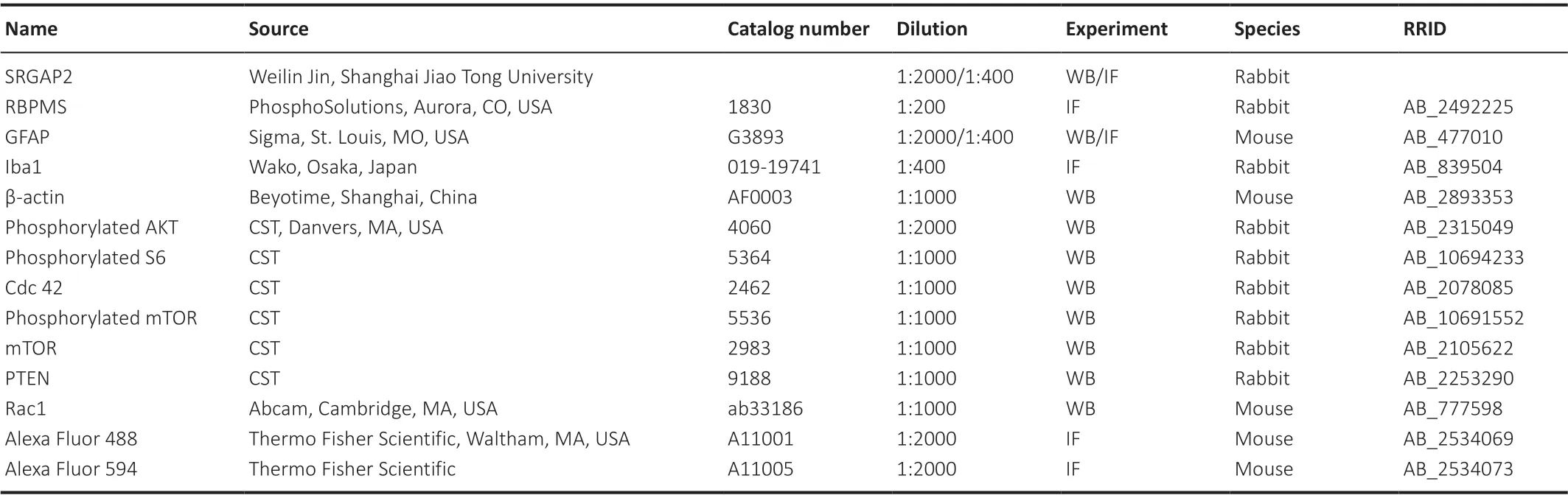
Table 1 |Antibody used in this study
Results

Table 2 |Primer sequences used in quantitative reverse transcription-polymerasechain reaction
Gene Ontology
The roles of the identified key genes were analyzed according to the functions of their regulated genes.These genes were submitted to Gene Ontology (GO) (http://www.geneontology.org/) analysis by ClusterProfiler R (https://bioconductor.org/packages/release/bioc/html/clusterProfiler.html) to identify enrichments in biological process, molecular function,and cellular component terms (Harris et al., 2004; Yu et al., 2012).Twosided Fisher’s exact test with false discovery rate correction was used to calculate thePvalue of each term, and those withP< 0.05 were considered statistically significant.
Western blot assay
Proteins were extracted by RIPA lysis and extraction buffer (P0013B,Beyotime) from retina at 14 days after ONC for glial fibrillary acidic protein and 7 days after ONC for mTOR signaling pathway and from PC12 cells at 72 hours after siRNA transfection.Concentrations were determined by bicinchoninic acid protein assay kit (Thermo Fisher Scientific).Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred to polyvinylidene difluoride membranes.Membranes were blocked with 5% skim milk containing 0.5% Tween-20 in Tris-buffered saline for 1 hour at 26°C and then incubated with primary antibodies overnight at 4°C.After washes with 0.5% Tween-20 in Tris-buffered saline, the membranes were incubated with secondary antibodies conjugated with horseradish peroxidase for 1 hour at 26°C and the images were captured using a ChemiDoc imaging system (Bio-Rad).The bands were analyzed using ImageJ software (Version 1.8.0).Antibody information is shown inTable 1.
Statistical analysis
No statistical methods were used to predetermine sample sizes; however,our sample sizes are similar to those reported in previous publications (Liu et al., 2014, 2022).No animals or data points were excluded from the analysis.All data were analyzed with the evaluator blinded to grouping and plotted using GraphPad Prism version 6.01 software (GraphPad, San Diego, CA, USA,www.graphpad.com).The data are presented as mean ± standard error of the mean.Two-tailed Student’st-test was used to compare the differences between groups.Differences were considered significant atP< 0.05.
