Influence of soil and elevation on roadside cryptogam diversity in the tropical Andes
Pul Slins,Mrin Mz′on,Viniio Crri′on-Plines,Nixon Cumius,Ptriio Guzm′n,Polo Giorni,′Angel Benítez,,*
a Maestría en Biología de la Conservaci′on y Ecología Tropical,Universidad T′ecnica Particular de Loja,San Cayetano Alto s/n,Loja,1101608,Ecuador
b Centro de Investigaciones Tropicales del Ambiente y Biodiversidad–CITIAB,Universidad Nacional de Loja,Ciudadela Universitaria,sector La Argelia,EC 110101,Loja,Ecuador
c Biodiversidad de Ecosistemas Tropicales-BIETROP,Herbario HUTPL,Departamento de Ciencias Biol′ogicas y Agropecuarias,Universidad T′ecnica Particular de Loja,San
Cayetano Alto s/n,Loja,1101608,Ecuador
d Departament of Pharmacy,University of Genoa,Viale Cembrano 4,16148,Genoa,Italy
Keywords:Bryophytes Indicator species Beta diversity Lichens Richness Ecuador
A B S T R A C T Background:The deforestation caused by road construction is one of the main drivers for both biodiversity and function loss in tropical ecosystems.Terricolous cryptogams are pioneers in colonizing roadside and they are limited by environmental and edaphic factors,thus,cryptogams may act as pioneers for ecosystem rehabilitation at roadside.
1.Introduction
Anthropic disturbances related to deforestation,such as agricultural expansion,mining activites and the creation of road infrastructure,are causing ecosystem services loss related with composition,structure and processes(Wallace,2007),having a significant impact on tropical areas(Lamb et al.,2005;Laurance et al.,2009;Wentink,2015).Road construction has been identified as an important driver of deforestation in tropical areas(Fearnside,2005;Perz et al.,2007;Laurance et al.,2009;Ahmed et al.,2013;Baraloto et al.,2015),since it causes multiple ecological impacts by altering the biotic and abiotic conditions that affect both the structure and functioning of ecosystems from local to regional scale(Forman and Lauren,1998;L′azaro-Lobo and Ervin,2019).For instance,roadside vegetation is the result of landscape fragmentation,where the original plant cover has been transformed and often replaced by exotic or invasive species that may cause adverse effects on ecosystem functions(Maynard et al.,2016;L′azaro-Lobo and Ervin,2019)related with reducing water retention,increasing soil compaction,inducing changes in the nutrient cycle and causing soil loss due to sedimentation which makes them highly vulnerable to erosion(Coffin,2007;García--Palacios et al.,2011)and might lead to landslides and soil desestabilization.
Vegetation restoration at roadsides to avoid landslides should consider native species,as well as the successional stages(Arenas et al.,2017).For doing so,it is important to know the native plant species and their relationship to environmental variables(Van Diggelen et al.,2001;Wentink,2015),which will allow for,in the long term,the land rehabilitation and consequently the soil estabilization(Jackson and Hobbs,2009).At large scales,elevation has been identified as a determinant factor on plant diversity at roadside,where diversity of both native and exotic plant species did not follow a common pattern with elevation(Ar′evalo et al.,2005;Lembrechts et al.,2014;Bacaro et al.,2015;Haider et al.,2018).On the other hand,the physicochemical properties of soils have also been recorded as potent drivers of vegetation diversity at roadside.For instance,some studies have shown that fertility of the upper soil layer accelerates biotic assemblages and ecosystems’functional recovery,and favors the establishing of native plant species(Arenas et al.,2017;L′azaro-Lobo and Ervin,2019).Other factors that determine plant diversity are the type,age,total area and topography of the roadside,the surrounding vegetation and the management and maintenance activities of the roads(Deckers et al.,2005;Arenas et al.,2017;L′azaro-Lobo and Ervin,2019).
