Effects of forest canopy density and epixylic vegetation on nutrient concentrations in decaying logs of a subalpine fir forest
Zhung Wng,Wnqin Yng,Bo Tn,Qin Wng,,Lifeng Wng,Chenhui Chng,Rui Co,Yurui Jing,J¨org Müller
a School of Life Science,Taizhou University,Taizhou,Zhejiang,318000,China
b Institute of Ecology and Forestry,Sichuan Agricultural University,Chengdu,611130,China
c Field Station Fabrikschleichach,Department of Animal Ecology and Tropical Biology(Zoology III),Julius Maximilians University Würzburg,Glashüttenstraße 5,96181,Rauhenebrach,Germany
d Bavarian Forest National Park,Freyunger Straße 2,94481,Grafenau,Germany
Keywords:Coarse woody debris Natural disturbance Forest canopy density Epixylic vegetation Nutrient concentrations
A B S T R A C T Background:Deadwood and the associated epixylic vegetation influence nutrient cycles in forest ecosystems.Open canopies strongly regulate deadwood decomposition and disrupt epixylic vegetation on logs.However,it is unclear how the forest canopy density and epixylic vegetation growth affect the nutrient concentrations in deadwood.
1.Introduction
Coarse woody debris(CWD)accounts for approximately 30% of the global forest carbon(C)stocks(Pan et al.,2011;Seibold,2021),and its decomposition promotes biodiversity and soil fertility in forest ecosystems(Harmon et al.,1986;Botting and DeLong,2009;Oghimian et al.,2017).CWD plays a vital role in nutrient cycling on the forest floor,and concentrations of major nutrients such as N and Mn in decomposing logs are closely related to the degradation of recalcitrant organic carbon,the improvement of underlying soil properties,and the provision of nutrients for organisms such as beetles and deadwood-associated fungi as well as tree seedlings(Laiho and Prescott,2004;Gorgolewski et al.,2020;Minnich et al.,2021).Therefore,a better understanding of the responses of nutrients in deadwood to decreasing forest canopy density and epixylic vegetation growth is necessary to provide a deeper insight into the linkages between the decomposing deadwood and the surrounding environments under different climate conditions.
Wood traits,leaching processes,and microbial nutrient translocation/fixation(e.g.Ca translocation of fungi and N fixation by bacteria)are recognized as prevalent factors affecting the nutrient concentrations of decaying deadwood in forest ecosystems(Boddy and Watkinson,1995;Hafner et al.,2005;Herrmann and Bauhus,2018).They are associated with changes in climate conditions and forest structures,some of which are directly or indirectly influenced by forest canopy density and epixylic vegetation growth(Rosier et al.,2015;Haughian and Frego,2017;Zellweger et al.,2020).
Forest gap formation and decreasing canopy density are important ecological phenomena that lead to environmental heterogeneity within most forest ecosystems(McCarthy,2001;De Frenne et al.,2019;Heidrich et al.,2020).Recently,concerns have been raised regarding the responses of decomposition rates and carbon dynamics of decaying logs to gap-associated microclimate changes,particularly in some climate-sensitive areas,e.g.,the eastern Qinghai-Tibetan Plateau(Chang et al.,2020)and the Malaysian rainforest(Griffiths et al.,2021).Nutrient concentrations in decaying logs may also be affected by gap associated environmental heterogeneity.For example,the extent of leaching and soil biota activities can greatly vary with locations within forests(Brazee et al.,2014;Rosier et al.,2015;Krah et al.,2018).Theoretically,the decomposing deadwood under open canopies generally experiences a stronger leaching effect and more active invertebrate invasion(Tan et al.,2018;Vogel et al.,2020).Despite the occurrence of changes in nutrient concentrations in the decaying logs related to these processes(Edmonds and Eglitis,1989;Laiho and Prescott,2004;Kuehne et al.,2008),research addressing the effects of forest canopy gaps on such processes has been relatively rare.
Epixylic vegetation is an association of small vascular plants,bryophytes,lichen,and fungi living on the deadwood surface.Nutrient concentrations in the epixylic vegetation are governed to a considerable extent by atmospheric depositions rather than the substrates including deadwood and soil(Huang et al.,2019;Porley and Hodgetts,2005).Epixylic vegetation can affect the nutrient concentration in decaying logs through the cover and nutrient release from mosses and lichens under natural conditions(Yuan et al.,2017;Lasota et al.,2018).Greater epixylic moss coverage increases the nutrient losses from decaying logs by promoting water infiltration into deadwood and the subsequent leaching processes(Krankina et al.,1999).However,the decomposition of litter from epixylic vegetation may be a potential factor in increasing some of the nutrient concentration in deadwood(Li et al.,2014).The composition and biomass of epixylic vegetation differ between forest locations(Dittrich et al.,2013;Wang et al.,2019),with higher nutrient concentrations such as N and K and lower epixylic biomass vegetation observed in sun-exposed forest areas(Sardans and Pe~nuelas,2013;Wang et al.,2018a).However,a detailed understanding of the various roles of epixylic vegetation in the nutrient cycling of decaying logs under different microenvironments is currently lacking.