Srgap2expression in the developmental retina and optic nerve injury modelSlit/Robo signaling has been shown to be involved in the regulation of RGC axon guidance (Erskine et al., 2000).We found increased expression of Slit2/Robo1 in the retina, and decreased expression in the optic nerve after ONC(Additional Figure 1B–E).As the downstream molecule of Slit/Robo signaling,SRGAP2 plays significant roles in the central nervous system, but its function is unclear in normal neuroretina and optic neuropathies (Fossati et al., 2016).Therefore, we first determined the expression pattern ofSrgap2during the developmental stage of retina.Immunofluorescence staining showed SRGAP2 protein expression in embryonic day 14.5, postnatal day 0 (P0), P5, P25,and adult mice, with distribution particularly in the retinal nerve fiber layer,GCL, and inner nuclear layer during retinal development (Figure 1A).qPCR confirmed thatSrgap2was expressed from E14.5 to adult retina (Figure 1B).Immunohistochemistry showed that SRGAP2 protein level was higher in the retina and optic nerve 7 days after ONC compared with that in the control group (Figure 1CandD).Srgap2mRNA levels also were increased 7 to 14 days after ONC (Figure 1E).
Srgap2gene knockout leads to embryonic mortality
To explore the role ofSrgap2, we establishedSrgap2knockout mice using CRISPR/Cas9 technology.The gene knockout strategy and gene identification are shown inAdditional Figure 2AandB.SRGAP2 protein levels inSrgap2+/–retina were approximately 50% of the levels in WT mice (Additional Figure
2CandD).No homozygote mice were obtained in this study.Therefore, we analyzed the birth rate of a total of 718 mice from WT × HET and HET × HET breeding as shown inAdditional Figure 2E.The average number of pups per litter was 4.25 ± 0.16 in the HET × HET breed, which was approximately 25%less than in WT × HET with an average of 5.82 ± 0.37 (Additional Figure 2F).Moreover, the ratio of WT and HET was about 1:2 in HET × HET breeding(Additional Figure 2G).These results suggest that homozygousSrgap2–/–mice may be embryonic lethal.Thus, we used HET mice (Srgap2+/–) in the present study.
Srgap2suppression improves RGC survival after ONC
Progressive RGC loss was observed from 5–7 days after ONC.Histological analysis did not show abnormality of the retina inSrgap2+/–mice.However,after ONC,Srgap2+/–mice had increased RGC survival compared with that in WT (Figure 2AandB).RBPMS is widely used for specific labeling of RGCs(Rodriguez et al., 2014).Similar to the results of the histological analysis, there was no difference in RBPMS-positive RGCs in the uninjured retina between WT andSrgap2+/–mice, whereas more RBPMS-positive RGCs were observed inSrgap2+/–mice than in the WT group after ONC (Figure 2CandD).These results were further verified using immunostaining with antibodies against β-III tubulin (Tuj1), another neuronal marker (Zhou et al., 2021;Additional
Figure 3).Transmission electron microscopy of uninjured optic nerve showed that the cytoplasm and axoplasm in the optic nerve bundle were filled with organelles, whereas ONC models had a thinner myelin sheath, swelling and vacuolization of the cytoplasm and axoplasm 14 days after ONC.These pathologic features were less apparent inSrgap2+/–mice compared with WT mice, as shown inFigure 2E.
ONC reduced the retinal thickness in mice.We used optical coherence tomography to compare retinal thickness in WT andSrgap2+/–micein vivoand found that the total thickness, ganglion cell complex (GCC) thickness and inner thickness were thicker inSrgap2+/–mice than in WT mice 7 days post-ONC.No significant difference was observed in the thickness of the outer retinal layer between the groups (Figure 2FandG).We concluded thatSrgap2suppression provided a neuroprotective effect in the injured retina and optic nervein vivo.