Most research dealing with vegetation diversity at roadside and their relationship with environmental variables have been carried out in temperate areas(Ar′evalo et al.,2005;Fekete et al.,2017,2020).In tropical zones,these kind of studies are relatively scarce(Maynard et al.,2016),with most focusing on vascular plants(Bernes et al.,2017).For instance,in Ecuador studies evaluated changes in plant diversity on roadside in northern and southern related to elevation and soil physicochemical properties,however,these insights may not be equally extrapolated to terricolous cryptogams(bryophytes and lichens),due to its peculiar characteristic.Bryophytes and lichens are the pioneers in colonizing disturbed areas,and thus they are commonly found growing along roadside(Concostrina-Zubiri et al.,2019),depending on the soil properties,elevation and surrounding vegetation(Concostrina-Zubiri et al.,2013).These organisms are keystones at roadside,acting as a seedbed for vascular plants and as a soil stabilizer,reducing nutrient loss and water overflow,which,together with bacterias,are known as biological soil crusts or biocrusts(Groeneveld et al.,2007;Concostrina-Zubiri et al.,2019).Therefore,cryptogams may act as pioneers for ecosystem rehabilitation at roadside,a function that has been relatively well documented in temperate areas,including biocrusts(Groeneveld et al.,2007;García-Palacios et al.,2011;Concostrina-Zubiri et al.,2019;Monteiro et al.,2020),but not so well studied in tropical ecosystems.In this context,indicator species analysis with cryptogams,including biocrusts has been used widely in previous studies related with forests succession(D′eleg et al.,2021),disturbance(Holz and Gradstein,2005),fragmentation(Gignac and Dale,2005)and climatic changes(Qian et al.,1999;Ponzetti and McCune,2001).
Considering the scarcity of the information on cryptogam colonization of roadside in tropical areas,we proposed this research aimed to:1)analyze the relationship of elevation and soil properties with the diversity and species composition of terricolous cryptogams at roadside,and 2)establish indicator species for early successional stages in these ecosystems as a tool for monitoring ecological processes.We hypothesize that changes in soil properties at every altitudinal level will determine the alpha and beta diversity of cryptogams at roadsides.
2.Methods
