Astrocyte-neuron communication mediated by the Notch signaling pathway: focusing on glutamate transport and synaptic plasticity
Ke-Xin Li, Meng Lu, Meng-Xu Cui, Xiao-Ming Wang, Yang Zheng
AbstractMaintaining glutamate homeostasis after hypoxic ischemia is important for synaptic function and neural cell activity, and regulation of glutamate transport between astrocyte and neuron is one of the important modalities for reducing glutamate accumulation.However, further research is needed to investigate the dynamic changes in and molecular mechanisms of glutamate transport and the effects of glutamate transport on synapses.The aim of this study was to investigate the regulatory mechanisms underlying Notch pathway mediation of glutamate transport and synaptic plasticity.In this study, Yorkshire neonatal pigs (male, age 3 days, weight 1.0–1.5 kg, n= 48) were randomly divided into control (sham surgery group) and five hypoxic ischemia subgroups, according to different recovery time, which were then further subdivided into subgroups treated with dimethyl sulfoxide or a Notch pathway inhibitor (N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester).Once the model was established, immunohistochemistry, immunofluorescence staining, and western blot analyses of Notch pathway-related proteins, synaptophysin, and glutamate transporter were performed.Moreover, synapse microstructure was observed by transmission electron microscopy.At the early stage (6–12 hours after hypoxic ischemia) of hypoxic ischemic injury, expression of glutamate transporter excitatory amino acid transporter-2 and synaptophysin was downregulated, the number of synaptic vesicles was reduced, and synaptic swelling was observed; at 12–24 hours after hypoxic ischemia, the Notch pathway was activated, excitatory amino acid transporter-2 and synaptophysin expression was increased, and the number of synaptic vesicles was slightly increased.Excitatory amino acid transporter-2 and synaptophysin expression decreased after treatment with the Notch pathway inhibitor.This suggests that glutamate transport in astrocytes-neurons after hypoxic ischemic injury is regulated by the Notch pathway and affects vesicle release and synaptic plasticity through the expression of synaptophysin.
Key Words:astrocyte; astrocyte-neuron communication; glutamate; glutamate transporter; hypoxic-ischemic injury; magnetic resonance spectroscopy;neonate; Notch signaling pathway; plasticity; synapse
Introduction
Neural network activity in the central nervous system is mainly regulated by a combination of communication between neurons and glial cells and nutritional support provided by the extracellular matrix and the vasculature(vascular system) (Elorza Ridaura et al., 2021; Peguera et al., 2021).Astrocytes provide energy and metabolic support to neurons by releasing energy substrates (Magistretti and Allaman, 2018; Beard et al., 2021), transporting neurotransmitters (Andersen et al., 2020), and secreting cytokines (Jha et al.,2019).Furthermore, astrocytes regulate neuronal excitability and promote neuronal survival, regeneration, and neurite growth (Allen and Eroglu,2017).In addition to releasing signaling molecules, the interaction between astrocytes and neuron affects neural stem cell proliferation (Engler et al.,2018) and neuronal regeneration (Wilhelmsson et al., 2012) by signaling through intercellular receptor-ligand binding, which regulates neurological homeostasis under physiological and pathological states.It has been suggested that astrocytes are not limited to providing protection and support to neurons, but are also involved in synaptic information transmission; in other words, astrocytes, presynaptic, and postsynaptic neurons together constitute“tripartite synapses”(Araque et al., 1999).Communication between astrocytes and glutamatergic neurons, both components of the“tripartite synapse,”occurs mainly in the form of glutamate (Glu) uptake and release of signaling molecules by astrocytes (Lalo et al., 2021), which further modulates synaptic plasticity (Liu et al., 2021).The main route of Glu uptake by astrocytes is through the excitatory amino acid(EAA) transporter-2 (EAAT2) (Pajarillo et al., 2021).The proposed notion of“tripartite synapses”broadens the scope of astrocyte action from“point-topoint”to“point-to-network.”However, there are several aspects of astrocyte function within the neural network that warrant further investigation.For example, it is unknown how astrocyte-neuronal communication is regulated in pathological states or how astrocyte metabolites regulate neural circuit function (Hirrlinger and Nimmerjahn, 2022).Hypoxic ischemia (HI) is one of the leading causes of neonatal encephalopathy(Rodriguez et al., 2021; Xiong et al., 2021).In a HI state, astrocytes maintain neurotransmitter homeostasis by transporting Glu.As Glu is the main EAA in the central nervous system and also a core component of excitatory synaptic energy and amino acid metabolism (Andersen et al., 2021), during HI, the increase in extracellular Glu causes excitotoxicity.Under these conditions,the Glu-glutamine (Gln) cycle between neurons and astrocytes becomes important for maintaining neurotransmitter homeostasis.Astrocytes take up Glu via the Glu transporter EAAT2 to reduce the accumulation of Glu in the synaptic cleft, and the Glu is then converted to Gln for uptake by neurons(Macaisa et al., 2019).Within the neurons, Gln is then converted again to Glu and transported into synaptic vesicles (SVs) by the vesicular Glu transporter-1(vGLUT1) (Du et al., 2020).Synaptophysin, one of the most abundant membrane proteins in SVs and a marker protein of synapses during neuronal development (Ahnert-Hilger et al., 1996), can also serve as a marker of synaptic changes after HI injury.However, the nature of the dynamic changes that occur in Glu transport status after HI injury, the molecular mechanisms that regulate this process, and its effects on synaptophysin, warrant further study.