Natural disturbances accelerate the formation of canopy gaps and treefall,altering the distribution of epixylic vegetation(Wu et al.,2013;Xiao et al.,2016).Studying changes in the nutrient concentration of decaying logs in response to different microclimate conditions can help predict the contribution of deadwood decomposition to nutrient cycling in subalpine forests with more frequent natural disturbances.To test the effects of the forest canopy density,epixylic vegetation,and their interactions,we exposed logs in gaps,at the edge of gaps,and under closed canopy conditions and removed the epixylic vegetation from half of the logs.We tested two hypotheses:(1)Nutrient concentrations in logs are lower in the gap than under the closed canopy because of the stronger leaching process and invertebrate activity,particularly for barks.(2)The epixylic vegetation cover increases the nutrient concentrations in decaying logs due to the higher element concentrations in epixylic mosses.We also contrasted the epixylic vegetation effects between forest locations.
2.Materials and methods
2.1.Site description
The study was conducted at the Long-Term Research Station of Alpine Forest Ecosystems(102°53′–102°57′E,31°14′–31°19′N,altitude 2,458‒4,619 m)in Li County,Sichuan,southwestern China.The region is a typical transition zone from the Tibetan Plateau to the Sichuan Basin.The mean annual temperature is approximately 2–4°C,with a maximum of 23.7°C(July)and a minimum of‒18.1°C(January).The mean annual precipitation including rainfall and snowfall is approximately 850 mm,occurring predominantly in the wet season from May to August and the winter season from November to April the following year(Tan et al.,2018).The dominant tree species at our study site is Faxon fir(Abies fargesiivar.faxoniana[Rehder & E.H.Wilson]Tang S.Liu),which accounts for approximately 80%of all the tree species present(Xiao et al.,2016).Shrubs includingSalix paraplesiaC.K.Schneid andRhododendron lapponicum(L.)Wahlenb.and herbs includingCystopteris montana(Lam.)Desv.andBerberis sargentianaC.K.Schneid were also present.The soils are categorized as being Cambisols.The diversity,composition,and biomass of epixylic vegetation have been investigated in a previous field experiment(Wang et al.,2018a,2019).The epixylic mosses account for the greatest proportion of the epixylic vegetation,andNeckera pennataHedw.is the prime moss species found on decaying logs.Higher diversity and biomass of epixylic vegetation on decaying logs have been observed under the closed canopy than in the gaps.Nutrient concentrations in the epixylic vegetation and soil were previously analyzed in different forest locations with the higher nutrient concentrations often being recorded in the gaps(Wang et al.,2018b;Tang et al.,2018).
2.2.Experimental design
In a Faxon fir primary forest,we selected three 25 m×25 m plots around three forest gaps with an area of approximately 50–60 m2each(Fig.1S).These plots were located on a sunny slope and successively distributed along a corridor between mountain and river.Each of the three plots represents a gradient of forest canopy density from the gap to the edge to closed canopy conditions along the downwind direction.We placed the experimental logs at nine systematic positions(3 locations×3 plots)on August 2013.
A system of five decay classes was used in our study to define the decay stages of logs according to the modified criteria of Rouvinen(Rouvinen et al.,2002;Chang et al.,2020).The decay classes are defined as:I)cambium still fresh and almost without epixylic vegetation;II)slightly decayed xylem with sparse moss or vegetation;III)relatively intact shape but with loose xylem and the epixylic moss extending further;IV)heartwood rotten and oval-shaped,and covered with moss or other vegetation;V)partially collapsed structures under the thick moss dominated vegetation cover.In the decay classes IV and V,the bark was absent or difficult to distinguish from the highly decayed wood.Therefore,the bark from decay classes IV and V were excluded.The newly harvested tree logs were used to represent decay class I for ensuring a precise starting decomposition state.Fallen logs,which mainly originated from windthrow that were each approximately 30 cm in diameter,were collected as decaying log samples for the decay classes II–V.Each fallen log that had been collected was cut into sections of 120 cm in length,which were carefully transferred into the plot sites.Two sets of decay class I–V sections were placed in each forest location at least 3 m away from each other.Parts of the sections were selected for manual removal of the epixylic vegetation(epixylic vegetation removal)using a knife,while the remaining sections were kept in their natural state(epixylic vegetation growth).Sections from each decay class were removed from the logs at the same or under similar decomposition conditions.This was undertaken to avoid initial differences in nutrient concentrations in the decaying logs under different epixylic vegetation treatments and among forest locations.We collected a total of 90 log samples(5 decay classes×3 forest locations×2 epixylic vegetation treatments×3 replication plots)and positioned them for our incubation experiment.Each month,newly established epixylic vegetation on the logs in the first group was removed manually except for during the snow cover period.As an individual experimental object,each log of given decay class represents the response of the decaying log at different decomposition stages to the canopy gap and the epixylic vegetation.After all the log samples have been arranged and placed to their designated positions,a small wood disk sample was removed from each log and taken back to the laboratory to measure the initial nutrient concentrations in wood and barks(Table 1S).