2.1.Study area
The study was carried out along the Loja-Zamora road,located in southern Ecuador,the asphalt road(ca.10–12 years)has large volumes of traffic(e.g.heavily trucks).We selected five areas within an altitudinal range(Table 1),from 2700 to 1000 m a.s.l.,with two replicates for every area(Fig.1).Vegetation corresponds to the Evergreen low montane forest from the southeastern Andes mountains in the high-elevation areas(E1,E2 and E3),and Evergreen low piedmontane forest from the southeastern Andes in the low-elevation areas(E4 and E5).The most predominant species in both vegetation types areAlchornea grandiflora,
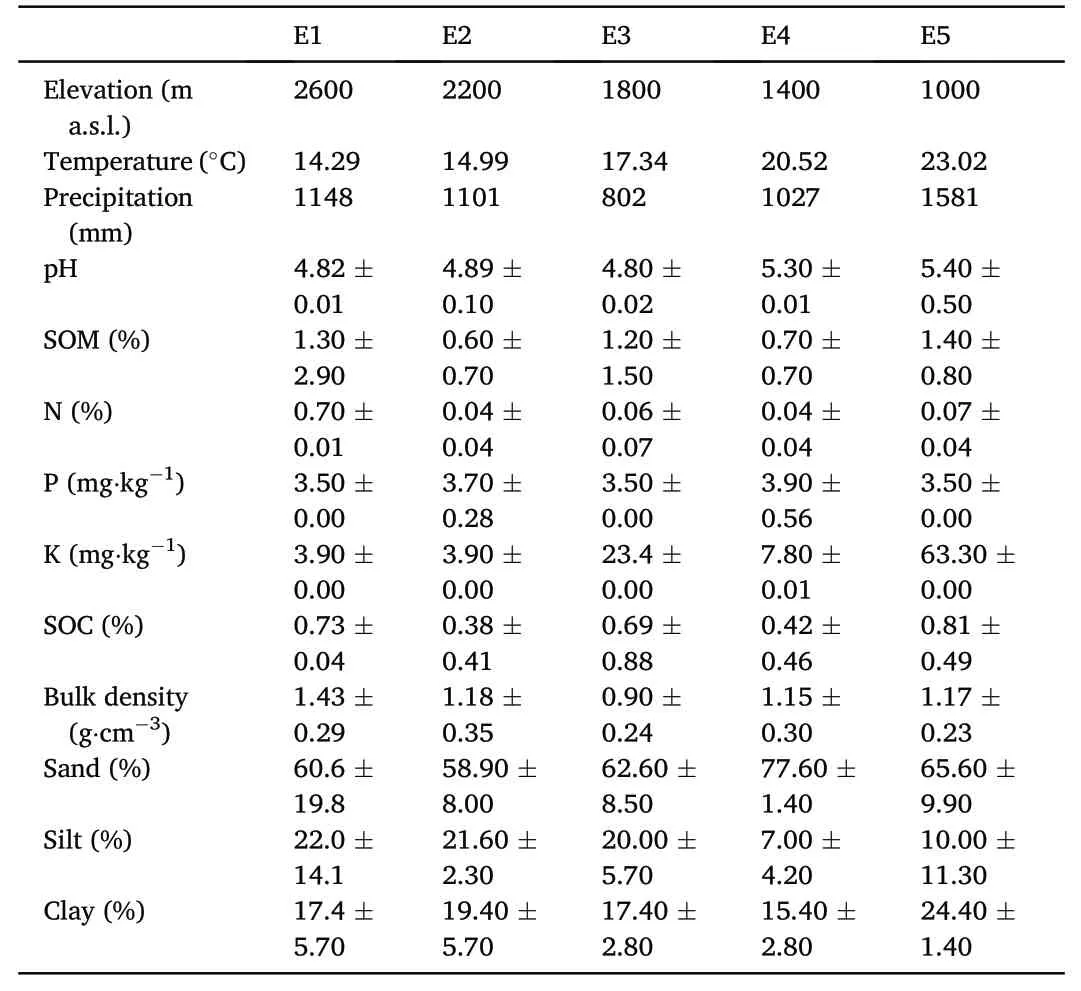
Table 1Description of the soil variables in every altitudinal gradient(E1‒E5).Mean values and the standard deviation are given.SOM=soil organic matter;SOC=soil organic carbon;Bd=bulk density.
Calyptranthes pulchella,Cedrela montana,Ceroxylon parvifrons,Cinchona mutisii,Clethra ovalifoliaandClusia alata(Ministerio del Ambiente del Ecuador,2013).The field phase was conducted from November 2019 to May 2020.The bioclimatic data of annual temperature(°C)and annual total precipitation(mm)were extracted from Worldclim(Hijmans et al.,2005).
2.2.Sampling design and data collecting
Along the studied area,five zones with different elevations were considered(hierarchical experimental design),which were separated±400 m a.s.l.from one zone to another(2600,2200,1800,1400,1000 m a.s.l.).The selected roadsides are characterized by moderately steep slopes(20%–30%),that were measured with a clinometer(PM-5/360 PC Clinometer,Suunto,Finland).In each area,two replicas were considered,where for each replicate,one 10 m2(2 m×5 m)transects were established perpendicular to the road(Vittoz et al.,2010;Guzm′an et al.,2022).Each transect was subdivided into 10 nested plots of one square meter,of which five nested plots were considered for sampling(2,4,6,8 and 10).The presence and cover of lichens and bryophytes were estimated using five 20 cm×30 cm grids(250 grids)nested in plots(Castillo-Monroy and Benítez,2015;Castillo-Monroy et al.,2016;Benítez et al.,2019).Additionally,elevation,slope(calculated with a clinometer,PM-5/300 PC clinometer,Suunto,Finland),and vascular plant cover(thecover of the vascular species was quantified in each nested plot,using a 1 m×1 m,divided into 0.10 cm×0.10 cm cell)were recorded.