Jagged1 is highly expressed in neurons (Marathe et al., 2017), whereas Notch signaling proteins are highly expressed in astrocytes, and secreted Jagged1 activates Notch signaling mechanisms in astrocytes, thereby regulating astrocyte regeneration (Hu et al., 2019).Notch signaling is important for maintaining the functional connectivity of neural networks (Zhang et al.,2018; Yuan et al., 2020).Notch signaling is activated in mammals through the binding of Notch receptors (Notch1–4) to ligands, such as Jagged and Delta/Serrate/Lag2, with subsequent cleavage by α- and γ-secretase and release of the Notch intracellular domain (NICD) as an active signaling fragment that regulates the transcription of target genes (Nonneman et al., 2018).γ-Secretase inhibitors such asN-[N-(3,5-difluorophenacetyl)-L-alanyl]-Sphenylglycine t-butyl ester (DAPT) activate protein production and block the Notch signaling pathway (Li et al., 2016).Jagged1 is highly expressed in the hippocampal dentate gyrus during postnatal development, while other ligands are not (Irvin et al., 2004), and Notch signaling proteins are highly expressed in astrocytes; Jagged1 binding to Notch receptors activates signaling mechanisms in astrocytes and regulates astrocyte regeneration (Hu et al., 2019).However, whether Jagged1 similarly mediates Notch pathway activation in astrocytes and promoting Glu transport after HI injury remains not well-characterized.
Newborn pig brain anatomy and maturity are highly similar to those of human neonates; thus, the neonatal pig model of HI is representative of neonatal HI (Zheng and Wang, 2018).In this study the neonatal pig model of HI was used to investigate regulation of Glu transport and its effect on synaptic structural plasticity by analyzing dynamic changes in expression of the Glu transporter EAAT2 and synaptophysin after HI injury.In addition, the regulatory mechanism underlying astrocyte-neuron Glu communication and synaptic plasticity was investigated by intervening the experimental animals with a Notch pathway inhibitor (DAPT) and transiently down regulating EAAT2 or synaptophysin.
Methods
Animal model
To exclude the effects of sex on HI (Smith et al., 2014), the animals for research used in this study were 48 male 3-day-old Yorkshire neonatal pigs(body mass, 1.0–1.5 kg).The neonatal animals were housed in a facility with a temperature of 26–30°C, humidity 40–70%, and 12/12-hour light/dark cycle.The animals were randomly divided into the control group and the following groups based on recovery time after HI: 0–6, 6–12, 12–24, 24–48,and 48–72 hours.Each HI group was then randomly and equally divided into an HI + dimethyl sulfoxide (DMSO) group and an HI + Notch pathway inhibitor(DAPT) group withn= 4 animals per subgroup.All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Shengjing Hospital and China Medical University on February 23, 2018(approval No.2018PS43K).The process of establishing the HI model, as well as the whole-brain1H-magnetic resonance spectroscopy (MRS) scanning and data postprocessing procedures, were performed as previously described(Li et al., 2022).In brief, HI injury was achieved by temporarily blocking the bilateral common carotid arteries for 40 minutes, while the animals received 6% oxygen by mechanical inhalation.A detailed description of the HI model and the MRS process are provided in Additional file 1.The study design andtimeline are shown in Additional Figures 1 and 2.
Drug treatment
A DMSO stock solution (CAS: 67–68–5, Solarbio, Beijing, China; purity >99.5%) was diluted in saline to generate a 4% DMSO working solution; DAPT(S2215, Selleck; purity, 99.57%) was then dissolved in 4% DMSO to generate the DAPT working solution (solubility < 86 mg/mL).Thirty minutes after the completion of hypoxia and occlusion, animals in the Notch pathway inhibition group were injected intraperitoneally with the DAPT working solution at a concentration of 3.33 mg/mL, and animals in the control group (sham surgery) were injected intraperitoneally with an equal volume of 4% DMSO.
Immunohistochemistry and fluorescence staining
The animals were sacrificed by intramuscular injection of an overdose of Su-Mianxin anesthetic (0.6 mL/kg ; Changchun Institute of Military Medical Research, Changchun, China).The brains were removed, fixed, and sectioned using a Microm HM340E rotary microtome (thickness 3.5 μm; Microm,Walldorf, Germany).The sections were stained according to previous studies (Li et al., 2021), and details regarding the two-step detection kit(Zsbio, Beijing, China, Cat# PV-9001, RRID: AB_2868452) that was used are provided in Additional file 1.The primary antibodies and dilutions used for immunohistochemistry were as follows: rabbit polyclonal anti-activated Notch1 antibody (1:200, Abcam, Cambridge, UK, Cat# ab8925, RRID:AB_306863) and rabbit monoclonal anti-vGLUT1 antibody (1:2000, Abcam,Cat# ab227805, RRID: AB_2868428).