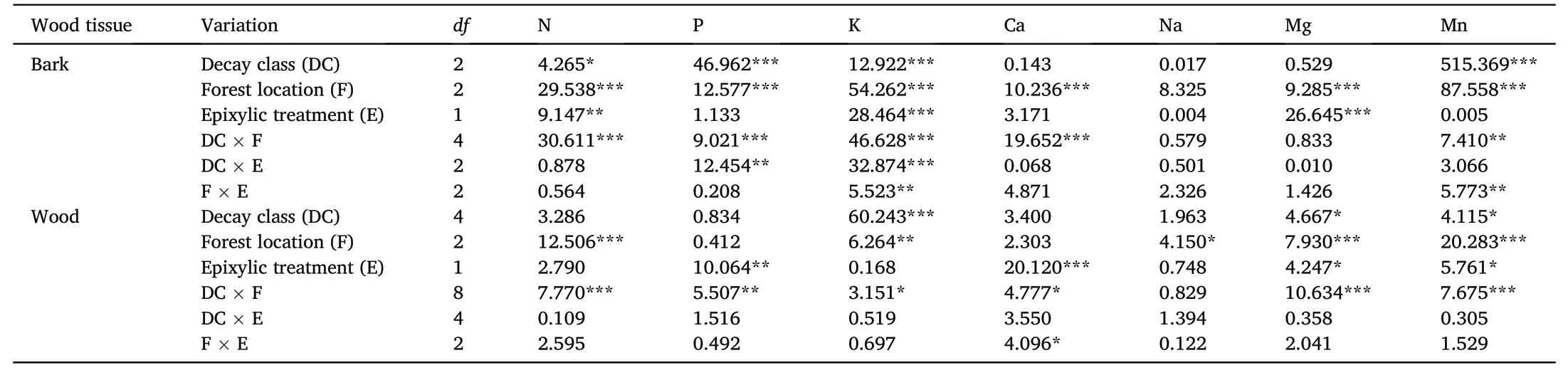
Table 1Multivariate analysis of variance(MANOVA)for the effects of the decay classes,forest locations,and the epixylic vegetation treatments and their interactions on the nutrient concentrations of the decaying wood and bark.The concentration of each nutrient was estimated in the decaying wood and bark for two epixylic vegetation treatments(epixylic vegetation cover vs.epixylic vegetation removal)exposed to three forest locations(gap,edge,and canopy).The F-values shown and asterisks represent the significance(*p<0.05,**p<0.01,and***p<0.001).

Fig.1.Estimated linear mixed-effects models for the effect of the forest location on the nutrient concentrations of decaying wood and bark in different decay classes.The nutrient concentrations in logs of different decay classes in the three forest locations(gap,edge,and canopy)were tested using this model.Different uppercase letters indicate significant differences between the forest locations.
2.3.Sampling and chemical analysis
After four years of incubation,we started sampling during August 2017.A disk sample of 2 cm in thickness was removed from each decomposing log with a saw for the decay classes I–III on each plot.For the decay classes IV and V,fragmented wood samples were collected.All the samples were placed into ziplock plastic bags and then transported to the laboratory for analysis.
The disk samples were divided into wood and barks.Five cubes(2 cm×2 cm×2 cm)and larger pieces of bark fragments were cut from each disk.All the log samples were classified based on the forest location,epixylic vegetation treatment,wood traits,and the decay class.They were oven-dried to constant weigh at 65°C and weighed separately to determine the relative moisture content.The dried wood cubes and bark fragments were ground and filtered using a 2.0-mm mesh.The pH analysis was performed using a pH meter(PHS–25CW,Bante Instrument,Shanghai,China).The concentrations of carbon(C),nitrogen(N),and phosphorous(P)in the log samples were measured using dichromate oxidation,Kjeldahl digestion(KDN,Top Ltd.,Zhejiang,China),and phosphomolybdenum yellow spectrophotometry(TU-1901,Puxi Ltd.,Beijiang,China),respectively.To measure the concentrations of potassium(K),calcium(Ca),sodium(Na),magnesium(Mg),and manganese(Mn)in the decaying logs,the oven-dried samples were digested with a concentrated acid mixture of HNO3–HClO4(5:1,v/v)and heated to 160°C for 5 h.After the digestion had been undertaken,the concentrations of the cations were analyzed using atomic absorption spectrometry(AAS,AA-7000,Shimadzu Corporation,Kyoto,Japan).
