Neuroprotective effect of mesenchymal stem cellderived extracellular vesicles on optic nerve injury in chronic ocular hypertension
Fei Yu, Yao Wang, Chang-Quan Huang, Si-Jie Lin, Ru-Xin Gao, Ren-Yi Wu,5,*
AbstractMesenchymal stem cells have neuroprotective effects that limit damage to the retina and photoreceptors, and which may be mediated by extracellular vesicles(or exosomes) released by mesenchymal stem cells.To investigate the neuroprotective effect of extracellular vesicles derived from umbilical cord mesenchymal stem cells on glaucoma, we established rat models of chronic ocular hypertension by injecting conjunctival fibroblasts into the anterior chamber to mimic optic nerve injury caused by glaucoma.One week after injury, extracellular vesicles derived from umbilical cord-derived mesenchymal stem cells were injected into the vitreous cavity.We found that extracellular vesicles derived from mesenchymal stem cells substantially reduced retinal damage, increased the number of retinal ganglion cells, and inhibited the activation of caspase-3.These findings suggest that mesenchymal stem cell-derived extracellular vesicles can help alleviate optic nerve injury caused by chronic ocular hypertension, and this effect is achieved by inhibiting cell apoptosis.
Key Words:animal model; apoptosis; chronic glaucoma; chronic ocular hypertension; extracellular vesicles; mesenchymal stem cells; neuroprotection; rat;retinal ganglion cells; umbilical cord
Introduction
Glaucoma, the leading cause of irreversible blindness worldwide (Quigley, 2011;Weinreb et al., 2014; Lam et al., 2016; Jonas et al., 2017; Stein et al., 2021) is a chronic degenerative optic neuropathy (Doozandeh and Yazdani, 2016; Jonas et al., 2017; Liu et al., 2022).Its early pathological changes include reduction in axon transport, axon loss, and dendritic reconstruction, which eventually lead to retinal ganglion cell (RGC) apoptosis and thinning of the retinal nerve fiber layer, eventually resulting in visual field defect and vision loss (Liu and Margeta, 2019; Mélik Parsadaniantz et al., 2020; Liu and Lee, 2021).In patients with glaucoma, optic nerve protection therapy aims to reduce damage to or to replace RGCs (Liu and Margeta, 2019; Stein et al., 2021).The predominant risk factor for glaucoma occurrence and development is pathologically increased intraocular pressure (IOP), and, at present, the only effective methods for glaucoma treatment are medications, laser, and/or surgery interventions to reduce abnormally high IOP (Quigley, 2011; Zaharia et al., 2022).Although these treatments can effectively control glaucoma progression in some cases,they cannot restore lost vision (Chamling et al., 2016; Fenwick et al., 2020).In recent years, there has been an increased enthusiasm for exploring new therapies to treat or prevent RGC degeneration and death (Almasieh and Levin,2017; Nucci et al., 2018; Khatib and Martin, 2020).
Mesenchymal stem cells (MSCs), which are self-renewable stem cells,originate from the mesoderm.They have a wide range of sources in the body and can be easily isolated and proliferatedin vitro(Uccelli et al., 2008; Ding et al., 2011; Fu et al., 2019).MSCs from bone marrow, fat tissue, and the human umbilical cord (UC) have been reported to have protective effects against injuries to the cornea (Ljubimov and Saghizadeh, 2015; Shojaati et al., 2019),lacrimal gland (Zoukhri, 2010; Lu et al., 2020), retina (Mead and Tomarev,2017; Gaddam et al., 2019; Mathew et al., 2019; da Silva-Junior et al., 2021;Sharma and Jaganathan, 2021), and photoreceptor cells (Soleimannejad et al., 2018; Usategui-Martín et al., 2020; Deng et al., 2021).Stem cell-based retinal cell replacement in the eye is thus a promising approach for triggering neuroprotection and regeneration (Mathew et al., 2017; Li et al., 2021).In our recent study, the intravitreous transplantation of human UC-derived MSCs(UC-MSCs) decreased RGC loss and attenuated retinal injuries in a rat model of chronic ocular hypertension (COH) (Wang et al., 2021), which indicates that MSCs have potential in treating optic neuropathy such as glaucoma.However,relatively low cell integration rate and aberrant growth, among other factors,limit the potential of MSCs in clinical applications (Johnson et al., 2010).