To perform double immunofluorescence staining, equal proportions of two types of primary and secondary antibodies were mixed by vortexing.The primary antibodies used for immunofluorescence staining and their dilutions were as follows: mouse monoclonal anti-Notch1 antibody (1:50, Abcam,Cat# ab44986, RRID: AB_776840); rabbit polyclonal anti-Jagged1 antibody(1:50, Abcam, Cat# ab7771, RRID: AB_2280547); rabbit polyclonal anti-EAAT2 antibody (1:50, Abcam, Cat# ab203130, RRID: AB_2924794); and rabbit polyclonal anti-synaptophysin antibody (1:100, Abcam, Cat# ab14692, RRID:AB_301417).The secondary antibodies and their dilutions were as follows:goat anti-rabbit IgG (Alexa Fluor® 488) (1:200, Abcam, Cat# ab150077,RRID: AB_2630356) and goat anti-mouse IgG (Alexa Fluor® 594) (1:200,Abcam, Cat# ab150116, RRID: AB_2650601).4′,6-Diamidino-2-phenylindole,dihydrochloride (DAPI) (Abcam, Cat# ab104139) was used for nuclear staining.Immunohistochemical images were acquired using an Ni-U orthofluorescence microscope and an NIS-Elements F image acquisition system(Nikon, Tokyo, Japan); immunofluorescence images were acquired using a laser confocal microscope (LSM-880, Zeiss, Oberkochen, Germany).Images of the same protein were acquired using the same microscopy parameters.Image analysis was performed using ImageJ software 1.6.0 (National Institutes of Health, Bethesda, MD, USA; Schneider et al., 2012).Further details are provided in Additional file 1.Protein co-expression, as assessed based on immunofluorescence double staining, was quantified as the percentage of double-positive cells.Protein expression was reported as the average optical density.
Western blotting
For tissue protein extraction, 100 mg of hippocampal tissue from each neonatal pig in each group was weighed, and 1 mL of radioimmunoprecipitation assay (Solarbio, Cat# R0010) and 10 μL of phenylmethanesulfonyl fluoride (Solarbio, Cat# P0100) were added in turn before subjecting the sample to ultrasonic homogenization.The homogenized tissue was then placed on ice for 30 minutes to allow lysis to occur.Subsequently, the tissue was centrifuged at 12,000 ×gfor 5 minutes at 4°C,and the supernatant was collected.
For protein denaturation, loading buffer was added to each tube at a ratio of four volumes of protein to one volume of loading buffer (4:1).The mixture was then vortexed to mix and allowed to denatured at 100°C for 5 minutes.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)gels with a 4–20% gel gradient were used (GenScript, Piscataway, NJ,USA, M42015C).The loading volume was calculated based on the protein concentration, and 30 μg of protein was added to each lane.The SDS-PAGE conditions were set at 140 V for 60 minutes.
For the transmembrane transfer procedure, a polyvinylidene fluoride (PVDF)membrane (IPVH00010, Millipore, Darmstadt, Germany) was placed in methanol and incubated for 3 minutes, and the gel was then transferred to the PVDF membrane using constant voltage at 100 V for 60 minutes.
Next, the membranes were blocked with skim milk powder dissolved at a concentration of 5% in tris-buffered saline with Tween-20.The PVDF membrane was then sealed in a plastic sleeve containing the skim milk solution and incubated for 2 hours at room temperature on a low-speed horizontal shaker (40 r/min) (Qilin, Haimen, Jiangsu Province, China, TS-1000).The loading control for the western blots is mouse monoclonal anti-β-actin antibody (1:1000, Abcam, Cat# ab8226, RRID: AB_306371).The primary antibodies used were rabbit polyclonal anti-EAAT2 antibody (1:1000,Proteintech, Wuhan, China, Cat# 22515-1-AP, RRID: AB_2879112) and rabbit monoclonal anti-synaptophysin antibody (1:1000, Abcam, Cambridge, UK,Cat# ab52636, RRID: AB_882786); the membranes were incubated with the appropriate antibodies overnight at 4°C, followed by incubation with horseradish peroxidase (HRP)-conjugated anti-mouse, rabbit IgG (1:10,000,Proteintech, Cat# SA00001-1, RRID: AB_2722565; Cat# SA00001-2, RRID:AB_2722564) for 2 hours at room temperature.
An Amersham Imager 680 (GE Healthcare, Chicago, IL, USA) was used for imaging, and the exposure was set to auto mode.Quantitative analysis of the results was performed using ImageJ software.