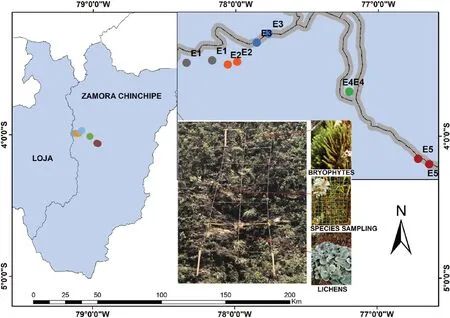
Fig.1.Study area in southern Ecuador,with location of the ten transects at five altitudinal gradients(G1‒G5)for evaluating the cryptogams at roadside.
Lichens and bryophytes were identified with a Zeiss PRIMO STAR microscope and a Zeiss Stemi DV4 stereoscope,together with specific taxonomic keys(Churchill and Linares,1995;Gradstein and Costa,2003;Lücking et al.,2013;Gradstein,2021).The identified samples were placed in the HUTPL herbarium(Universidad T′ecnica Particular de Loja).For the nomenclature of the species,we mainly followed,for bryophytes,the Liverworts and Hornworts of Colombia and Ecuador(Gradstein,2021),the World Checklist of Hornworts and Liverworts(S¨oderstr¨om et al.,2016)and the Catalogue of the Plants and Lichens of Colombia(Bernal et al.,2016),while the database MycoBank for lichens.
2.3.Soil sampling and analytical methods
To determine the bulk density(Bd),three soil samples were taken at a depth of 0–5 cm,using standardized metal cores(5.5 cm in diameter,5 cm high,119 cm3in volume).Thus,6 individual samples were obtained for bulk density analysis at each elevation gradient,obtaining 30 samples in total.In addition,two samples were taken at a depth of 0–10 cm with large standardized metal cylinders(6 cm in diameter,10 cm in height,283 cm3in volume)in each plot,and both were then mixed to obtain a composite sample(one composite sample per transect,two per elevation gradient,ten in total)that were used for the texture and chemical analysis(Munkholm et al.,2002).After collection,the soil samples were packed into separate plastic bags and were properly labeled.
In the laboratory,bulk density was first determined using the cylinder method for which the individual samples of Bd were oven-dried for 48 h at 105°C.Samples for textural and chemical analysis were then dried at room temperature for 72 h.Subsequently,all visible roots were removed and the samples were sieved through a 2-mm mesh.Soil texture was determined using the Bouyoucos hydrometer method(Black et al.,1965)while soil pH was measured with a pH meter applying the standard method(Black et al.,1965).Furthermore,soil organic carbon(SOC)and soil organic matter(SOM)were determined using the Walkley and Black method(Page et al.,1982),for which a thermostat at 125°C was inserted for 45 min after the samples were oxidized with a K2Cr2O7/H2SO4solution.Total nitrogen(TN)was determined by the Kjeldahl method,the phosphorus content(mg⋅kg-1)by the modified Olsen method(Bremner,1966),and the potassium content(mg⋅kg-1)by atomic absorption spectrophotometry(Tan et al.,2012).