Although not fully understood, it has been reported that the neuroprotective effect of MSCs in some tissues may be at least partially mediated by extracellular vesicles (EVs) released by MSCs (Dai et al., 2007; Gnecchi et al., 2008; Yao et al., 2015).EVs are vesicular particles with double-layer membrane structures containing RNAs and proteins.By using MSC-derived EVs, it is feasible to overcome the limitations and/or complications of stem cell treatment inside the eye, whereby neuroprotection and regeneration could be facilitated by simply by transplanting EVs into target ocular tissues(Mead and Tomarev, 2020).When intravitreally injected, EVs derived from bone marrow MSCs have been found to enhance RGC survival and promote axon regeneration in a model of optic nerve crush (Mead and Tomarev, 2017;Pan et al., 2019; Seyedrazizadeh et al., 2020; Cui et al., 2021), a genetically modified glaucoma DBA/2J mouse model (Mead et al., 2018b), and in rats with glaucoma induced through either the injection of microbeads into the anterior chamber, or photocoagulation of the circumferential limbal vessels by laser (Mead and Tomarev, 2017; Mead et al., 2018a).The UC is considered to be one of the best sources of MSCs, and, compared with bone marrowderived MSCs, its collection is noninvasive and effortless, and the cells from the UC are more capable of growth and prolific (Reyhani et al., 2020).The neuroprotective effect of EVs derived from UC-MSCs, an ideal source of EVs,on optic nerve degeneration has not been studied.This study therefore investigated the protective effects of EVs derived from rat UC-MSCs on RGC injury in a rat model of COH.
Methods
Animals
This study adhered to the Statement of the Association for Research in Vision and Ophthalmology for the Use of Animals in Ophthalmic and Vision Research (Association for Research in Vision and Ophthalmology, 2021).All research protocols were ratified by the Experimental Animal Ethics Committee of Xiamen University, School of Medicine in March 2019 (approval No.20190306155209).We used 40 male and 2 pregnant wild-type Sprague-Dawley (SD) rats aged 8–12 weeks, weighing 180–200 g, which were obtained from the Experimental Animal Center of Xiamen University (license No.SYXK(Min) 2018-0010).Two male SD rats were used to obtain the conjunctival fibroblasts (CFs).Animals were maintained on a 12-hour light-dark cycle and were dark-adapted for at least 2 hours before the experiments.Before surgical procedures, deep anesthesia was introduced by an intraperitoneal injection of pentobarbital sodium (30 mg/kg, Sinopharm Chemical Reagent Co., Ltd.,Shanghai, China).The animals were sacrificed by anesthetic overdose with an intraperitoneal injection of pentobarbital sodium (150 mg/kg).
Isolation and cell culture of rat UC-MSCs
The rat UCs were dissected from the placenta of newborn SD rats and blood vessels were removed.The fragments of Wharton’s Jelly tissues were rinsed with normal saline and were further sliced into pieces, followed by treatment of 0.1% type-IV collagenase (Life Technologies, Carlsbad, CA, USA).After centrifugation, the cells were collected and cultured in Dulbecco’s modified Eagle’s medium (Gibco BRL, Grand Island, NY, USA)/F12 supplemented with 10% fetal bovine serum (deprived of EVs) and 1% penicillin plus streptomycin at 37°C.The 3rdor 4thpassage cells were used for the following experiments.The expression of CD90, a typical marker for UC-MSCs (T et al., 2016; Pham et al., 2018), was assessed using a Western blot assay.
MSC-EV isolation
As described in the related literature, EVs were isolated from the supernatant of rat UC MSCs (Shao et al., 2018).After the UC-MSCs reached about 80%confluency, the culture medium was carefully collected and centrifuged at 300 ×gfor 10 minutes, followed by a 2000 ×gcentrifugation for 15 minutes(4°C).The cell supernatant was then collected, and the cellular debris was discarded.The supernatant was then centrifuged at 10,000 ×gfor 45 minutes, followed by a centrifuge at 120,000 ×gfor 70 minutes at 4°C with an ultracentrifuge.The precipitation was collected and resuspended with 3 mL phosphate-buffered saline (PBS), and centrifuged again at 120,000 ×gfor 70 minutes (4°C).After supernatant removal, the precipitation was resuspended with 200 μL PBS.The protein content of the EV suspension was calibrated using a bicinchoninic acid quantitation kit (Beyotime, Shanghai, China).The expression of CD81, a specific marker for EV (Kowal et al., 2016; Kowal et al.,2017), was tested using a Western blot assay.The culture medium deprived from EVs was also assessed for CD81 expression as the negative control.