Transmission electron microscopy imaging
Immediately after the animals were sacrificed, the hippocampal tissues were harvested, fixed for 24 hours, then placed in 2.5% glutaraldehyde solution for 2 hours and then 1% reagent enzyme for 2 hours.After fixation, thetissues were rinsed with distilled water and placed in a gradient of ethanol and acetone solutions for dehydration; after dehydration, the tissues were placed in a mixture of epoxy resin and acetone overnight (16 hours) at room temperature, embedded in epoxy resin, and dried for storage using a thermostat.The tissue blocks were trimmed, cut into 100-nm ultrathin sections using a Leica EM UC7 ultramicrotome (Leica, Weztlar, Germany),and stained with uranyl acetate and lead citrate (Zhongjingkeyi Technology Co., Ltd., Beijing, China) for 10 and 5 minutes, respectively.The sections were allowed to dry and observed by transmission electron microscopy (TEM) (JEM-1400Flash, JEOL Japan Electronics Co., Ltd., Tokyo, Japan).For each group of animals, four thin-layer sections, each obtained from a different animal, were placed in a copper mesh for TEM acquisition.First, five fields were acquired at ×2000.Next, for each field, we took two random photographs at ×8000 and ×10,000.For each group of animals, > 50 synapses were included in the assessment.Image capture and TEM assessment were performed by two pathologists blinded to group information, and disagreements were resolved by negotiated consensus.
Statistical analysis
No statistical methods were used to predetermine sample sizes; however, our sample sizes are similar to those reported in a previous publication (Li et al.,2021).A one-way analysis of variance with homogeneity of variance testing was performed to analyze subgroup differences.Tukey’spost hoctest was used forpost hoctests among multiple groups.Dunnett’st-test was used for groups with unequal variances.The least significant difference test was used forpost hoctests between two groups.Statistical significance was set atP<0.05.Statistical software (SPSS, version 22.0; IBM, Armonk, NY, USA) was used as previously described (Li et al., 2022).Data are expressed as the mean ±SEM.
Results
Changes in EAAT2 and vGLUT1 expression and in Glu content after hypoxic ischemic injury
To investigate changes in Glu transport after HI, we first analyzed changes of EAAT2, vGLUT1 expression and Glu content.The trends in EAAT2 expression after HI is shown in Figure 1.Compared with the control group, EAAT2 expression was decreased at 6–12 hours (P< 0.001), increased at 12–24 hours (P< 0.001), decreased at 24–48 hours, and increased again at 48–72 hours (P< 0.001) after HI.vGLUT1 expression (Figure 2) was decreased at 0–6 hours after HI (P= 0.042), then increased, peaking at 12–24 hours after HI (P< 0.05), and decreased again at 24–48 hours (P= 0.011).Compared with the control group (5.398 ± 0.476 mM), Glu levels as measured by 1H-MRS were significantly higher 24–48 hours after HI (10.850 ± 2.192 mM;P= 0.019).
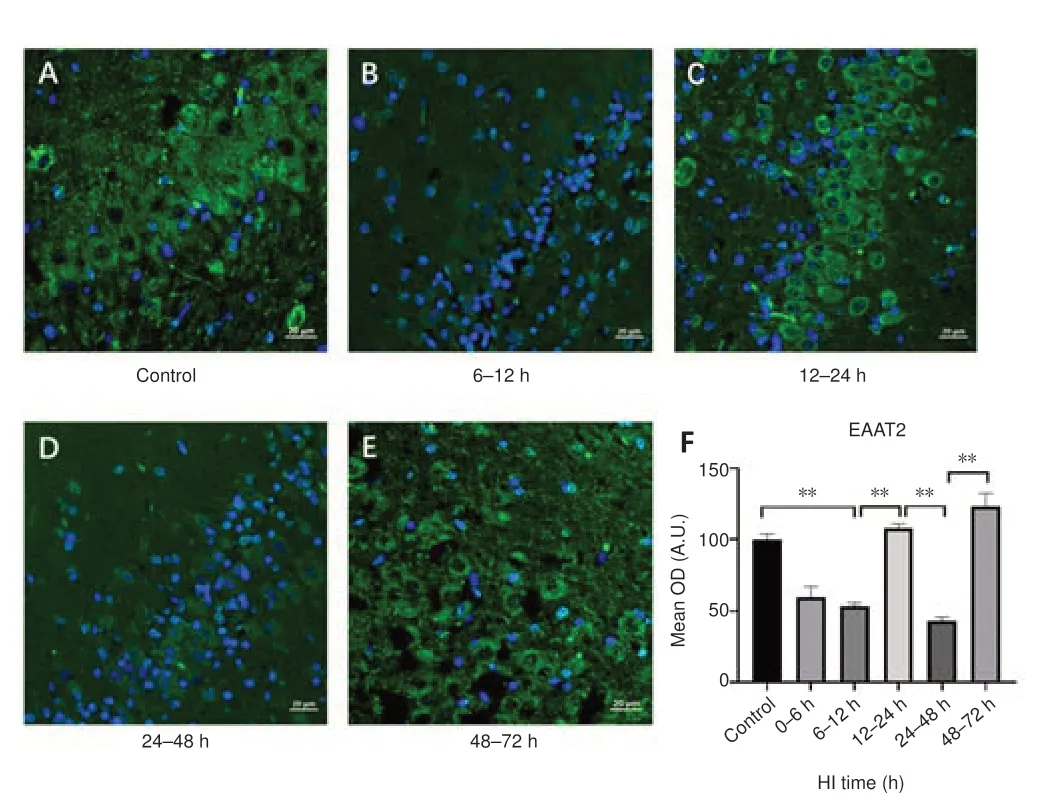
Figure 1|Effect of HI on EAAT2 expression in the hippocampal region in pigs.