2.4.Data analysis
To test the effects of the decay class,forest location,epixylic treatment,and their interactions on nutrient concentrations in decaying logs and to compare the extent to which each factor affected the nutrient concentrations,we applied multivariate analysis(MANOVA).The effects of forest location,epixylic vegetation,and their interaction on nutrient concentrations in decaying logs were separately tested by applying linear mixed-effects models(package lmer4).The forest location effects in different decay classes of decaying logs were estimated in the first model with plot identity and epixylic vegetation treatment as random effects.The epixylic vegetation effects in different decay classes of decaying logs were estimated in the second model with plot identity and forest location as random effects.The epixylic vegetation effects in different forest locations were estimated in the third model with plot identity and decay class as random effects.To simultaneously test the significant differences in nutrient concentrations in decaying logs between different forest locations or epixylic vegetation treatments in all models,such as gap vs.canopy and epixylic vegetation cover vs.epixylic vegetation removal,multiple comparisons with the adjustment ofp-values were carried out based on the‘glht’function(package multcomp;Frank et al.,2010).We also performed redundancy analyses(RDA)using the‘rda’functions in the add-on package‘vegan’to explore the relationship between the nutrient concentrations in wood and barks with the deadwood quality in different forest locations and the epixylic vegetation treatments.One-way analysis of variance(ANOVA)was used to analyze the differences in nutrient concentrations among the epixylic vegetation,soil,decaying barks,and the decaying wood at our study site.All the statistical analyses were conducted in R 4.0.2(http://www.r-project.org).
3.Results
3.1.Effect of forest canopy density on the nutrient concentrations of decaying logs
The concentrations of most nutrients in the decaying wood and barks were significantly affected by the forest location and the decay class(Table 1).The concentrations of K,Na,Mg,and Mn in barks were higher under the closed canopy than in the gap and edge and a significant difference between forest locations was observed for Mn concentration(Fig.1).The effects of the forest location on the concentrations of N,P,and Ca in barks differed between decay classes.The forest location had little or no effect on nutrient concentrations in the decay classes I–III wood.Almost all nutrient concentrations(except Na)in the decay classes IV and V wood were higher under the closed canopy than in the gap and edge and the significant differences between forest locations were observed for N,Mg,and Mn concentrations.
3.2.Effect of epixylic vegetation on nutrient concentrations in decaying logs
As shown in Fig.2,the significant differences in concentrations of P,K,Mg,and Mn in barks between epixylic vegetation treatments were found.Among these,the bark K concentration increased after epixylic vegetation removal and the effects of epixylic vegetation on other nutrient concentrations depended on the decay class.The concentrations of N,P,Ca,and Mn in the decay class IV wood decreased after epixylic vegetation removal.There were no significant changes in the wood Na and Mn concentrations after epixylic vegetation removal.The effects of epixylic vegetation on the wood K concentration differed between decay classes II and III.
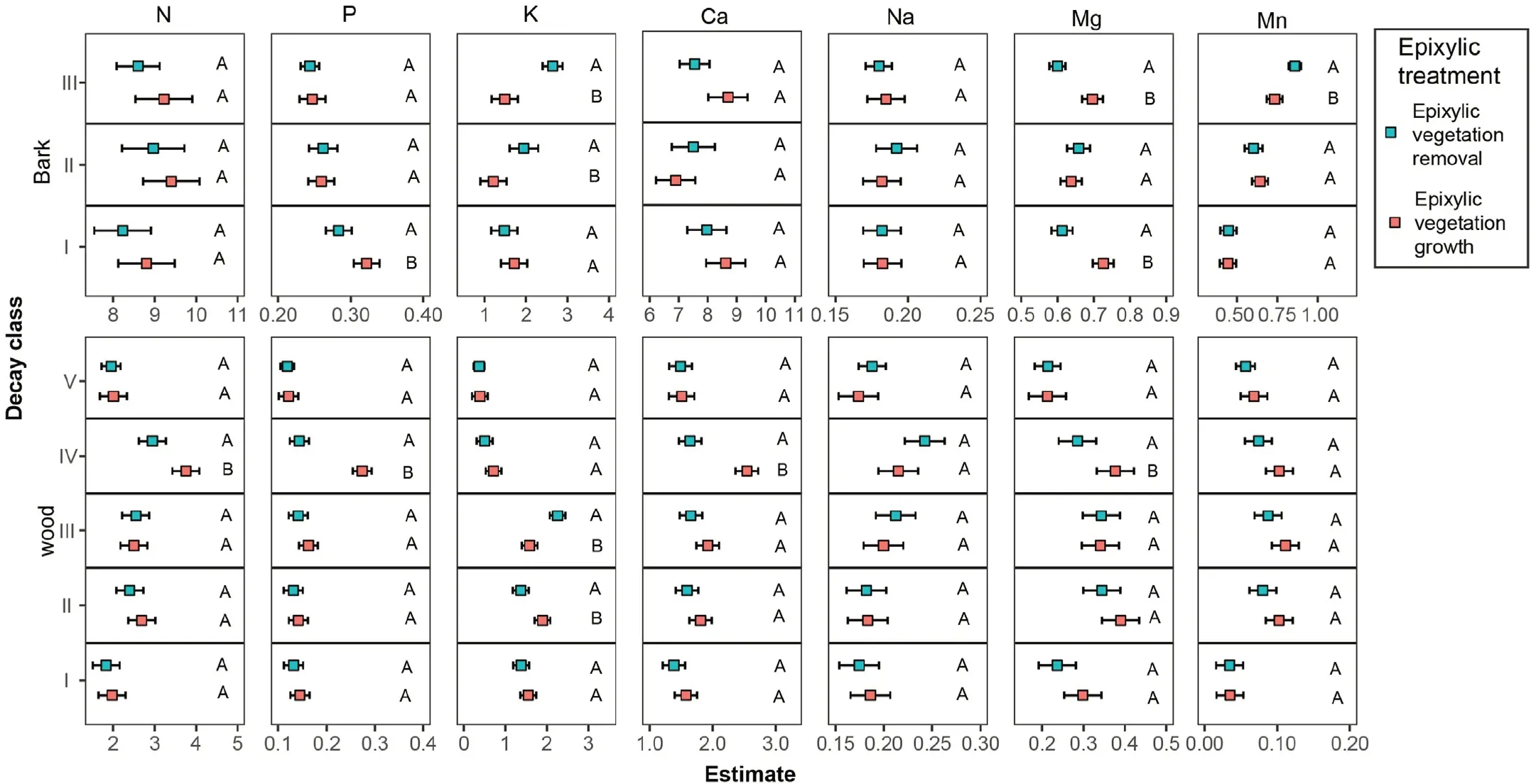
Fig.2.Estimated linear mixed-effects models for the effects of epixylic vegetation treatments on the nutrient concentrations of decaying wood and bark in different decay classes.The nutrient concentrations in the logs of different decay classes in two epixylic vegetation treatments(epixylic vegetation cover vs.epixylic vegetation removal)were tested using this model.Different uppercase letters indicate significant differences between the epixylic vegetation treatments.