Electron microscopic identification of MSC-EVs
MSC-EVs were adsorbed onto carbon-Formvar film grids and double-fixed using 2.5% glutaraldehyde (pH 7.4) and 0.5% osmium tetroxide (Thermo Fisher Scientific, Tewksbury, MA, USA), followed by embedding with epoxy resin.Sections of MSC-EVs with a 90-nm thickness were laid onto 200-mesh copper grids and air-dried.These sections were doubly stained (uranyl acetate/lead citrate) and photographed under a transmission electron microscope (JEOL JEM-1010, JEOL Ltd., Tokyo, Japan).
Nanoparticle tracking analysis of MSC-EVs
The particle size of MSC-EVs was assessed using nanoparticle tracking analysis on a NanoSight NS300 system (Malvern Panalytical, Malvern, UK).MSCEVs were diluted with PBS (1:10) before being loaded into the laser module sample chamber of the system.Five readings were recorded for each MSC-EV sample (100 μL) to calculate the mean size.
Cell culture of CFs
CFs were harvested from SD rats.In brief, rats were deeply anesthetized.Under a surgical microscope (Carl Zeiss, Oberkochen, Germany), the superficial layers of the conjunctiva in the eyes of the rats were dissected and rinsed with Ca2+- and Mg2+-free Hank’s solution (Gibco BRL).CFs were digested using 0.2% dispase II (1.10 unit/mg, Gibco BRL) and then incubated under 95% humidified air + 5% CO2.When 80% confluence was reached, CFs were detached using trypsin (Thermo Fisher Scientific, Tewksbury, MA, USA)and ethylenediaminetetraacetic acid (Thermo Fisher Scientific), and further diluted to a ratio of 1:3.Conventional Dulbecco’s modified Eagle’s medium(Gibco BRL) was used for cell culture with a supplement of 10% fetal bovine serum and 1% penicillin plus streptomycin.
Induction of COH in the rat eye
Male animals are relatively stronger and more tolerant to surgeries than females, so the COH model was established using male rats.The procedure to establish a rat model of COH has been previously described (Wang et al.,2021).In brief, after general anesthesia, 0.5% tropicamide eyedrops (Alcon Inc., Fort Worth, TX, USA) were applied to the right eye to dilate the pupil.With a 30-gauge needle (Hamilton, Reno, NV, USA), CF suspension (10 μL,about 2000 cells/μL) was carefully injected into the anterior chamber of the right eye.The left eye was only injected with 10 μL PBS (control).After sustained IOL elevation had been observed for 7 days, the animals were randomly divided into two groups, as follows: (1) the treatment group (COH+ MSC-EV group,n= 20), which received intravitreous EV injection into the right eye; and (2) the COH group (n= 18), which received intravitreous PBS injection into the right eye.
IOP measurement
The IOP of the rat was measured daily after COH using a TonoLab-type tonometer (Icare, Helsinki, Finland) while animals were awake.According to the user guide, the mean of the 2ndthrough 6threadings was automatically calculated and recorded after six consecutive manual measurements.For each rat, the IOP was assessed at the same time on the day of the measurement.
MSC-EV injection
One week after COH induction, 5 μL MSC-EV suspension (200 μg/mL, ~5 × 104particles) was carefully transplanted into the vitreous using a 30-gauge needle(Hamilton, Reno, NV, USA) via the pars plana of the COH eye.The injection site was checked to avoid any leakage before being covered with erythromycin ointment.
Histological assessment
Eight weeks after COH, the rats were euthanized by overdose anesthesia and perfused intracardially with cold 4% paraformaldehyde in 0.1 M PBS at pH 7.4.The eyeballs were immediately enucleated and frozen in the optimal cutting temperature compound (Sakura Finetechnical, Tokyo, Japan).The eyeballs were sectioned along the meridian at a thickness of 10 μm.For the hematoxylin and eosin (HE) staining (Auragene, Changsha, China) of the retina, the eyeballs with intact optic nerves were quickly excised and fixed in 4% paraformaldehyde with 0.1 M PBS for 2 hours.The corneas and lenses were dissected, and the rest of the globe was left in the same fixation solution overnight.Sections were cut (10 μm thickness) with a cryostat (CM1900,Leica, Wetzlar, Germany) and adhered onto SuperFrost (Fisher Scientific,Loughborough, UK) coated slides and stored at –20°C.The sections were proceeded for HE staining and examined under a light microscope (Olympus,Tokyo, Japan).