Changes in synaptophysin expression and synaptic microstructure after HI injury
To investigate changes in synaptic protein expression and synaptic microstructure, we next analyzed synaptophysin expression after HI injury(Figure 3A–L).Compared with that observed at 6–12 hours (Figure 3C) after HI, synaptophysin expression was transiently increased at 12–24 hours (Figure 3D) after HI (P= 0.003).Moreover, TEM showed an increase in the number of intrasynaptic vesicles at this time point (Figure 3J).In addition, synaptophysin expression was decreased significantly at 24–48 hours (Figure 3E) after HI (P= 0.009), at which time TEM showed disruption of synaptic structures (Figure 3K) and a decrease in the number of intrasynaptic vesicles.
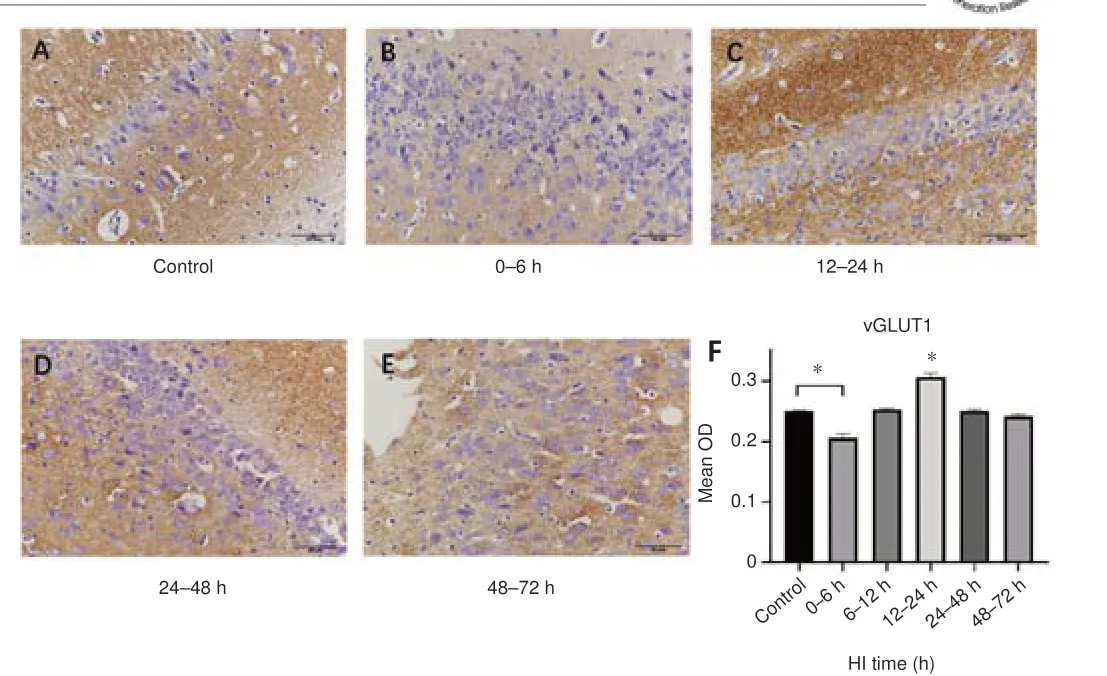
Figure 2| The effect of HI on vGLUT1 expression in the hippocampal region in pigs.