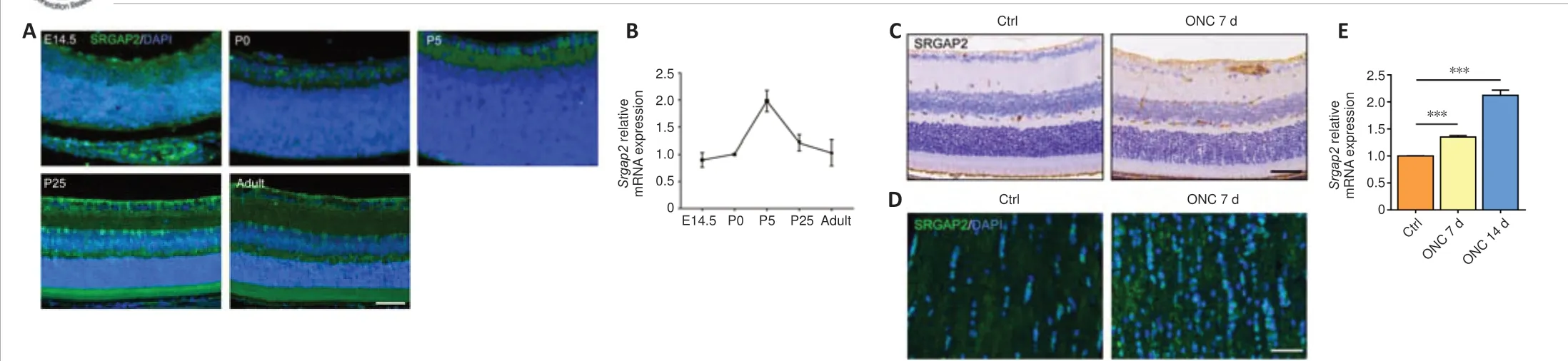
Figure 1|Srgap2expression in the developmental retina and optic nerve injury mice.
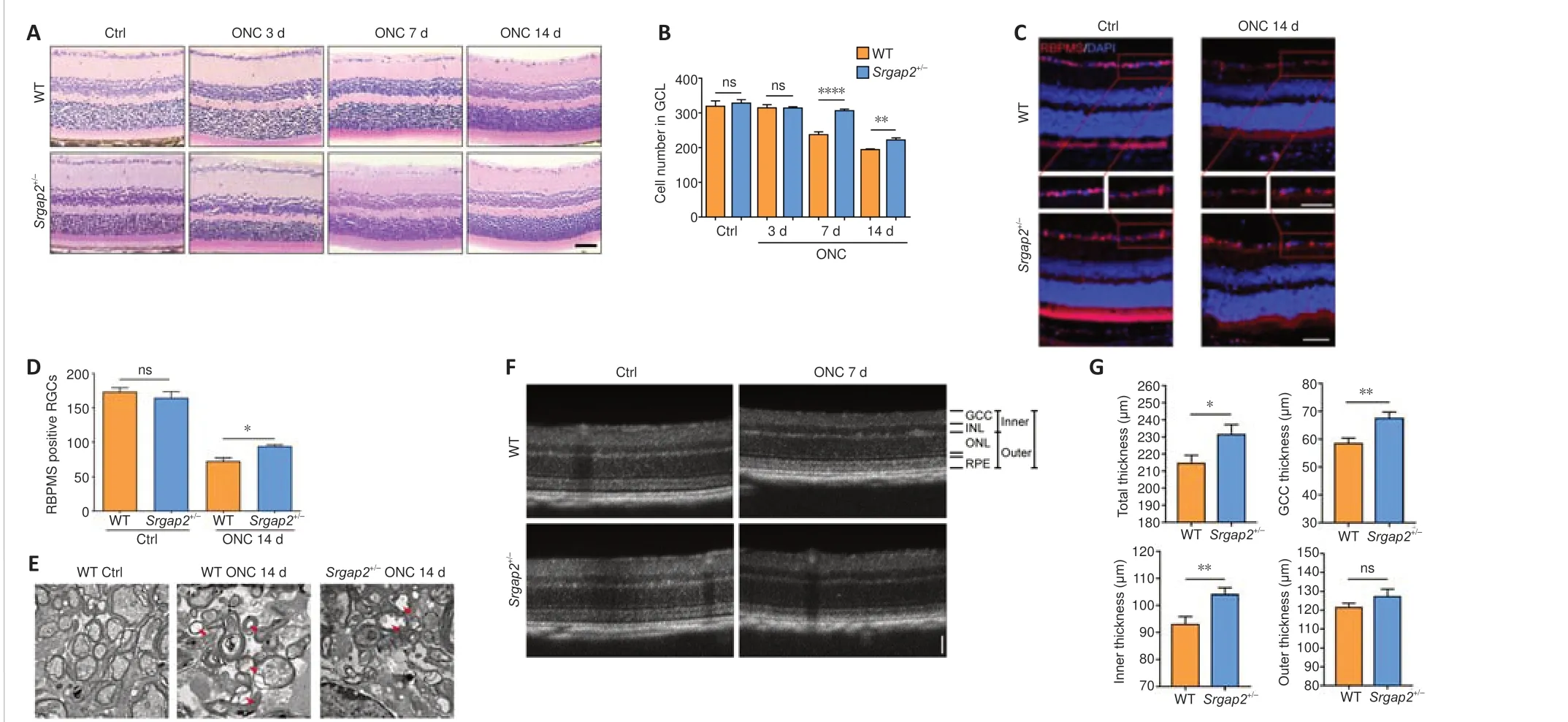
Figure 2|RGC survival after ONC in Srgap2+/–mice.