Immunostaining
RNA binding protein with multiple splicing (RBPMS) has been reported to be a specific marker of RGCs (Kwong et al., 2010).The retinal sections were thawed and washed twice with PBS-Triton X-100 (10 minutes) and incubated at room temperature (~20°C) overnight with a primary rabbit anti-RBPMS antibody.After washing twice with PBS, the sections were incubated with the secondary antibody (Alexa Fluor 594-conjugated donkey anti-rabbit antibody)for 60 minutes at 37°C.All information on antibodies is shown in Table 1.Staining of the nuclei with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA) was performed.All slides were mounted with Fluoromount-G™ (SouthernBiotech, Birmingham, AL, USA).
RBPMS-positive (RBPMS+) RGCs were counted in the ganglion cell layer across a retinal section taken in line with the optic disc on 20× magnification images under a fluorescence microscope (Leica).A total of six images were analyzed from two sections per rat with software ImageJ (version 1.52, National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012).
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining
The eyes were then enucleated and prepared for paraffin embedding.The embedded tissues were sectioned to thicknesses of 7 μm using a semimotorized rotary microtome (Leica DM2500).Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining was performed on the retina tissues using a TUNEL assay kit (Promega, Madison, WI, USA),and the cell nucleus was dyed using DAPI.The positive control was incubated with a concentration of 0.5 μg/mL of DNase I in the retinal section before the addition of equilibration buffer.The terminal deoxynucleotidyl transferase reaction mixture was replaced with distilled water in the negative control.The apoptotic cells from the different groups were observed and counted in the same area under fluorescence microscopy.The TUNEL-positive cells in the ganglion cell layer of each section were counted and quantified as per mm of retina.A total of six images on 20× magnification were used from two sections per animal.
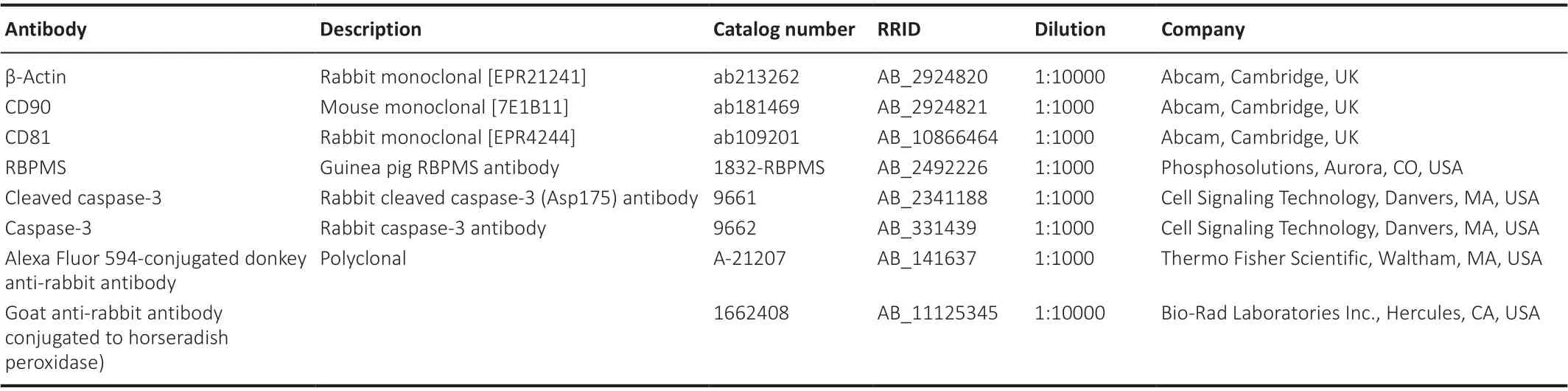
Table 1 | Primary and secondary antibodies
Fluorogold labeling of RGCs
The procedure for retrograde labeling of the RGCs in both eyes with fluorogold has been described previously (Hu et al., 2015; Lu et al., 2020;Wang et al., 2021).Briefly, 7 weeks after COH, animals were anesthetized and mounted on stereotactic equipment (RWD Life Science, Shenzhen, China).Injection of 3.0 μL 4% fluorogold (Fluorochrome LLC, Denver, CO, USA) was performed to the superior colliculus on both sides via the points 6.0 mm caudal to the bregma and 1.0 mm lateral to the midline (5.0 mm depth from the skull surface (Hu et al., 2015; Wang et al., 2021)).