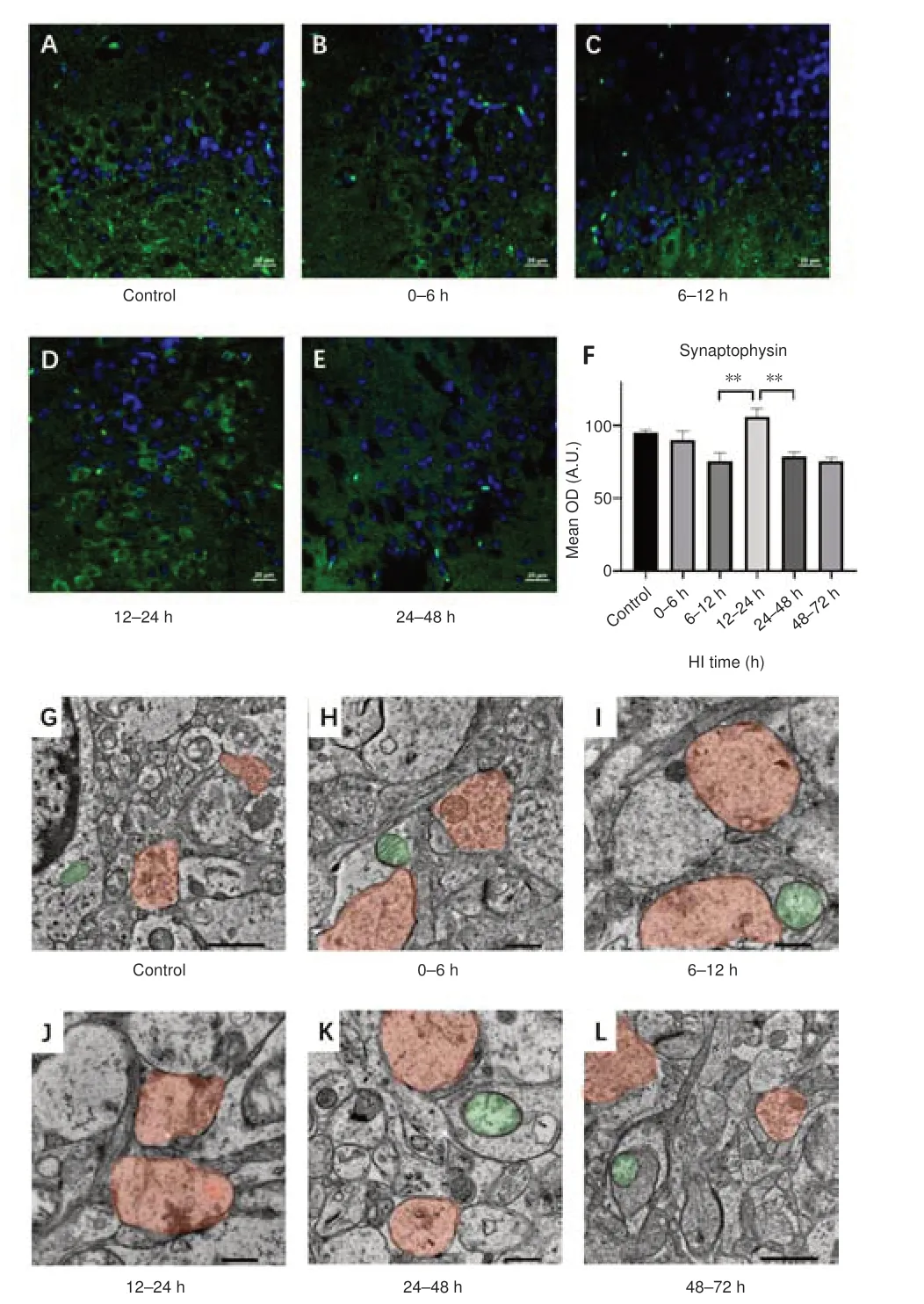
Figure 3| Effect of HI on synaptophysin expression and synaptic transmission electron microscopy images in the hippocampal region in pigs.
Changes in Notch1, Jagged1, and NICD expression after HI injury
To investigate changes in Notch signaling pathway, we analyzed Notch1,Jagged1, and NICD expression after HI injury.The changes in Notch1 and Jagged1 expression and in the percentage of Notch1/Jagged1 double-positive cells after HI injury are shown in Figure 4.Notch1 expression (P= 0.001)and the percentage of Notch1/Jagged1 double-positive cells (P= 0.002)were decreased at 0–6 hours compared with the control group.Notch1 (P= 0.014) and Jagged1 (P= 0.009) expression and the percentage of doublepositive cells (P= 0.048) were increased at 12–24 hours.The trends in NICD expression after HI injury over time are shown in Figure 5.NICD expression was significantly higher at 12–24 hours than it was at 0–6 hours and 24–48 hours after HI (P= 0.026 andP= 0.001, respectively).
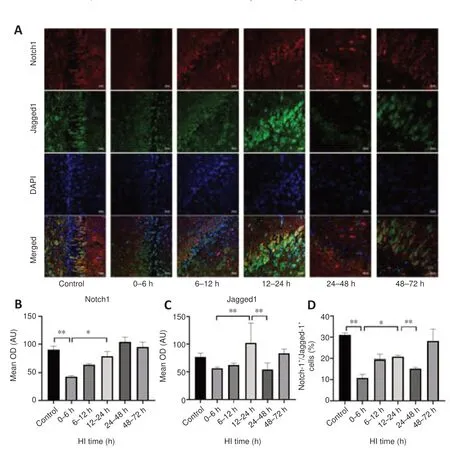
Figure 4|Effects of HI on Notch1 and Jagged1 expression and Notch1/Jagged1 double-positive immunofluorescence in the hippocampal region in pigs after HI.
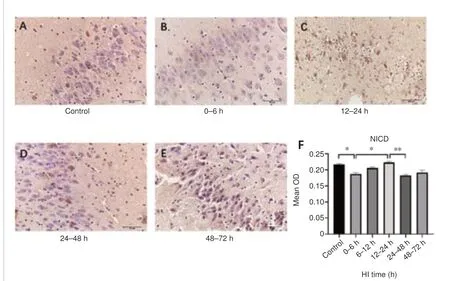
Figure 5|Changes in NICD expression in the hippocampal region in pigs after HI.