Srgap2suppression ameliorates glial activation in the retina after ONC
It was reported that robust activation of Müller glia and microglia was occurred after ONC (Au and Ma, 2022).Immunofluorescence staining of glial fibrillary acidic protein, a specific marker of astrocytes and activated Müller cells (Bulirsch et al., 2022), showed positive labeling only in the GCL in the uninjured retinas associated with non-activated astrocytes.However, there was robust activation of astrocytes in the GCL and Müller cells throughout the retina after ONC.Glial activation was inhibited significantly inSrgap2+/–mice compared with WT (Figure 3A).Furthermore, activation of Iba1-positive microglia in the retina after ONC was inhibited inSrgap2+/–mice (Figure 3B).We confirmed these results using western blot with antibodies against glial fibrillary acidic protein (Figure 3CandD).Quantification of Iba1-positive microglia also showed inhibition in the retina after ONC in Srgap2+/–mice(Figure 3E).These findings indicate thatSrgap2suppression ameliorated glial activation after optic nerve injury.
Srgap2suppression improves RGC survival in chronic ocular hypertension
We used a second RGC injury model to verify the involvement of Srgap2 in RGC protection.SO was injected into the mouse anterior chamber, which leads to a sustained increase in IOP, resulting in chronic RGC damage (Zhang et al., 2019).IOP was elevated to 30 mmHg from 12 mmHg at baseline after SO injection and stayed at this level for 6 weeks (Figure 4AandB).Optical coherence tomography scanning showed that the anterior chamber angle was closed after SO injection (Figure 4C).Similar to the ONC results, more RGCs survived inSrgap2+/–mice compared with WT mice 6 weeks after SO injection,observed with both hematoxylin and eosin and immunofluorescence staining(Figure 4D–G).These results indicated that suppression ofSrgap2may play a protective role during neuroretinal damage.
Srgap2 suppression relieves the impaired retinal function after ONC
To assess retinal function, we performed qOMR behavioral analysis to monitor visual function in control andSrgap2+/–mice before and after SO injection(Figure 5A).In spatial frequency ranging from 0.012 to 0.5 cycles/degree,the optomotor index had a maximum value of approximately 1.5 in 0.05, 0.1 and 0.2 cycles/degree in both WT andSrgap2+/–groups, with no significant difference between the groups (Figure 5B).The maximum optomotor index decreased to 1.1 in WT mice, which was significantly less than the value of 1.3 inSrgap2+/–mice 3 weeks after SO injection (Figure 5C).The result suggests thatSrgap2+/–mice had improved visual function preservation compared with WT mice, and thus thatSrgap2suppression provided a protective role in IOPinduced visual loss.In the ONC model, the qOMR index quickly decreased to flat (at about 1) at all spatial frequencies in both groups (data not shown), so we did not compare qOMR in the ONC model.
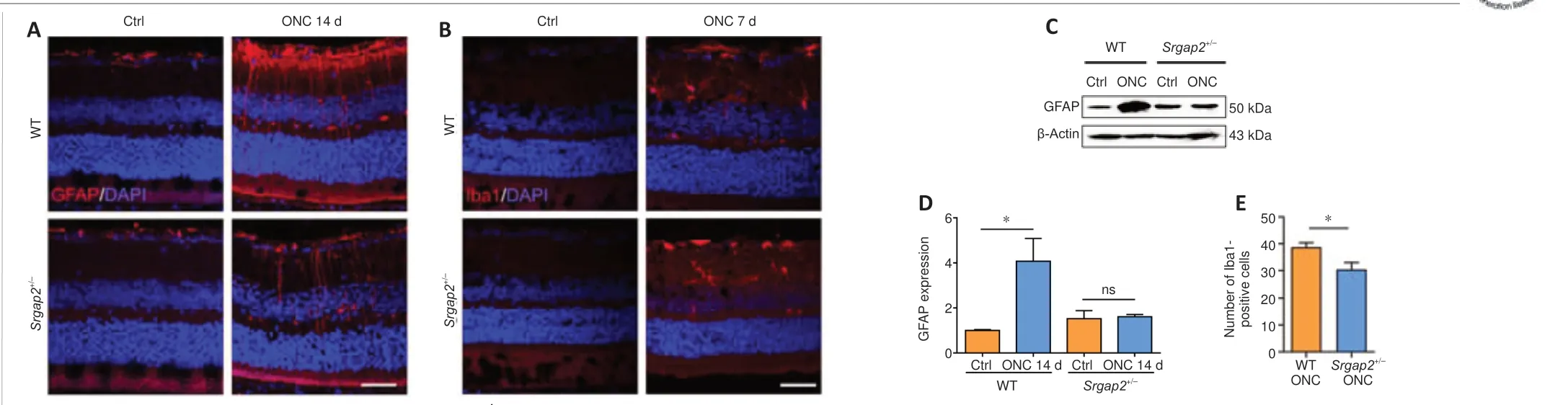
Figure 3|Glial cell distribution after ONC in Srgap2+/–mice.