For RGC counting, rats were sacrificed under anesthesia, and eyes were enucleated and fixed in 4% paraformaldehyde (1 hour, 4°C) in the dark.The retina was carefully dissected and flat-mounted onto a glass slide.One image with 40× magnification was taken under the fluorescence microscope at a distance of 2, 3, and 4 mm, respectively, radially away from the optic disc in each of the four quadrants of the retina.Manual RGC counting was performed by an investigator blind to the experimental conditions using the image analysis program ImageJ (version 1.52, National Institutes of Health,Bethesda, MD, USA) (Schneider et al., 2012).Cells considered to be microglia or macrophages were excluded from the RGC counting (Lu et al., 2020).
Western blot analysis
Protein extraction and Western blots assay of the retina were carried out as previously reported (Soleimannejad et al., 2018; Wang et al., 2021).The retinas were dissected after global enucleation and homogenized with lysis buffer (Solarbio Science & Technology, Beijing, China).The total protein (20 μg) of the retina, MSCs, or EVs was dissolved in 12% sodium dodecyl sulfate gels, transferred to polyvinylidene fluoride membranes (Millipore, Watford,UK), and incubated overnight at 4°C with antibodies against CD90, CD81,cleaved caspase-3, caspase-3, or β-actin.The target protein bands were further incubated for 2 hours at 20°C with secondary antibodies (horseradish peroxidase-conjugated goat anti-rabbit IgG) visualized with enhanced chemiluminescence reagents (Advansta, Menlo Park, CA, USA) and quantified with the Quantity One software (Bio-Rad Laboratories Inc.).The data were normalized by β-actin.All information on antibodies is shown inTable 1.
Statistical analysis
No statistical methods were used to predetermine sample sizes; however, all experimental sample sizes are similar to those reported in the literature (Hu et al., 2015; da Silva-Junior et al., 2021; Wang et al., 2021).All data are based on at least three independent experiments.All measure data are summarized as mean ± standard deviation, and the statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows, version 19.0, IBM Corp., Armonk,NY, USA).One-way analysis of variance was performed followed by Tukey’spost hoctests for multiple comparisons with aP-value < 0.05 considered to be statistically significant.
Results
Characterization of cultured rat UC-MSCs and EVs
The growth of UC-MSCs was observed in the UC fragments several hours after incubation.The fusiform and polygon-shaped cells proliferated within 24 hours and reached confluency in 8 days (Figure 1A).Approximately 2.5 mL MSC-EVs were obtained from 50 mL MSC supernatant at a concentration of 1510.6 μg/mL determined by the bicinchoninic acid protein quantification kit (Beyotime).The cup-shaped UC-MSC-derived EVs were visualized under electron microscopy (Figure 1B).MSC-EVs were analyzed using nanoparticle tracking analysis; their concentration was 3.66 × 1011particles/mL with a mean diameter of 174.9 ± 21 nm (Figure 1C).The western blot assay revealed a specific positive expression of CD90 on UC-MSC and CD81 on EVs(Figure 1D).
IOP changes induced by COH after CF injection
As shown in our previous report (Wang et al., 2021), the transplantation of CFs into the anterior chamber induced a significant and sustained IOP increase (Figure 2).This IOP elevation lasted throughout the experimental period, for at least 8 weeks.The mean IOP averaged over the length of the experiment for the COH eyes was 25.45 ± 1.80 mmHg (n= 16), which was significantly higher than that in the control eyes (12.17 ± 1.19 mmHg,n= 16)(P< 0.001).Intravitreous injection of EVs into the COH eyes (IOP 25.35 ± 1.70 mmHg) did not influence the IOP significantly compared with the eyes that received PBS (P> 0.05).
Neuroprotective effect of MSC-EVs on COH-induced retinal injury
HE and immunostaining
Compared to the normal rats, changes in the HE staining of the retina were observed in the COH rats, including marked swelling of the ganglion cell layer,the inner plexiform layer, the outer nuclear layer, and the RGC layer of the retina.In addition, in the RGC layer, the number of cells was reduced, with irregularity in the cell arrangement, darkened staining, and condensation of the cell nuclei.In the MSC-EV treatment group, the swelling of the retinal layers was alleviated, and the morphological anomaly was improved in the RGC layer (n= 3/group;Figure 3A).