Effects of a Notch pathway inhibitor on EAAT2 and synaptophysin expression
To investigate the regulatory role underlying Notch pathway mediation of Glu transport and synaptic vesicle release.We study the effects of a Notch pathway inhibitor on EAAT2 and synaptophysin expression.EAAT2 was downregulated after 12 hours of applying a Notch pathway inhibitors, and significant differences were observed at 12–24 hours and 48–72 hours post-HI compared with the DMSO group (P= 0.001 andP= 0.030, respectively).Synaptophysin expression was significantly inhibited by treatment with the Notch pathway inhibitor at 12–24 hours and 48–72 hours after HI (P= 0.043,P= 0.013, respectively; Figure 6).

Figure 6|EAAT2, synaptophysin, and β-actin expression in hippocampal tissue of pigs in the absence or presence of a Notch pathway inhibitor.
Discussion
The key to neural network repair after neonatal HI lies in synaptic reconstitution, which requires epigenetic changes caused by gene expression and the regulation of signal pathways mediated by intercellular communication (Boulanger and Dura, 2022).Regarding the regulatory role of intercellular communication, astrocytes and neurons have two mechanisms of interaction and crosstalk, including neurotransmitter-mediated messaging and membrane surface receptor-mediated signaling pathways, both of which play an important role in neuroprotection and network reconstruction after HI injury (Martín et al., 2007; Jiao et al., 2020; Lee et al., 2021).The aims of this study were to investigate the dynamic changes in astrocyte-neuron neurotransmitter transmission after HI injury and to investigate the molecular mechanisms of astrocyte-neuron neurotransmitter transmission mediated by membrane receptors, as well as their effects on synaptic plasticity.
Dynamic regulation of astrocyte-neuron Glu transport after HI injury and the effect on synaptic plasticity
Glu is the predominant EAA in the central nervous system and plays a crucial role in neural circuit organization (Rusakov and Stewart, 2021).However, HI causes increased Glu release and decreased Glu reuptake (Pregnolato et al.,2019).After HI, the Glu transporter EAAT2 plays a crucial role in maintaining Glu homeostasis in the brain, helping to remove the excessive Glu released from the synaptic gap (Pregnolato et al., 2019; Andersen et al., 2021).In the present study, we found that EAAT2 expression was increased in the 12–24 hours group, implying increased Glu transport during this period; after Glu is take by EAAT2, it may further enter neurons via the Glu-Gln shuttle and be transported to SVs, a process that is mediated by the vGLUT1.Here we found that vGLUT1 expression was transiently increased 12–24 hours after HI,implying that increased storage of Glu in vesicles may mitigate the neuronal damage caused by its excessive release.In contrast, 24–48 hours after HI,EAAT2 and vGLUT1 were downregulated, and the Glu concentration was increased, implying a decrease in Glu transport and an increase in Glu release,causing excitatory toxicity.As reported in our previous study (Li et al., 2021),after HI, the number of hippocampal neurons increased in the 12–24 hours group compared with the 6–12 hours group and decreased again in the 24–48 hours group.These changes appear to be related to the Glu changes, but this needs further confirmation.
After HI, maintenance of intersynaptic neurotransmitter signaling depends on effective fusion of SVs with the presynaptic plasma membrane and subsequent recycling (Cousin, 2021).Synaptophysin is a key protein involved in this process and has previously been shown by immunoelectron microscopy to be mainly distributed in the SVs of neuronal cells (Navone et al., 1989;Yu et al., 2019), to be an important marker of synaptic regeneration and synaptic plasticity (Wang et al., 2019).In this study, we further investigated the effects of HI on synaptic structural plasticity by analyzing changes in synaptophysin expression and its relationship with Glu transporter changes after HI.The results showed that the expression of the Glu transporters EAAT2 and vGLUT1 was decreased from 0–6 hours and increased from 12–24 hours after HI, and that the trend in synaptophysin expression was consistent with that of the Glu transporters during this period.Structurally,this suggests that there is a compensatory increase in synaptogenesis after HI.Functionally, as Glu transport to vesicles by vGLUT1 is accompanied by an inward flow of H+and an outward flow of Cl–(Martineau et al., 2017), 12–24 hours after HI vGLUT1 expression increases, Glu flows inward, and Cl–flows from vesicles to the endochylema.This flow increases the negative potential of the presynaptic endochylema, limiting the opening of voltage-gated calcium channels, reducing the binding of calcium ions to synaptophysin, and reducing vesicle release.While this sequence seems plausible, it needs to be confirmed in future electrophysiologic studies.In contrast, at 24–48 hours after HI, decreased Glu transport, increased brain Glu content, and decreased synaptophysin expression were observed.Moreover, electron microscopy showed vacuolated swelling of synapses, suggesting that excessive Glu accumulation caused secondary synaptic damage.