2.4.Data analysis
Alpha diversity(i.e.,diversity within every altitudinal area)was analyzed by means of species richness,Shannon-Weaver and Simpson indices(Magurran,2004),and was graphically represented in a violin plot.The effects of altitude on richness,Shannon-Weaver and Simpson indices were analyzed separately using generalized mixed linear models(GLMMs)at grid level.In these models,altitude was used as predictor(fixed factor),whereas locality was included as random source of variation.We assumed Poisson errors for the response variables with the log link function.Effects of random factors were tested using the WaldZ-statistic test,and GLMMs were fit using the“lme4”R package with the function“glmer”(Bates et al.,2014).We used the Laplace approximation for likelihood estimates(Bolker et al.,2009).For GLMMs,the minimal adequate model was selected based on Akaike's information criterion(AIC).Finally,we used heatmap and Spearman's nonparametric correlation analysis(rho)to test the relationship between soil factors and species richness and diversity(Shannon-Weaver and Simpson indices).Beta diversity(i.e.,the species turnover between altitudinal areas)was evaluated by means of non-metric multidimensional scaling(NMDS)with Bray-Curtis distance and 999 Monte Carlo permutations with the“vegan”statistical package(Oksanen et al.,2019).To analyze the effect of environmental variables on beta diversity,a correlation was run between both axes and the environmental variables using the“envfit”function.Finally,to determine the indicator species for every elevation area,an indicator species analysis(ISA)was performed(Dufr^ene and Legendre,1997),using the IndVal function of the“labdsv”package(Roberts,2013).The indicator value ranges from 0(one species was absent from one elevation)to 1 or 100(one species occurred in all grids of one elevation and was absent from other grids).All the analyses were calculated utilizing the statistical software R 3.2.2.(R Core Team,2015).
3.Results
3.1.Alpha diversity
A total of 72 species were recorded,distributed into 44 bryophytes and 28 lichens(Table S1).The violin plot showed that the highest species richness was found in the highest elevations,gradually decreasing as elevation decreased(Fig.2).
On the one hand,soil factors such as bulk density,silt and nitrogen were greater in the highest elevations(Table 1).Conversely sand and pH were higher at lower elevations(Table 1).
This relationship was confirmed with the GLMMs,with elevation(E1‒E3)positively influencing species richness(p<0.0001)and Shannon-Weaver(p<0.0001)and Simpson indices(p=0.03).Besides,Spearman correaltions(Fig.3),represented by pH and K and sand,significantly and negatively affected cryptogam species richness and Shannon-Weaver index.
3.2.Beta diversity
The NMDS analysis showed the cryptogam species grouping according to elevations,with stronger clustering within and greater distance between the highest and lowest elevations(Fig.4).Soil properties and elevation were influencing the cryptogam composition(Table 2).The pH was explaining most variability in species composition(66%),followed by silt content(42%)and elevation(41%).The other variables explained less than 30%(Table 2).In the highest elevation areas(E1 and E2),silt content,bulk density and slope limited the terricolous cryptogam composition,while pH and sand content,which were determinant for the lowest elevation composition.
3.3.Indicator species
A total of 25 indicator species were reported,with 16 belonging to bryophytes and 9 to lichens.From the 25,18 species were selected as best suited for indicators by having an indicator value higher than 20(Table 3).

Table 2Data from the NMDS ordination for environmental factors and roadside cryptogam composition of the Loja-Zamora road.Squared correlation coefficients(R2)for axes 1 and 2,p-value and the contribution of every variable to the variability in every axis are shown.SOM=soil organic matter;Bd=bulk density.

Table 3Roadside cryptogam species selected as indicators of altitudinal gradients at the Loja-Zamora road.