When stained with the RGC-specific marker RBPMS (Kwong et al., 2010;Rodriguez et al., 2014), the number of RGCs decreased significantly (P< 0.001,n= 4/group,Figure 3BandC) in the COH eyes.With the treatment of MSCEV, this decrease in RGC number was significantly smaller than that of the COH eyes (P< 0.01,n= 4/group).
Cell apoptosis
TUNEL staining of the retina revealed a significant increase in cell apoptosis in rats with COH (P< 0.001,n= 3,vs.control group).After treated with MSCEV injection, the number of apoptotic cells significantly decreased in the COH rats (P< 0.001,n= 3,vs.COH group;Figure 4AandB).
The Western blot assay revealed that the expression of cleaved caspase-3,which is an apoptosis-related protein (Julien and Wells, 2017; Asadi et al.,2022) was significantly up-regulated in the retina of COH rats (P< 0.001,n= 3/group,vs.control group).After MSC-EV treatment, cleaved caspase-3 expression was significantly inhibited (P< 0.001,n= 3/group,vs.COH group;Figure 4C and D).
RGC labeled by fluorogold staining
Representative images of RGC fluorogold staining are shown inFigure 5A.Quantitative calculation revealed that fluorogold-labeled RGC in the retinas of COH rats decreased significantly at week 8 (P< 0.001,n= 3/group,vs.control group).For the COH eyes that received MSC-EV injection, the RGC density was significantly higher than those without the MSC-EV treatment (P< 0.001,n= 3/group,Figure 5B).
Discussion
In this study, we successfully isolated EVs derived from rat UC-MSCs using serial high-speed centrifugation and ultracentrifugation.In a rat model of COH induced by transplantation of cultured CFs into the anterior chamber of the eye, we tested the neuroprotective effect of MSC-EV.UC-MSC-EV treatment significantly alleviated the retinal injuries and prevented the RGC decrease.Furthermore, transplantation of UC-MSC-EVs inhibited the protein expression of caspase-3 and cell apoptosis in the retinas of COH rats.These findings suggest that the neuroprotection conferred by UC-MSC-EVs in COH rats is partially mediated by the inhibition of cell apoptosis in the retina.

Figure 1|Cell culture of MSCs from rat umbilical cord and collection of EVs.
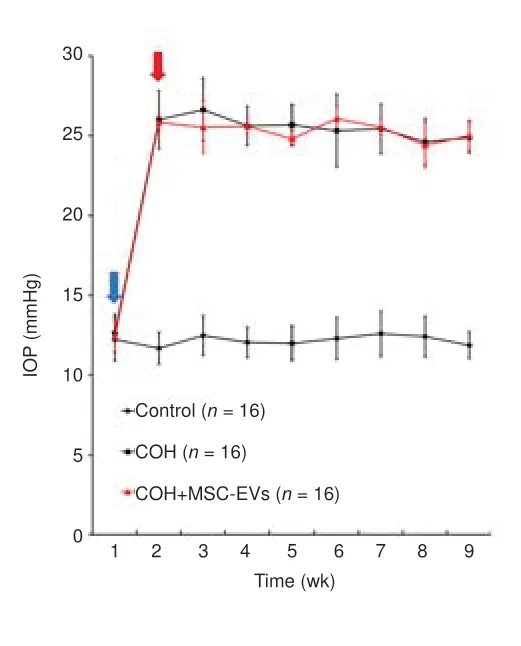
Figure 2|COH was induced by transplantation of allogeneic CFs into the anterior chamber of rat eyes.
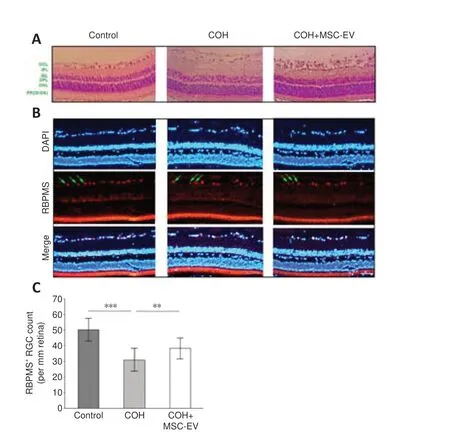
Figure 3|Protective effect of umbilical cord-derived MSC-EV treatment on retina damage and ganglion cell loss induced by COH.