3.3.Interactions between the forest location and epixylic treatment
The nutrient concentrations in the decaying wood and barks were most strongly affected by the forest location,followed by epixylic vegetation(Table 1).A significant interactive effect between forest location and epixylic vegetation was observed.Except for K and Na,nutrient concentrations in barks and wood after epixylic vegetation removal decreased in the gap and edge but did not change under the closed canopy(Fig.3).The concentrations of N,Ca,and Mg in barks and wood between epixylic vegetation treatments only in the gap showed significant differences.K concentration in all decay classes barks after epixylic vegetation removal was higher under the edge and closed canopy than in the gap.
4.Discussion
Our results showed a strong influence of forest locations and epixylic vegetation on nutrient concentrations in decaying logs.Open canopies decreased nutrient concentrations in decaying logs except for P and Na,which partly supported our first hypothesis.The negative effect of canopy opening was significant only for the specific decay class barks and the highly decayed wood.These results are consistent with the disturbance-related nutrient loss in the decomposition of CWD(Laiho and Prescott,2004).The positive effects of epixylic vegetation on nutrient concentrations of decaying logs were only observed under open canopies,which partly agreed with the second hypothesis.This result suggested that the epixylic vegetation effects depend primarily on gap-associated conditions.
4.1.Effects of forest canopy density
Forest canopy density can affect nutrient concentrations in logs in the decomposition process.The decreased nutrient concentrations in decaying logs under the open canopies may mainly result from the leaching effect.The strength and amount of precipitation reaching the surface of decaying logs gradually increase from the closed canopy to the forest gaps(Tan et al.,2018).The leaching process has been considered to be an important driver of nutrient loss of CWD in earlier studies(Gorgolewski et al.,2020;Minnich et al.,2021;Palviainen et al.,2004).Johnson et al.(2014)and Ganjegunte et al.(2004)also suggested that leaching can decrease bark K and Ca concentrations in log-bark decomposition over time.The significant effects of open canopies on nutrient concentrations in the highly decayed wood were observed.It is likely that nutrients are more easily leached only when the decomposing wood is permeable and porous(Lasota et al.,2018;Gorgolewski et al.,2020).In the case of K,which is a highly mobile cation in the CWD,a negative correlation of K concentration with logs moisture was found(Fig.2S).This correlation has also been described by Kuehne et al.(2008),who demonstrated that the amount of K in the leachate increased as the CWD decay progressed but the amount of leachate decreased due to the stronger water holding capacity of the highly decayed CWD.
Changes in N,P,Ca,and Mn concentrations in decaying logs are also related to deadwood-related fungal and bacteria activity(Laiho and Prescott,2004;Tl′askal et al.,2017).Most fungi prefer to live in a humid environment under the closed canopy within forests and require more nutrients for reproduction and development(B¨assler et al.,2021;Müller et al.,2020).Higher N and P concentrations in the decay classes IV and V wood under the closed canopy may be in part due to the N and P imports by cord-forming fungi through a mycelial network(Boddy and Watkinson,1995).Fungal activity can also increase Ca and Mn concentrations in the highly decayed wood under the closed canopy because Ca and Mn can be immobilized effectively in interstitial mycelium growing in the highly decayed wood(Harmon et al.,1994;Herrmann and Bauhus,2018).In contrast,open canopies accelerate the nutrient release from the highly decayed wood by promoting the invasion and colonization of invertebrates or microorganisms.Gap conditions with a warmer microclimate relative to the closed canopy usually correspond with the higher number of beetles living in the large diameter logs(Vogel et al.,2020)and with greater abundance and diversity of soil fauna(Tan et al.,2020).Boddy and Watkinson(1995)and Edmonds and Eglitis(1989)highlighted that the invertebrates colonized in deadwood could result in nutrient loss from decomposing logs not only by destroying the mycelia structures within deadwood but also by accelerating fragmentation.The Ca and Mn released from decaying wood may be one of the most important reasons for the higher Ca and Mn concentrations in soils at the gap and edge than under the closed canopy in our site(Wang et al.,2018b).