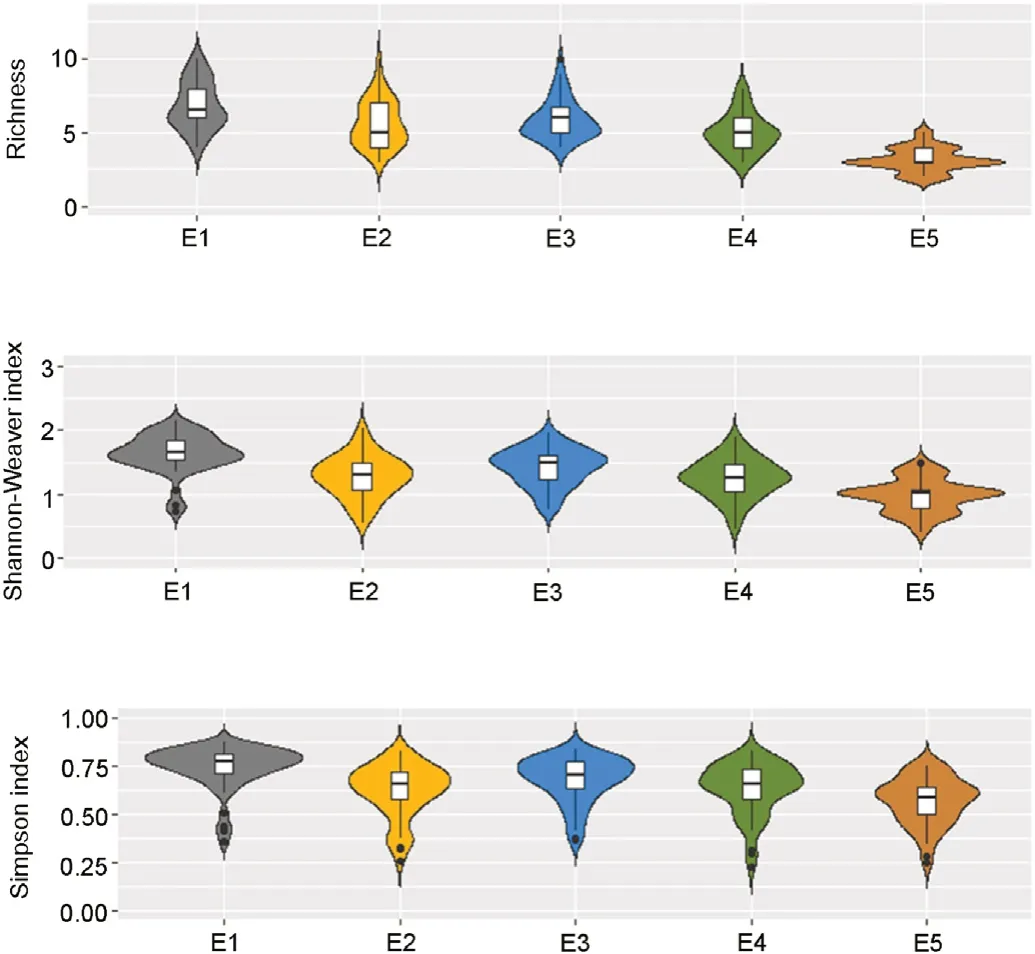
Fig.2.Violin plot for species richness and diversity indices(Shannon-Weaver and Simpson)of cryptogams in every elevation level of roadside(E1‒E5)along the Loja-Zamora road.Boxes span the first to third quartiles;the horizontal line inside the boxes represents the median.
4.Discussion
Our results indicated that elevation and soil properties such as bulk density,sand and silt content,total nitrogen,and pH,were the main properties responsible for the cryptogam diversity along the roadside of this tropical area.Both elevation and soil properties have been proven to influence roadside plant species diversity,not only for cryptogams but also for vascular plants in temperate zones(Ar′evalo et al.,2005;García-Palacios et al.,2011;Haider et al.,2018;Concostrina-Zubiri et al.,2019).
Usually,vascular plants species richness on roadside decreases with increasing elevation,or shows the highest values at mid-elevations(Ar′evalo et al.,2005;Haider et al.,2018),with only a few exceptions(Haider et al.,2018).However,our results were the opposite,with species richness being higher with higher elevation,a common trend recorded for terricolous cryptogams and biocrusts(Austrheim,2002;Bruun et al.,2006;Ah-Peng et al.,2007;Castillo-Monroy et al.,2016).In our study,species richness and diversity(Shannon-Weaver and Simpson indices)were positively correlated not only with elevation but also with silt content,and negatively with sand content.The establishment of non-vascular plant that benefit from silt content can be explained by two reasons:1)fine particles provide a nutrition source(Danin et al.,1989;Kidron et al.,2008)and 2)silt can absorb more moisture(Lan et al.,2015),creating a more favorable environment for them.