Figure 4|RGC survival after SO injection in Srgap2+/–mice.
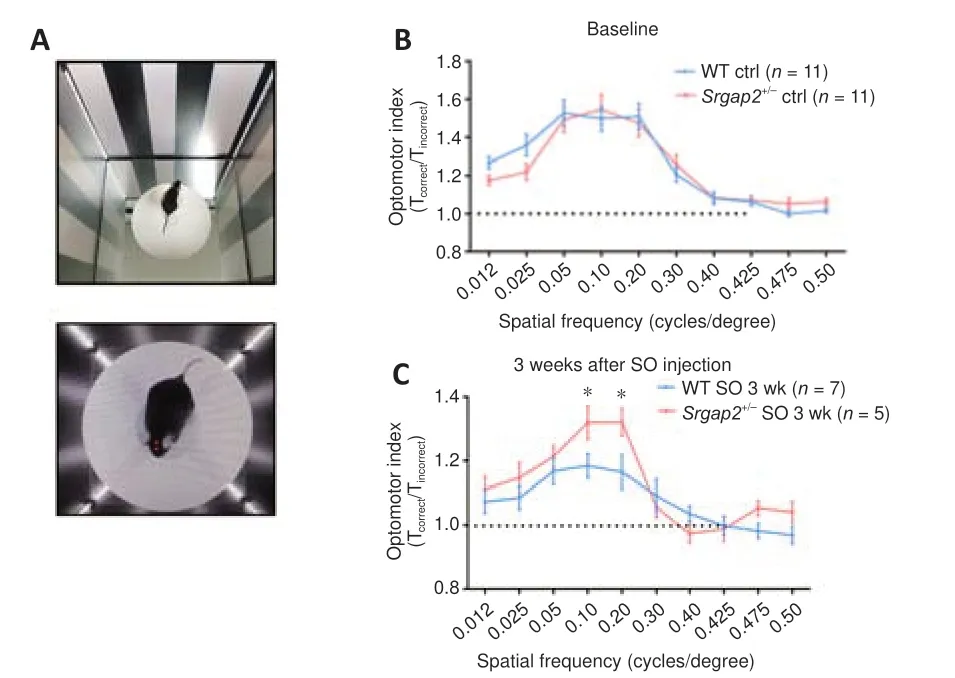
Figure 5|Visual function of WT and Srgap2+/–mice.
Srgap2suppression activates the mTOR signaling pathway after ONC
The above results indicated thatSrgap2+/–mice demonstrate neuroprotective characteristics after optic nerve injury.To identify the molecular mechanisms underlying the neuroprotection, we knocked downSrgap2in PC12 cells using RNA interference.We initially observed SRGAP2 expression in PC12 cells by immunofluorescence staining (Figure 6A).After siRNA transfection,SRGAP2 expression was reduced to about 20% of the control level in PC12.In addition, SRGAP2 suppression increased the expression levels of Cdc42 and Rac1 (Figure 6BandC), which belong to the Rho GTPase family and are downstream proteins of SRGAPs (Wong et al., 2001) that play an important role in neurotoxicity.
PI3K/AKT/mTOR signaling pathways are important for neuroprotection,and phosphatase and tensin homolog deleted on chromosome ten (PTEN)negatively regulates this pathway (Cuenca et al., 2014).We found significantly increased levels of phosphorylated AKT (p-AKT) and p-mTOR/mTOR, and increased phosphorylation of the downstream protein ribosomal s6 kinase(pS6) afterSrgap2siRNA transfection, suggesting activation of the mTOR signaling pathway (Figure 6DandE).Furthermore, PTEN expression was decreased inSrgap2+/–retina compared with WT retina after ONC (Figure 6F),and pS6 expression was increased inSrgap2+/–retina after ONC (Figure 6G).These findings indicate that Srgap2 suppression may protect the neuronal retina from damage through activation of the mTOR signaling pathway.