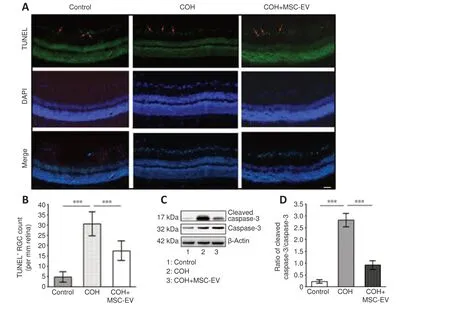
Figure 4|Inhibition of apoptosis by MSC-EV treatment in the COH rat retina.
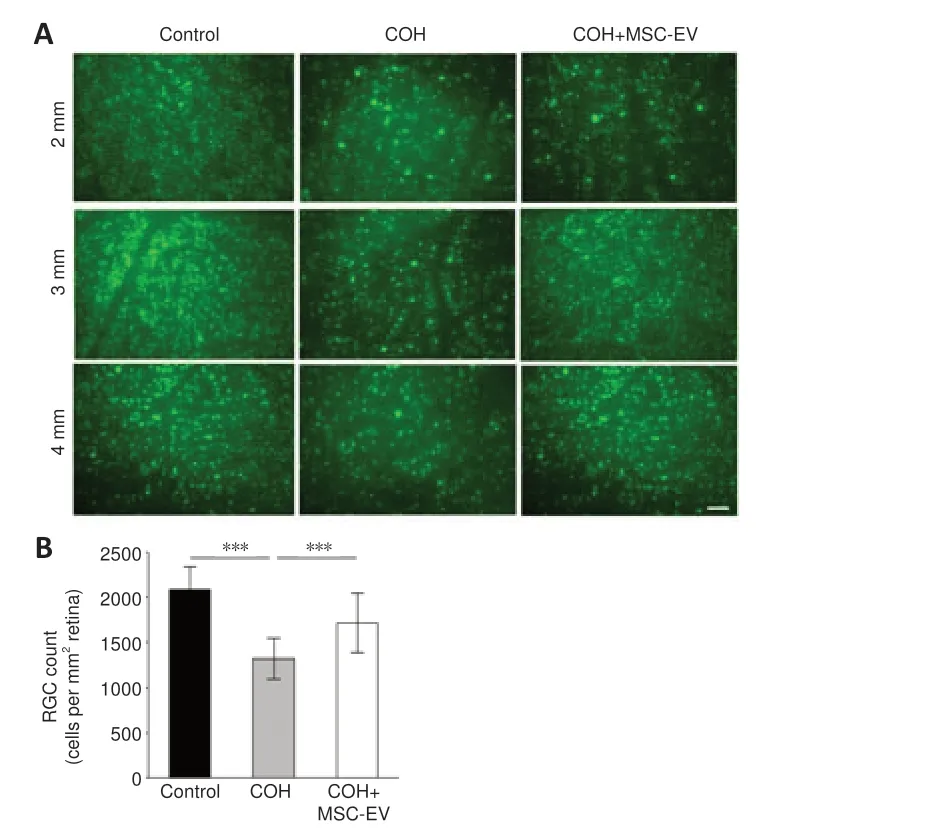
Figure 5|Neuroprotective effects of MSC-EV treatment on RGCs in the COH rat retina.
In recent years, the rapid development of stem cell therapy has provided a novel approach to the repair and functional restoration of damaged tissues and organs.Due to its low immunogenicity and harmlessness to donors during tissue collection and cell harvest, the UC is an ideal source of MSCs(Ding et al., 2015; Li et al., 2015; Reyhani et al., 2020; Xie et al., 2020).Previous studies have reported successful MSC isolation and culture methods from the Wharton’s Jelly of the human UC (Ding et al., 2015; Li et al., 2015;Reyhani et al., 2020).However, collecting UC-MSCs from rats’Wharton’s Jelly is more difficult, as it is difficult to remove the tiny blood vessels of the rat UC.In this study, by culturing Wharton’s Jelly with a low-glucose medium,the contamination of cells with anything other than MSCs was avoided.The purified UC-MSCs were confirmed by the expression of CD90, the marker for MSCs (Zhang et al., 2016; Voga et al., 2020).In our previous study on a rat model of COH, the intravitreous transplantation of human UC-MSCs migrated to the retinal surface, survived for at least 8 weeks, alleviated retinal damage, and reversed the loss of RGCs (Wang et al.,2021).However, the integration of these stem cells with retinal tissue could not be detected by the GFP-labeling method (Wang et al., 2021).The eye is considered to be a relatively immune-privileged site (Caspi, 2008; Johnson et al., 2010; Benhar et al., 2012; Chen et al., 2019), and UC-MSCs possess immune privilege properties, which promise a low rate of immunological rejection after transplantation into the ocular tissue.Nevertheless, the structural integration of UC-MSCs into the retinal tissue is complicated and difficult due to the barrier effect of inner limiting membrane of the retina and intercellular junction (Wang et al., 2021).In addition, the transplantation of MSCs in tissue reconstruction comes with the risk of tumorigenesis and immune rejection (Sohn et al., 2015; Kuriyan et al., 2017; da Cruz et al.,2018; McGill et al., 2018).Transplanting MSC-derived EVs may overcome some of these obstacles in future applications.EVs have been reported to be able to penetrate obstacles such as small vessels and successfully reach the target tissue or cells (Yu et al., 2016).Moreover, MSC-derived EVs express substances such as signaling proteins, heat shock proteins, and adhesion molecules, which are thought to play an essential role in tissue repairment/reconstruction of the target tissues (Harrell et al., 2018; Keshtkar et al., 2018;Qiu et al., 2019; Abbaszadeh et al., 2020).