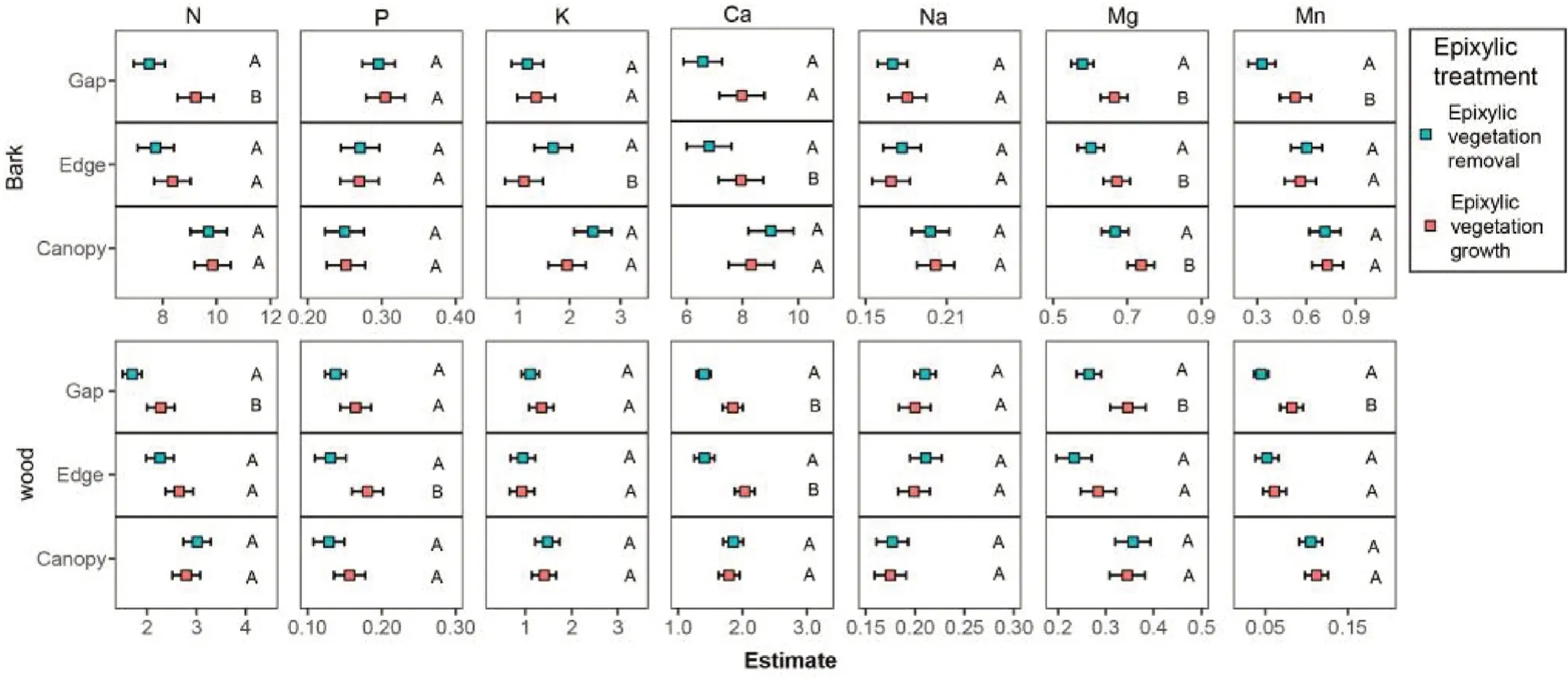
Fig.3.Estimated linear mixed-effects models for the effects of epixylic vegetation treatments on the nutrient concentrations of decaying wood and bark in different forest locations.The nutrient concentrations in logs with two epixylic vegetation treatments in three forest locations(gap,edge,and canopy)were tested using this model.Different uppercase letters indicate significant differences between epixylic vegetation treatments.
Na and Mg concentrations in CWD are relatively insensitive to mechanical leaching and are less influenced by decay-associated microbes compared with N,P,K,and Mn(Edmonds and Eglitis,1989;Holub et al.,2001;Laiho and Prescott,2004).In the present study,the Mg concentration in the decaying wood and barks was correlated with concentrations of N,Ca,and Mn(Fig.2S),in agreement with results described by Garcia-Palacios et al.(2015)as well as Laiho and Prescott(2004).Saunders et al.(2011)also showed that cord-forming fungi transfer Mg between the decaying logs and ambient environments along with N,P,and K.Apart from no difference in the Na concentration of decomposing logs between forest locations,the Na concentration is almost identical between soils and decaying logs at our study site(Fig.4).This likely implies the relatively low importance of Na for deadwood decomposition and decomposer activity.This implication is inconsistent with the positive effect of Na on the decomposition of litter and woody debris.Experiments conducted in subtropical and tropical forests have shown that increasing Na supply can facilitate termite-driven plant residue decomposition(Kaspari et al.,2009;Ji et al.,2020).This inconsistent finding suggests that Na may not be a crucial element for deadwood-associated microorganisms and invertebrates in the subalpine forest.
4.2.Effects of epixylic vegetation
Our results partly confirmed that epixylic vegetation positively influences nutrient concentrations in decomposing wood but has varying effects on those in different decay class barks.These observations were inconsistent with earlier studies(Harmon et al.,1994;Krankina et al.,1999;Lasota et al.,2018).The epixylic vegetation cover is widely thought to increase some nutrient releases from the deadwood at the advanced decay stages.A common explanation for the epixylic vegetation related nutrient releases from deadwood is that the epixylic mosses growing on the highly decomposed logs accelerates water penetration toward the wood inside,increasing the leaching effect.However,we found that epixylic vegetation did not change nutrient concentrations of decaying logs under the closed canopy despite the highest coverage and biomass of epixylic mosses on logs under the closed canopy(Wang et al.,2019).This finding suggests that the impact of epixylic vegetation on the nutrient concentrations of Minjiang fir deadwood is not directly related to the leaching effect.
The effects of epixylic vegetation on nutrient concentrations in barks exhibited high variability.Theoretically,the bark is more easily influenced by the epixylic vegetation due to direct contact.Bade et al.(2015)showed that epixylic vegetation can alter nutrient concentrations in barks because of frequent exchanges of water and cations between barks and the epixylic vegetation through stemflow.This process may depend on the nutritional requirements of the epixylic vegetation under different climate conditions.The epixylic mosses can effectively assimilate K released from barks due to the increasing demand for the K element in drought conditions in forests(Sardans and Pe~nuelas,2013).Thus,the epixylic vegetation cover decreased K concentration in barks in the present study.In contrast,we found that bark N concentration increased with epixylic vegetation cover.The increase in N concentration in barks may be due to N fixation by cyanobacteria growing in epixylic mosses or lichens(Tl′askal et al.,2017;Huang et al.,2019).
Higher nutrient concentrations in decaying wood and barks with epixylic vegetation cover may result from the nutrients releases of epixylic vegetation.During(1979)indicated that the nutrient availability of stumps increased after the death of epixylic mosses.Li et al.(2014)observed the most rapid releases of N and P from moss species although they had the slowest decay rate compared with litters and lichens in a subtropical montane forest.Rieley et al.(1979)contrasted some nutrient concentrations of the leachate from a bryophyte mat on the tree-bole with those of rainfall and throughfall in a Welsh woodland,suggesting a net loss of Mg from the moss layer.Most importantly,the N,P,Ca,and Mn concentrations in mosses are higher than in the decaying wood in the studied area(Fig.4).Therefore,epixylic vegetation may serve as a nutrient source for deadwood decomposition in subalpine forests.These findings indicate that decaying logs and the associated epixylic vegetation constitute a nutritional regulator for maintaining a nutrient-rich environment relative to surroundings,thereby enhancing the deadwood-related biodiversity and the underlying soil fertility.
4.3.Interactions between forest canopy density and epixylic vegetation
We observed an interactive effect between forest canopy density and epixylic vegetation on nutrient concentrations in barks and wood(Table 1).A positive impact of epixylic vegetation in the gap can be because the highest nutrient concentration in epixylic mosses in the gap potentially increases nutrient concentrations in decaying logs(Wang et al.,2018b).Open canopies promote nutrient translocation from the nutrient-rich epixylic vegetation to decaying logs through the leaching effect,invertebrate transport,and the mixture of fragmented deadwood and epixylic vegetation litter.This mechanism is vital to alleviate the lack of nutrients in decaying logs during decomposition under gap conditions.Although epixylic vegetation can be a nutrient source,there was almost no difference in nutrient concentrations in decaying logs between epixylic vegetation treatments under the closed canopy.It is likely that the cold condition under the closed canopy may freeze epixylic mosses during winter and that the nutrient released after the death of epixylic mosses is initially absorbed by the newly established epixylic vegetation(Huang et al.,2019;Fern′andez Martínez et al.,2021).These findings indicate that the forest canopy density plays a dominant role in governing the effects of epixylic vegetation on the nutrient cycle in deadwood.