Srgap2suppression increases crystallins expression

Figure 6|Downstream signaling pathways after inhibition ofSrgap2.
To further understand the molecular mechanisms ofSrgap2, we conducted high-throughput RNA sequencing using neuroretinal tissue ofSrgap2+/–and WT mice.RNA sequencing and bioinformatical analysis identified a total of 446 genes differentially expressed betweenSrgap2+/–and WT mice, including 214 upregulated genes and 232 downregulated genes.GO analysis showed thatSrgap2suppression mainly affected processes such as signal transduction, regulation of apoptotic process, cell migration, and eye development (Figure 7A).It is worth noting that all of these biological processes are closely related to neurogenesis and neuroprotection.Figure 7B shows the genes that were associated with optic neuropathy or retinal development.Among the differentially expressed genes, we focused on the crystallin-related genes, which were previously reported to play significant roles in retinal neuroprotection and axonal regeneration (Piri et al., 2016).We verified the expression changes ofCryaa,Cryba4andCrygs.RT-qPCR confirmed increased expression of several crystallin genes in the retina after ONC, with much higher upregulation inSrgap2+/–mice than in WT mice (Figure 7C).Additionally, we confirmed the downregulation ofCxcl5, a chemokine that is involved in inflammatory and cellular responses.
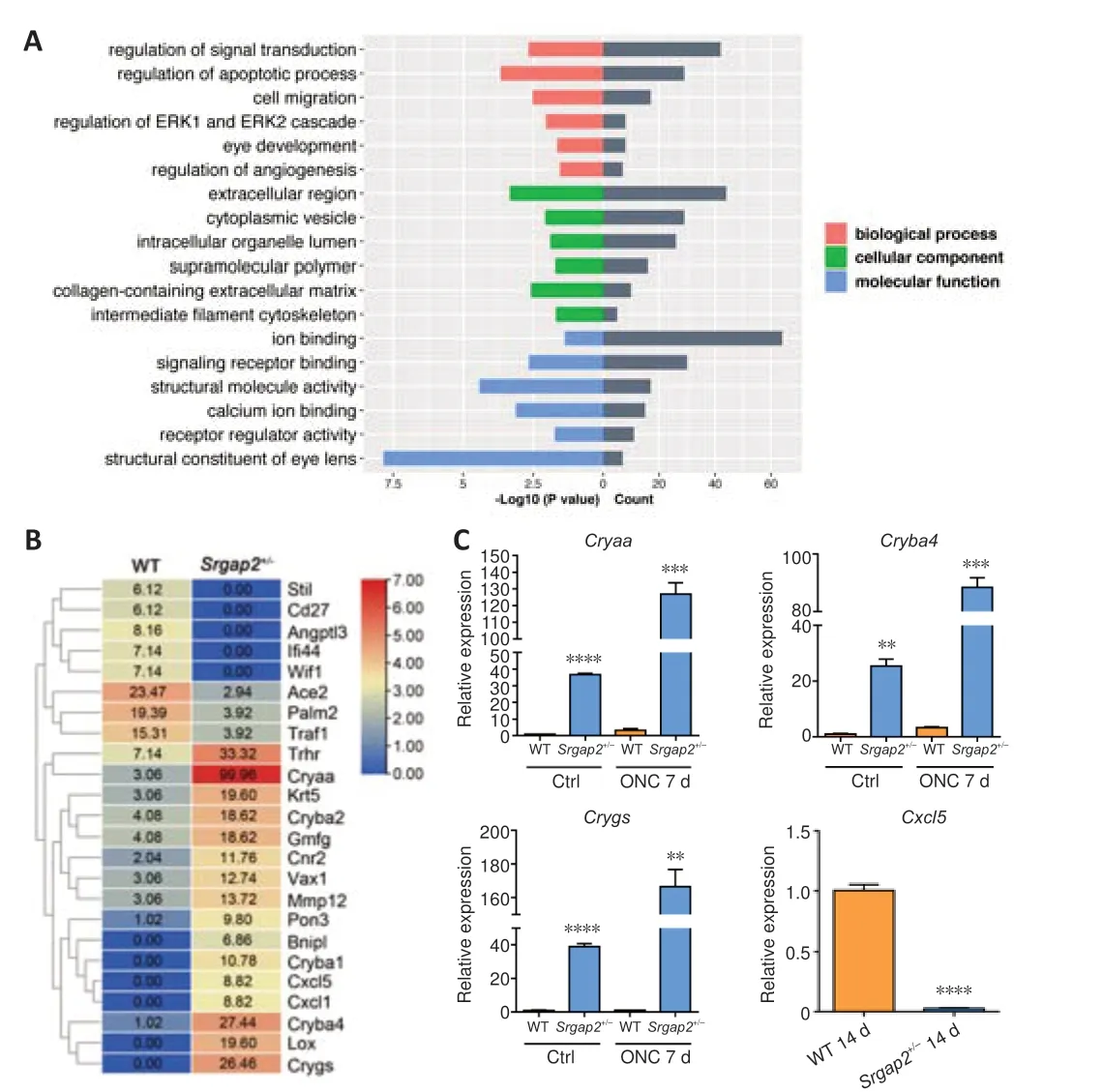
Figure 7|Gene regulation of retina in Srgap2+/–mice.