For the first time, we found that the intravitreous injection of UC-MSCderived EVs significantly reversed the retinal tissue damage and RGC loss induced by chronically elevated IOP, which underlines its potential in the prevention or treatment of glaucomatous optic neuropathy.Our study corroborates previous findings that MSC-derived EVs have a neuroprotective effect for RGCs in some optic neuropathy rat models (Mead and Tomarev,2017; Mead et al., 2018a; Pan et al., 2019; Seyedrazizadeh et al., 2020; Cui et al., 2021).The exosomes from bone marrow-derived stem cells have been found to augment the survival of RGCs and facilitate axonal regeneration(Mead and Tomarev, 2017; Mathew et al., 2019; Mead and Tomarev, 2020;Seyedrazizadeh et al., 2020; da Silva-Junior et al., 2021).This neuroprotective effect of EVs from bone marrow-derived stem cells has also been observed in a genetically-modified DBA/2J mouse model of glaucoma (Mead et al.,2018b).Furthermore, in rats with optic nerve crush, Pan et al.(2019) found that UC-MSC-derived exosomes enhanced the survival of RGCs.However,a promotion of axonal regeneration was not observed in that study.The authors reported that, when intravitreally injected and tracked with the green lipophilic fluorescent dye PKH67, UC-MSC-derived exosomes were found around the DAPI+nuclei of the inner retinal cells and targeted cells in the RGC layer.This is suggestive of an integration of EVs into the target tissues following intravitreous transplantation, thus exhibiting an advantage over MSCs for future clinical application.
There is much evidence for the involvement of caspase signaling-dependent apoptosis in the pathophysiological process of glaucomatous neuropathy(McKinnon et al., 2002; Almasieh et al., 2012).The activation of caspase-8 and -3 could represent sequential events in the apoptosis cascade in RGCs(Mayordomo et al., 2003; Tezel and Yang, 2004; Spalding et al., 2005;Stein-Streilein, 2008; Liu et al., 2015).Another mechanism underlying the neuroprotective effects of MSC-derived EVs could include immune regulation.EVs have been reported to be rich in cytokines including transforming growth factor-β, mothers against decapentaplegic homolog 2 and 4 proteins, which could activate the downstream signaling pathways in CD4+T cells, thereby regulating neuroinflammation in the event of nerve injury (Xia et al., 2021).
This study has several limitations.The survival and integration of EVs into intraocular tissues were not monitored after intravitreous transplantation, and so details on the destination of EVs are unknown.Furthermore, for a better understanding of the neuroprotection of EVs on retinal tissues in COH rats, a more extended period of post-treatment follow-up is needed.A combination of any functional evaluation with histological assessment would be valuable.Additionally, the mechanism underlying the neuroprotective effect of EVs needs further investigation—in particular, the involvement of cytokine components inside the EV cargoes must be clarified.Nevertheless, our study demonstrated that UC-MSC-derived EVs promote the neuroprotection of RGCs in a rat model of COH/glaucoma.In addition, the intravitreous implantation of EVs had little influence on the IOP, and such a neuroprotective benefit makes them a promising candidate for adjunctive treatment of glaucoma control in the future.
Author contributions:Study conception and design: FY, RYW; experimental studies, data acquisition and analysis: FY, CQH, SJL, RXG; manuscript preparation, editing and review: YW, RYW.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no competing interests.
Data availability statement:No additional data are available.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain