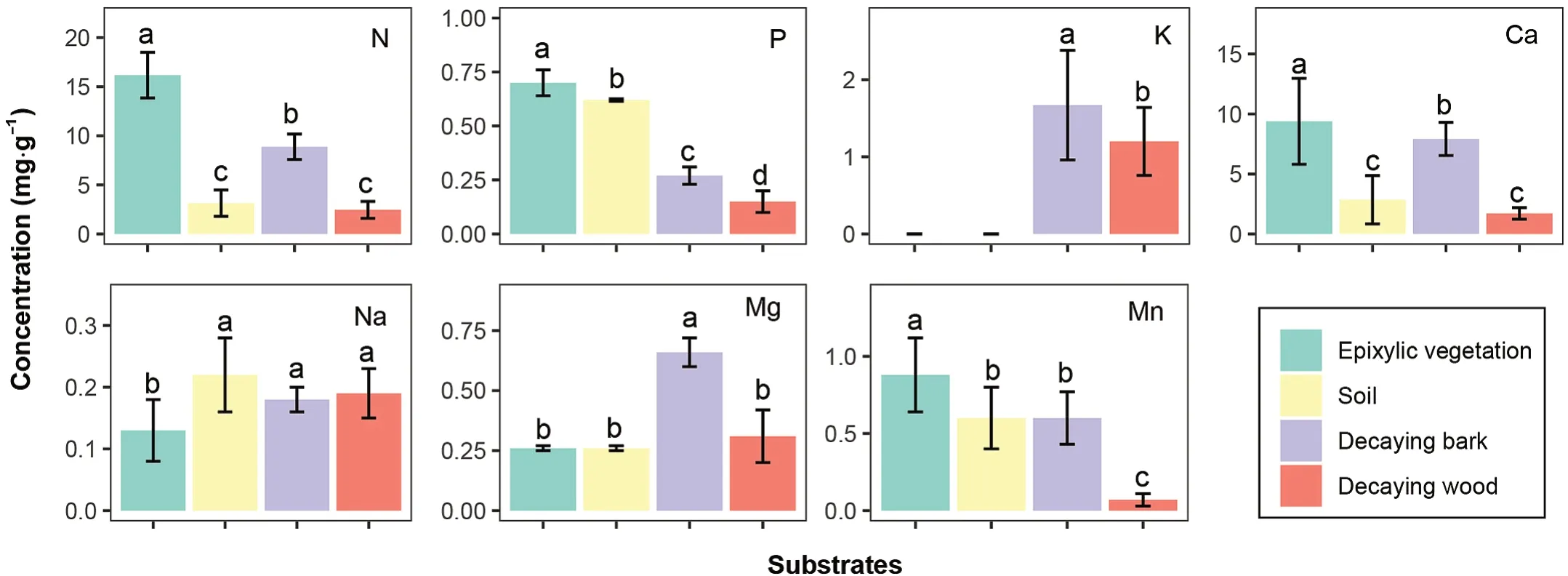
Fig.4.Comparisons of the mean nutrient concentrations(mg⋅g-1)among epixylic vegetation(mainly epixylic moss),soil,decaying bark,and the decaying wood at our study site.Different lowercase letters indicate a significant difference(p<0.05)in the concentration of the same nutrient among substrates.
5.Conclusions
This study focused on the effects of forest canopy density and epixylic vegetation on the nutrient concentrations in decaying logs.Our results indicated that open canopies had a negative impact on nutrient concentrations in decaying logs,especially for the highly decayed wood.The epixylic vegetation cover had a positive or no effect on the nutrient concentrations in decaying logs,depending on the decay class of logs and forest locations.The forest canopy density directly modulated the epixylic vegetation effect with a significant positive effect of epixylic vegetation on nutrient concentrations in decaying logs in the gap.These findings suggest that(1)the nutrient concentrations in decaying logs are sensitive to changes in microclimate;and(2)the forest canopy density,epixylic vegetation,and their interactions can directly alter the nutrient concentrations in decaying logs.Given that the effects of epixylic vegetation on nutrient concentration in decaying logs differed between forest locations,open canopies likely accelerated the rate of nutrient cycling between the epixylic vegetation and decaying logs in subalpine forests.
Author contributions
WQY and BT conceived and designed the experiment and contributed resources.ZW completed laboratory analysis and led the writing of the manuscript.ZW,QW,LFW,CHC,RC,and YRJ contributed to field work and laboratory analysis.WQY and JM provided writing assistance.All authors have read and agreed to the published version of the manuscript.
Funding
This work was jointly funded by the following grants:The National Natural Science Foundation of China(Nos.32071554,31870602,31901295);the National Key R&D Program of China(No.2017YFC0503906);and the Program of Sichuan Excellent Youth Sci-Tech Foundation(No.2020JDJQ0052).
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
This long-term field experiment was conducted in the subalpine forest.We thank Fan Yang and Ziyi Liang for their help in the field experiment.We are grateful for the financial support from the China Scholarship Council,China,and the office space provided by the Bavarian Forest National Park.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.i.org/10.1016/j.fecs.2022.100064.
- Forest Ecosystems的其它文章
- Multiple forest structural elements are needed to promote beetle biomass,diversity and abundance
- Diversity of click beetles in managed nonnative coniferous and native beech stands:Consequences of changes in the structural and species composition of tree stands in Central Europe
- Environmental and canopy conditions regulate the forest floor evapotranspiration of larch plantations
- Allometry-based estimation of forest aboveground biomass combining LiDAR canopy height attributes and optical spectral indexes
- Examining approaches for modeling individual tree growth response to thinning in Norway spruce
- Influence of soil and elevation on roadside cryptogam diversity in the tropical Andes