Discussion
Human-specific neuralSrgap2Ais orthologous to murineSrgap2.A previous study demonstrated that SRGAP2C dimerizes with SRGAP2A and inhibits its function in humans, which induces neoteny during spine maturation (Charrier et al., 2012).Mice expressing SRGAP2C display human-specific features and have sustained radial migration, demonstrating that suppression of SRGAP2 function is crucial for human brain development (Charrier et al., 2012).
In this study, we tried to generateSrgap2knockout mice and obtained only heterozygote but not homozygote mice through long-term breeding.Through the birth ratio analysis of 718 mice, we found that no homozygote mice were born, likely because of embryonic lethality.A previous study reported that the birth ratio of homozygousSrgap2knockout mice was significantly lower than the Mendelian ratio, and the homozygous mice had incomplete deletion of the gene with an approximately 90% reduction (Charrier et al.,2012).Another study reported thatSrgap2homozygosity caused early death owing to congenital heart disease and skeletal dysplasia (Armes et al., 2018).Furthermore, neonatal lethality was reported in global Slit2 and Robo2-knockout animal models (Rama et al., 2015).Taken together, the findings suggest thatSrgap2expression has a dynamic balance in different biological processes, where a high expression level promotes neuronal differentiation and maturation and a low expression level facilitates neural migration during brain development, implying thatSrgap2plays an important role in the nervous system.Conditional knockout or tamoxifen-induced gene deletion approaches may be beneficial to investigate in future studies.
Currently, optic neuropathy and vision loss cannot be reverted or stopped by either pharmacological or surgical interventions.In the present study,Srgap2gene expression was observed as early as E14.5 in developing neural retina.Its expression increased in development and reached a plateau during adulthood, especially in the GCL and inner nuclear layer.GO analysis supported thatSrgap2is closely involved in development, cell migration, and apoptotic processes.SRGAP2 protein levels increased markedly in retina and optic nerve after ONC, suggesting that it may be involved in optic nerve injury.Thus, we explored the pathological role ofSrgap2in RGC degeneration.
ONC is a well-recognized experimental disease model for traumatic or glaucomatous optic neuropathy, which causes damage to the axons across the optic nerve while keeping the dura and surrounding blood supply intact(Williams et al., 2020).SO injection into the anterior chamber is a recently developed model, which leads to ocular hypertension with chronic RGC loss and optic nerve degeneration (Zhang et al., 2019).In this study, we used both acute and chronic optic neuropathy models to investigate the role of SRGAP2in vivo.Both ONC and SO injection led to robust RGC death and optic nerve degeneration in WT mice, whereas theSrgap2+/–mice showed RGC protection and milder pathological changes, indicating that suppression of Srgap2 has a neuroprotective effect in optic neuropathy.RGC degeneration and axonal regeneration are related to processes such as RGC apoptosis (Maes et al., 2017; Gokoffski et al., 2020), the lack of neuronal regenerative capacity in mature central nervous system (Goldberg et al.,2002; Goldberg, 2004; Sun and He, 2010; Vignoles et al., 2019), inhibitory molecules located in myelin (Schwab, 2004; Ghorbani and Yong, 2021),inhibitory glial scarring at the injured site (Silver and Miller, 2004; Yiu and He,2006; Chen and Li, 2022), and activation of macrophages accumulating at damaged central nervous system tissues (Horn et al., 2008; Busch et al., 2009;Mesquida-Veny et al., 2021).We found thatSrgap2suppression modulated RGC survival and provided neuronal protection of visual function.Moreover,it inhibited the activation of Müller cells, astrocytes and microglial infiltration after retinal injury.