The composition of cryptogam communities being limited by elevation and soil properties,has also been found by other authors(Castillo-Monroy et al.,2016;Concostrina-Zubiri et al.,2019).In our study,species from the generaSyzygiella,Gongylanthus,HerbertusandStereocaulonwere abundant in soils with a higher bulk density,silt and nitrogen content,but with less sand content.Conversely,lower elevation composition,such as those consisting ofPlagiochilaandCorawere more commonly found in soils with a lower bulk density and higher values of sand content and pH.The pH is the most important variable to define plant species composition(Old′en et al.,2016),as seen in our study,since pH determines if a species can or can not grow and also affects their competitive abilities(Dupr′e and Ehrl′en,2002;L¨obel et al.,2016).Soil bulk density was also one of the more influential variables affecting the composition and establishment in the higher elevations,where density was lower.A higher bulk density implies a higher soil compaction,and soil compaction has a significant effect on plant growth by modifying the water and solute movement,as well as biological activity and soil airing(Atkinson et al.,2009).Nitrogen content also showed a relationship with cryptogam composition.According to several authors,some lichen(e.g.Stereocaulon)and bryophyte communities are able to fix tropospheric nitrogen and therefore can significantly increase the available nitrogen content(Gunther,1989;Gordon et al.,2001;Sottocornola et al.,2007;Pouliot et al.,2009;Hugron et al.,2013).Thus,Evans and Ehleringer(1993)and Lange et al.(1994)found that biocrusts can be an important sink aiding in the accumulation of nitrogen,which greatly contributes to soil stabilization(e.g.dune stabilization).
On the other hand,Zhang and Nie(2011)and Tavili et al.(2017)showed that a cover of cryptogams produces a direct effect on soil fertility in terms of constant increases towards optimal amounts of nutrients such as N,K,Ca,Mg and P,which leads to better seed germination,seedling emergence and initial growth.In addition,lichens and mosses have a great capacity to absorb elements from the soil due to their metabolic processes(Szczepanika and Biziuk,2003).Therefore,the species identified in this study have a potential use in restoration projects on the roadsides of southern Ecuador.
Cryptogams help in stabilizing soils(Arenas et al.,2017;Austrheim,2002),promoting soil fertility and improving microbial activity(García-Carmona et al.,2020),and reducing impacts of climate warming on soil properties(García-Carmona et al.,2020).They constitute a biological crust,which are currently being used as a promising tool for rehabiliting soils,especially in drylands,by translocation or by inoculation from laboratory(García-Palacios et al.,2011;Ballesteros et al.,2017;Antoninka et al.,2020;Rosentreter,2020).Lichens and bryophytes are important pioneer species,they play an essential role in the early stages of succession(Concostrina-Zubiri et al.,2019),which also help in the establishment of vascular plants(Hawkes,2004;Ah-Peng et al.,2007).Therefore,cryptogams should be further considered for ecological rehabilitation strategies(Lorite et al.,2020)especially among roadside in tropical areas,since these ecosystems are highly vulnerable to landslides,both natural and anthropic.This research helps in providing a combination of indicator species(both of bryophytes and lichens)with specific elevation and soil requirements,which may help in roadside stabilization.For instance,Syzygiella rubricaulis,Gongylanthus granatensis,Campylopus richardii,Isotachis multicepsandStereocaulon ramulosum
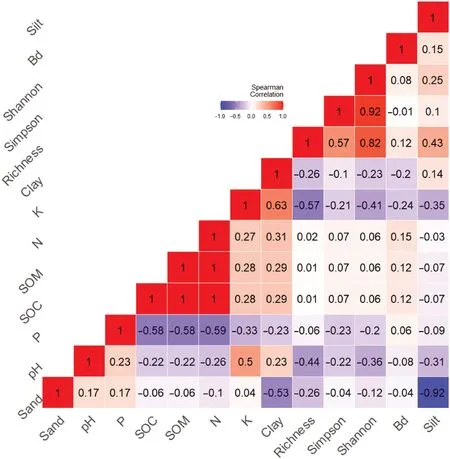
Fig.3.Correlation matrix(heatmap)of the richenss and diversity(Shannon-Weaver and Simpson indices)and soil factors.