After HI, the Notch pathway activates and regulates the expression of Glu transporters and synaptophysin
Notch signaling is affected by the binding state of ligands and receptors(Campbell et al., 2022).Previous studies have found that Notch1 receptors and Jagged1 ligands are upregulated after mature and neonatal brain injury and activate downstream target genes (Zhong et al., 2018; Gao et al., 2021).Accordingly, in the present study we performed double immunofluorescence staining for Notch1/Jagged1 to confirm colocalization of the receptor and its ligand; the results showed that the percentage of Notch1/Jagged1 doublepositive cells decreased from 0–6 hours, increased from 12–24 hours, and decreased again from 24–48 hours after HI, reflecting changes in Notch1-Jagged1 binding status.Notch pathway activation is followed by Notch1 receptor cleavage, which is mediated by proteinases such as γ-secretase,generating NICD (Guruharsha et al., 2012).Previous studies have found that γ-secretase activity and activation of protein expression are increased after hypoxic or ischemic injury, which causes enhanced Notch signaling (Cheng et al., 2014; Ren et al., 2018).The results of this study confirmed that NICD production continued to increase from 6–12 hours and 12–24 hours after HI injury, indicating that the Notch pathway was activated after HI during this period.In addition, we found that Notch1 receptor expression peaked at 24–48 hours after HI.In contrast, the expression of Jagged1, the number of Notch1/Jagged1 double-positive cells, and the expression of Notch activation protein NICD decreased significantly during this period.These findings suggest that the activation of the Notch pathway was affected by the binding status of Jagged1.
Further biological functions of NICD depend on regulation of the transcription of different target genes, and previousin vitroexperiments have revealed that Notch signaling between epithelium and astrocytes promotes EAAT2 mRNA and protein expression (Martinez-Lozada and Robinson, 2020).In turn, EAAT2 expression is dependent on γ-secretase activity (Lee et al., 2017).To explore the influence of the Notch pathway on Glu transport after HI injury, we treated the experimental animals with the γ-secretase inhibitor DAPT observed the changes in EAAT2 expression.The results showed that the expression of NICD decreased first, and then increased again after HI,with the first peak in expression observed at 12–24 hours and the second increasing trend detected at 48–72 hours after HI, which was consistent with the changes in EAAT2 expression over time.After treatment with the Notch pathway inhibitor, EAAT2 expression was significantly reduced in the 12–24 hours and 48–72 hours groups, whereas the changes in other groups were not significantly different.This implied that EAAT2 expression was increased at 12–24 hours and 48–72 hours after HI because of the increased NICD expression and the enhanced activation of the Notch pathway, which in turn influenced EAAT2expression.However, we unexpectedly found that EAAT2 expression was increased in the control and 0–6 hours groups treated with DAPT.Whether this is a reverse activation mechanism that occurs in the early stages in HI still needs further investigation.Additionally, the effect of the Notch pathway inhibitor in thein vivoexperiment was limited, and when NICD expression was low, its effect may not have been large enough to detect,resulting in the insignificant changes observed tin EAAT2 in the remaining time groups.In addition, the present study found that synaptophysin levels were also inhibited by DAPT at 12–24 hours and 48–72 hours after HI, implying that the Notch pathway regulates synaptophysin expression and affects synaptic plasticity during this period.Nevertheless, further experiments are needed to confirm whether the Notch pathway directly regulates synaptophysin expression or indirectly regulates synaptic plasticity by affecting neurotransmitter transport.
This study was a preliminary observational study designed to investigate the association among Notch, Glu transport, and synaptic structural changes after HI.However, our study was limited by the lack of electrophysiological analysis of synapses and investigation of neural function in the experimental animals.Exploring these two areas will help to further confirm the mechanism of synapse regulation by the Notch pathway, and we expect that its impact on physiological synapse function will be confirmed in subsequent experiments.
In conclusion, after HI injury, the Notch signaling pathway activated, and this activation seems to be Jagged1-related.Astrocyte-neuron Glu transport is regulated by the Notch pathway, which may further modulate vesicle release and synaptic structural plasticity by affecting synaptophysin expression.
Author contributions:KXL proposed the idea of this study, completed the main experimental content and was the major contributor in writing the manuscript.ML and MXC assisted in making the animal model.XMW and YZ refined the study design, guided the experimental process and edited the manuscript, and contributed equally to this study.All authors read and approved the submitted version.
Conflicts of interest:The authors declare that they have no competing interests.
Data availability statement:All data generated or analyzed during this study are included in this published article and its Additional files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:Additional file 1:Detailed methods.Additional Figure 1:Experimental design.Additional Figure 2:Experimental timeline.
- 中国神经再生研究(英文版)的其它文章
- From static to dynamic: live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy
- MicroRNAs in mouse and rat models of experimental epilepsy and potential therapeutic targets
- The generation and properties of human cortical organoids as a disease model for malformations of cortical development
- Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease
- Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning
- A pancreatic player in dementia: pathological role for islet amyloid polypeptide accumulation in the brain