The PI3K-AKT-mTOR signaling pathway plays a crucial part in neurodevelopment and neuroprotection.Its activity is closely associated with many neurodegenerative diseases.In this study, we demonstrated that inhibition of Srgap2 promoted mTOR pathway activationin vitroandin vivo.Rac1 and Cdc42 are downstream molecules of SRGAP2 in the Slit-Robo pathway, and it was previously reported that they were related to cell migration (Wong et al., 2001).SRGAP2 has specific GAP activity towards Rac1(Guerrier et al., 2009; Fossati et al., 2016), and SRGAP2 limits the duration of the tip-restricted Rac1 activity in regulation of contact protrusion collapse(Fritz et al., 2015).Previous studies have shown that activation of Rac1 and Cdc42 causes the actin cytoskeleton to reorganize into different structures(Govek et al., 2005).In addition, activated Ras and PI3K-induced neurite growth is Rac1- and Cdc42-dependent, and activated Cdc42-induced neurite growth is dependent on Rac1 activation (Sarner et al., 2000).Cdc42 activity is especially important for growth cone morphology and function, and is responsible for the formation of filopodia that senses the environment and orients growth (Brown et al., 2000; Chen et al., 2012).Our study also confirmed that inhibition of SRGAP2 increased the expression of Rac1 and Cdc42 proteins.
To further understand the molecular mechanisms of SRGAP2, we compared gene expression changes in the retina ofSrgap2+/–and WT mice by high throughput sequencing.Interestingly, we found that the crystallins, includingCryaa,Cryba4andCrygs, were significantly increased inSrgap2+/–mice without optic nerve injury and were further increased after ONC compared with WT mice.Mammalian crystallins are heterogeneous proteins including three main families: alpha, beta, and gamma crystalline, and play significant roles in RGC survival and axonal regeneration (Thanos et al., 2014; Liu et al., 2022).The alpha-crystallins primarily expressed in the GCL and the RGC protective effect were involved in the inhibition of microglial activation and inflammatory cytokines release (Piri et al., 2016).We also showed a significant reduction ofCxcl-5expression, a proinflammatory chemokine, in the retina ofSrgap2+/–mice.sHSPs are related to their chaperone functions, exhibit a robust anti-apoptotic property and are involved in many neurodegenerative diseases (Thanos et al., 2014; Selig et al., 2020; Webster et al., 2020;Rodriguez Ospina et al., 2022).As known sHSPs, alpha-crystallins protect cells from a variety of stress stimuli by inhibiting multiple pathways of apoptotic cell death, stabilizing the cytoskeleton, preventing protein misfolding,unfolding, or aggregation, reducing inflammation, and disposing of damaged or defective proteins (Piri et al., 2016).
In the present study, we carried out our experiments usingSrgap2heterozygote mice owing to the embryonic lethality of homozygosity.Future investigations should be conducted in a conditional knockout ofSrgap2.The present study used retinal slices for RGC quantification; however, whole mount retinas for RGC counting is more persuasive as it was counted in a more precise and thorough way.We only detected PTEN and pS6 in retinal tissue, and the regulatory relationship betweenSrgap2and the PI3K-AKTmTOR signaling pathway also needs to be further studied.Additionally, the relationship between crystallin-related genes and glial cell activation remains unclear.
In summary, our study suggests thatSrgap2suppression reduces RGC loss through inhibition of glial activation in RGC degeneration models.The crystallins and mTOR signaling pathways may be involved in RGC protection withSrgap2suppression.Srgap2should be further investigated for the treatment of neurodegenerative diseases.
Acknowledgments:The authors appreciate Dr.Stanislav Tomarev (National Eye Institute, National Institutes of Health) for kindly correction of manuscript,Prof.Wei-Lin Jin (Shanghai Jiao Tong University) for providing the anti-SRGAP2 antibody, and Prof.Jun Zhang (Wenzhou Medical University) for electron microscopy analysis.
Author contributions:Study design: ZLC, JQ; experiment implementation,data collection and analysis: YJG, YC, ZHZ, JZ, GC, LQD, TL, MXS; manuscript draft: YJG, ZLC.All authors reviewed and approved the final version of the manuscript.
Conflicts of interest:The authors declare no competing interests.
Data availability statement:All relevant data are within the paper and its Additional files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long asappropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1:Slit2 and Robo1 expression changes in the retina and optic nerve after ONC.
Additional Figure 2:Srgap2 knockout mouse generation and breeding.
Additional Figure 3:Immunofluorescence staining of Tuj1 after ONC and SO injection.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain