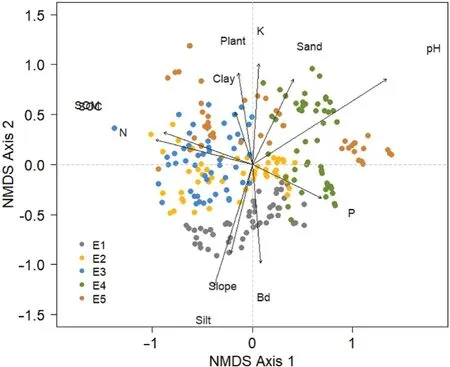
Fig.4.Non-metric multidimensional scaling analysis of roadside cryptogam species composition and environmental variables in the five studied elevations(E1–E5).
might be established in of high-elevation areas with a higher bulk density and silt as well as nitrogen content.However,for the study area,it is recommended that further research be applied to determine the development of these species through the evaluation of natural succession stages(over time)as developed in recent research(e.g.,Concostrina-Zubiri et al.,2019).In addition,future research should evaluate the effect of lichens and bryophytes in improving the physical-chemical properties of the topsoil,such as texture,nutrient content,and evaluate the interactions between the biological soil crust and vascular plants over time.
Several studies have been conducted on the role of biocrusts in enhancing the structural stability of soils in temperate zones(Issa et al.,2001;Li et al.,2002).However,cryptogams,and biocrusts in general,despite their potential,have been poorly studied in tropical areas(Castillo-Monroy et al.,2016;Antoninka et al.,2020).The lichen and bryophyte species that should be used as biological crust for ecological rehabilitation purposes should be site-appropriate(Rosentreter,2020),and for this reason more research,such as that presented here,is needed in order to understand the ecological variables that may favor cryptogam species establishment and to stabilize tropical roadside.
5.Conclusions
Our study provides new evidence for the drivers(elevation and soil properties)of cryptogamic diversity in Andean roadside that harbor a high diversity.Cryptogam diversity and composition were influenced by elevation and soil properties such as bulk density,pH,silt,sand and nitrogen content.We obtained a group of terricolous cryptogam species indicators for every set of environmental and soil conditions at roadsides,which may be used as a baseline for monitoring ecological processes in tropical areas.Cryptogam diversity could be an effective indicator for environmental and soil changes in roadsides,thus this study will be useful for supporting the management and stabilization of roadside.
Author contributions
PS,AB,VCP,PAG,NC,conceptualization,methodology,validation,investigation,data curation;′AB,PG analyzed the data;PS,AB,VCP,PAG,NC,MM,PG,writing–original draft,writing–review and editing.
Funding
This research was funded by Universidad T′ecnica Particular de Loja(UTPL-PROY_INV_CCBIO_2020_2773 and research scholarship I-II-III CONV).
Ethics approval and consent to participate
All the authors have approved the manuscript and agreed with submission to your esteemed journal.
Consent for publication
Not applicable.
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgements
We thank the Ministerio del Ambiente y Agua del Ecuador for providing access to the study areas and anonymous reviewers for constructive comments on the manuscript.Special thanks to Gregory Gedeon for English text revision on the manuscript.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.i.org/10.1016/j.fecs.2022.100061.
- Forest Ecosystems的其它文章
- Multiple forest structural elements are needed to promote beetle biomass,diversity and abundance
- Diversity of click beetles in managed nonnative coniferous and native beech stands:Consequences of changes in the structural and species composition of tree stands in Central Europe
- Environmental and canopy conditions regulate the forest floor evapotranspiration of larch plantations
- Allometry-based estimation of forest aboveground biomass combining LiDAR canopy height attributes and optical spectral indexes
- Examining approaches for modeling individual tree growth response to thinning in Norway spruce
- Patterns of species diversity and its determinants in a temperate deciduous broad-leaved forest

